苯丙胺
此條目可参照英語維基百科相應條目来扩充,此條目在對應語言版為高品質條目。 |
安非他命(英文名稱:Amphetamine[note 1]为一种中樞神經興奮劑,用來治療注意力不足過動症、嗜睡症、和肥胖症。“Amphetamine”一名擷取自 alpha‑methylphenethylamine。
安非他命於西元1887年被發現,以兩種對映異構體的形式存在[note 2] ,分別是左旋安非他命和右旋安非他命。
准确来说,安非他命指的是特定的化學物質-外消旋純胺類型態[24][25],這個物質等同於安非他命的的兩個對映異構體:左旋安非他命和右旋安非他命的等比化合物之純胺類型態。 然而,實際上安非他命一詞已被廣泛的用來表示任何由安非他命對映異構體構成的物質或安非他命對映異構體本身。[21][26][25]
安非他命是一种中樞神經興奮劑,適度適量地使用能提升整體抑制控制能力[27][28]。在醫療用的劑量範圍內,安非他命能帶來情緒以及執行功能的變化,例如:欣快感的增强、性欲的改變、清醒度的提升、大腦執行功能的進化。安非他命所改變的生理反應包含:減少反應時間、降低疲勞、以及肌耐力的增強。然而,若攝取劑量远超过醫療用的劑量範圍,將會導致大腦執行功能受損以及橫紋肌溶解症。 攝取過份超越醫療用劑量範圍的安非他命可引发嚴重的藥物成癮。然而長期攝取醫療劑量範圍的安非他命並不會產生上癮的風險。
此外,服用远超醫療用劑量範圍的安非他命會引起精神疾病(例如:妄想[參 1]、偏執[參 2])。然而長期攝取醫療劑量範圍的安非他命並不會引起上述疾病。
那些为享乐而摄入的安非他命通常会遠超過醫療用劑量範圍,且伴隨著非常嚴重甚至致命的副作用。 [sources 1]
历史上,安非他命也曾被用來治療鼻塞(nasal congestion)和抑鬱。
安非他命也被用來提升表現、促進大腦的認知功能及在助興時(非醫療用途情況下)被作為增強性慾[a]、和欣快感促進劑。
安非他命在許多國家為合法的處方藥[參 3]。然而,私自散布和囤積安非他命被視為非法行為,因為安非他命被用於非醫療用途的助興可能性極高。[sources 2]
首個藥用安非他命的藥品名稱為Benzedrine。當今藥用安非他命[參 4]以下列幾種形式存在:外消旋安非他命[參 5]、Adderall [note 3]。 、dextroamphetamine、或對人體無藥效的前驅藥物體[參 6]:lisdexamfetamine。
安非他命藉著自身作用於兒茶酚胺神經傳導元素:正腎上腺素及多巴胺的特點來活化trace amine receptor ,進而增加单胺类神经递质和神经递质(excitatory neurotransmitter)在腦內的活動。[sources 3]
安非他命屬於替代性苯乙胺類的物質。由安非他命衍伸出的物質被歸納在替代性苯乙胺[參 7]的分類中[note 4],比如說:安非他酮[參 8]、 cathinone、 MDMA、 和 甲基苯丙胺[參 9]。安非他命也與人體內可自然生成的兩個屬於痕量胺的神經傳導物質--特別是 phenethylamine 和 N-Methylphenethylamine--有關。 Phenethylamine 是安非他命的原始化合物,而N-methylphenethylamine則是安非他命的位置異構體(只有在甲基族中才會區分出此位置異構體)。[sources 4]
用途
醫療
安非他命是用來治療注意力不足過動症(ADHD)、嗜睡症(一種睡眠疾病)、和肥胖症。有時候安非他命會以仿單標示外使用的方式處方來治療頑固性憂鬱症及頑固性強迫症[1][10] [43] [50]。 在動物試驗中,已知非常高劑量的安非他命會造成某些動物的多巴胺系統和神經系統的受損。[51][52] 但是,在人體試驗中,注意力不足過動症患者在接受安非他命的治療後,則發現安非他命可促進大腦的發育及神經的成長。[53][54][55]
回顧許多核磁共振照影(MRI)的研究後發現,長期以安非他命治療注意力不足過動症患者能顯著降低患者大腦結構及大腦執行功能上的異常。並且優化大腦中數個部位,例如:基底神經節的右尾狀核。 [53][54][55]
眾多臨床研究的系統性及統合性回顧已確立長期使用安非他命治療注意力不足過動症的療效及安全。[56][57][58]
持續長達兩年的隨機對照試驗[參 10][b]結果顯示:長期使用安非他命治療注意力不足過動症,是有效且安全的。[56][58]
兩個系統性/統合性回顧的結果顯示長期且持續地使用中樞神經興奮劑治療注意力不足過動症能有效地減少注意力不足過動症的核心症狀(核心症狀即為:過動、衝動和分心/無法專心)、增進生活品質、提升學業成就、廣泛地強化大腦的執行功能。[note 5] 這些執行功能分別與下列項目有關:學業、反社會行為、駕駛習慣、藥物濫用、肥胖、職業、日常活動、自尊心、服務使用(例如:學習、職業、健康、財金、和法律等)、社交功能。[57][58]
一篇系統性/統合性回顧標誌了一個重要發現:一個為期九個月的隨機雙盲試驗中,持續以安非他命治療的ADHD患者,其智力商數平均增加4.5單位[註 1],且在專注力、衝動、過動的改善皆呈現持續進步的態勢。[56] 另一篇系統性/統合性回顧則指出:根據迄今為止為時最長的數個臨床追蹤研究[參 11],可以得到一個結論:即便從兒童時期開始以中樞神經興奮劑治療直到老年,中樞神經興奮劑都能持續有效地控制ADHD的症狀並且減少物質濫用的風險。[58] 研究表明,ADHD與大腦的執行功能受損有關。而這些受損的執行功能分別與大腦中部分的神經傳導系統有關[參 12]。[59] ;又此部分受損的神經傳導系統和中腦皮質激素-多巴胺[參 13]的傳導及藍斑核[參 14]和前額葉[參 15]中的正腎上腺素[參 16]的傳導相關。[59]
中樞神經興奮劑,例如:methylphenidate和安非他命對於治療ADHD都是有效的,因為中樞神經興奮劑刺激了上述神經系統中的神經傳導物質活動。[29][59] [60]
至少超過80%的ADHD患者在使用中樞神經興奮劑治療後,其ADHD的症狀可以獲得改善。[61]
使用中樞神經興奮劑治療的ADHD患者相較之下,普遍與同儕及家庭成員的關係較佳並且在學校擁有較好的表現。興奮劑能使ADHD患者較不易分心、衝動、且擁有較長的專注力時間和範圍。[62] [63]
根據考科藍協作組織[參 17]所提供的文獻回顧結果[note 6]指出:使用中樞神經興奮劑治療的ADHD患者即便其症狀改善,相較於使用非中樞神經興奮劑,仍因副作用而有較高的停藥率。[65] [66]
回顧結果也發現,中樞神經興奮劑並不會惡化抽動綜合症的症狀,例如:妥瑞氏症,除非服用dextroamphetamine[c]的劑量過高才有可能在部分妥瑞氏症合併注意力不足過動症患者身上觀察到抽動綜合症的症狀惡化。[67]
中樞神經興奮劑只要依照醫師指示用藥,都是相當安全的。[68][69][69][70] 中樞神經興奮劑,例如:利他能與專思達,可能導致:心悸、頭痛、胃痛、喪失食慾、失眠、因相對專注而變得冷淡(面無表情)等副作用,因此6歲以下的兒童不適宜服用。(副作用產生與否因人而異) [71]
隨著時間推進與各方的努力,中樞神經興奮劑的相關副作用已可藉由包括但不限於劑量調整、服藥時間、飯前飯後服用、服藥頻率等服藥模式之改變以及改變藥物組合等方式獲得相當程度的減少。[72] [73] [74] [69] [75]
提昇表現
- 認知方面(Cognitive)
西元2015年中,一篇系統性回顧[參 18]和一篇元分析/整合分析[參 19]回顧了數篇優秀的臨床試驗[參 20]報告後發現, 低劑量(醫療用劑量)的安非他命能適度但不強烈地促進一個人的認知功能,包含工作記憶(working memory)、長期的情節記憶(episodic memory)、抑制控制以及在一些方面的注意力(attention)。 [27] [28] 安非他命強化認知功能的效果已知是部分透過間接活化在大腦前額葉(prefrontal cortex)的dopamine receptor D1 和adrenoceptor α2。 [29] [27] 一篇2014年的系統性回顧發現低劑量(醫療用劑量)的安非他命能促進memory consolidation,進而提升一個人的recall of information。 [76] 低劑量(醫療用劑量)的安非他命也可增加大腦皮層(質)區的效率,這能讓一個人的工作記憶(working memory)獲得進步。 [29] [77] 安非他命和其他用於治療ADHD的中樞神經刺激劑能透過提升task saliency來增加一個人去做事情的動機、並強化一個人的警覺心(清醒度),因而能刺激一個人開始做「以目標為導向」的行為。 [29] [78] [79] 中樞神經興奮劑(例如:安非他命)能提升一個人在困難且枯燥的任務中的表現。 [29] [79] [80] 超過醫療用劑量範圍(包含其誤差範圍及容許最大上限)的安非他命劑量將不利於工作記憶(working memory)和其他的認知功能。 [29][79]
- 生理(physical)
雖然安非他命可以提升速度、耐力(延遲疲勞的發生)、肌耐力、身體素質和警覺心並減少心理反應時間。[30][34] [30] [81] [82] 然而,「非因醫療需求使用安非他命」在各種運動場合都是被嚴格禁止的。[83] [84]
安非他命藉由抑制多巴胺在中樞神經系統中的回收及外流來促進耐力和反應時間的提升。 [81][82] [85] 安非他命和其他作用於多巴胺系統的藥物一樣,都能增加在固定施力(levels of perceived exertion)下的動力(能)輸出。這是因為安非他命能奪取(override)體溫的「安全開關」的控制權並將身體核心溫度(core temperature limit)的上限提高以取得在體溫安全上限提高前被身體保留的能量。 [82] [86] [87] 於醫療用劑量範圍(包含其誤差範圍),安非他命的副作用不至於影響運動員的運動表現; [30][81] 然而,當攝取的劑量過多時,安非他命可能會引起嚴重的後果,例如:橫紋肌溶解症和體溫過高。 [31][33] [81]
醫療上的禁忌
根據International Programme on Chemical Safety (IPCS)和美國食品藥物管理局 (USFDA), [note 7]
安非他命不建議處方給有藥物濫用、心血管疾病、對於各種刺激嚴重反應過度、和嚴重焦慮歷史的人。 [note 8][89][90]
安非他命也不被建議處方給正經歷動脈血管硬化(血管硬化)、中度到重度高血壓、青光眼(眼壓過高)、或甲狀腺機能亢進(身體在體內製造出過量的甲狀腺 賀爾蒙/激素)的人。 [89][90][91]
曾對中樞神經刺激劑有藥物過敏的人以及正在服用單胺氧化酶抑制劑 (MAOI)或單胺氧化酶抑制劑類藥物 (MAOIs),可能不適合使用安非他命。即便曾有合併使用安非他命和單胺氧化酶抑制劑後仍一切平安的案例。 [89][90] [92][93] IPCS和美國食品藥物管理局也同意患有神經性厭食症(anorexia nervosa)、雙極性情感疾患(bipolar disorder)、憂鬱、高血壓、mania、思覺失調症、Raynaud's phenomenon、心臟病發(seizures)、抽動綜合症(tics)、妥瑞氏症(Tourette's disease)、和有甲狀腺問題、肝腎問題的人在使用安非他命時應密切追蹤上述疾病的變化。 [89][90]
人體試驗證明,醫療用劑量下的安非他命並不會導致胎兒或新生兒畸形(i.e., it is not a human teratogen)。然而超越醫療用劑量甚多的安非他命確實會增加胎兒或新生兒畸形的機會。 [90]
研究觀察發現,安非他命會進入母親的母乳中,因此建議母親不要在使用安非他命藥物的期間內授乳。 [89][90]
由於安非他命可能影響食慾繼而導致可反轉的身高及體重的成長遲緩, [note 9] ,因此建議兒童或青少年在用藥期間定期測量自己的身高及體重。 [89]
副作用
生理
心理
嚴重過量
安非他命過量使用會引起許多症狀,然而在適當的醫療照護下,不至於死亡。 [90][95]
藥物過量症狀的嚴重度與劑量成正比;與身體對安非他命的藥物耐受性成反比。 [34][90] 已知每天攝取達到5公克的安非他命(每天最大攝取量的五十倍)會導致身體對安非他命產生藥物耐受性。 [90] 嚴重過量的安非他命攝取所致的症狀列於下方;安非他命中毒一旦到達出現全身抽蓄(convulsion)和昏厥(coma)則必須立刻急救以避免死亡。 [31][34] 在2013年,安非他命、甲基安非他命和其他列於ICD-10 第五章:精神和行為障礙§使用化學藥物、物質或酒精引起的精神和行為障礙中的安非他命相關物質的過量使用在世界上共導致3788人死亡。(3,425–4,145 人死亡、 95% 信賴區間)。 [note 10][96]
被過度活化達到病態程度的mesolimbic pathway(一個連接腹側被蓋區(ventral tegmental area)和伏隔核(nucleus accumbens)的多巴胺通道),在安非他命的成癮中扮演著主要的腳色。 [97] [98]
當一個人經常服用嚴重過量的安非他命,將伴隨安非他命成癮的高度風險, 因為持續過量的安非他命會逐漸增加伏隔核的ΔFosB(「成癮」與否的分子開關和主控蛋白 原文:a "molecular switch" and "master control protein" for addiction.)的檔次。 [99][100][101] 一旦伏隔核的ΔFosB破表(over-expressed),這個人的「成癮性行為」[註 2](例如:出現試圖取得安非他命的衝動行為)將開始隨之增加。 [99][102] 雖然目前沒有治療安非他命成癮的有效藥物,但規律的且每次都有持續一定時間的有氧運動能降低安非他命的成癮風險也是治療安非他命成癮的天然療法。 [103][104] [sources 5] 運動能提升臨床治療的預後,且可能與認知行為治療(目前已知最有效的安非他命成癮的臨床治療法)相搭配為combination therapy。 [103][105][106]
| 生物系統 | 輕度、中度過量[31][34][90] | 過量[sources 6] |
|---|---|---|
| 心臟血管系統 | ||
| 中樞神經系統 | ||
| 肌肉骨骼系统 |
| |
| 呼吸系統 |
|
|
| 生殖泌尿系統 | ||
| 其他 |
成癮
| 「成癮及生理、心理依賴」的相關術語詞彙表[108][100][109][110] | |
|---|---|
| |
長期服用遠超醫療用劑量範圍的安非他命會導致安非他命成癮(Addiction)。然而長期攝取醫療劑量範圍的安非他命並不會引起上述問題。 [37][38][39] 安非他命濫用(例如:長期攝取嚴重過量的安非他命)會導致大腦對於該劑量產生藥物耐受性。漸漸地,濫用者必須服用更大量的安非他命以換取同樣的效果。 [111][112]
分子生物機轉(Biomolecular mechanisms)
當前關於「長期安非他命濫用所致的成癮」的模型(model)中,已知會改變一些腦部的結構(特別是伏隔核) [113][114][115]。 造成腦部結構改變的最重要的轉錄因子(transcription factor)為:ΔFosB、 cAMP response element binding protein (CREB)、和 nuclear factor kappa B (NF-κB)。 [note 11] [114] ΔFosB 在藥物成癮的發展過程中扮演著至關重要的腳色,主要的原因在於其在 伏隔核中D1-type medium spiny neurons的破表(over-expression),為「成癮」及「成癮衍生的行為」及「神經元為了適應新常態所做的調適」的充分且必要條件。 [note 12] [99][100][114]
一旦ΔFosB充分破表(sufficiently overexpressed),將誘發越來越嚴重的成癮狀態並伴隨ΔFosB值的持續創新高。 [99][100] ΔFosB已被證明與酒精成癮、大麻成癮、古柯鹼成癮、派醋甲酯成癮、尼古丁成癮、[鴉片]]成癮、phencyclidine成癮、異丙酚、和安非他命的替代性物質成癮、及其他成癮有關。 [sources 7] ΔJunD為一個轉錄因子;而G9a為組織蛋白甲基轉移酶的一種。ΔJunD和G9a直接與伏隔核中的ΔFosB值的升高成反比。 [100][114][119]
利用載體讓伏隔核中的ΔJunD充分破表,可以使由長期藥物濫用所致的漸進式神經元和行為改變完全停止。(比如說:ΔFosB所致的改變)。 [114] ΔFosB也在人們於天然酬賞(natural reward)中的行為反應調節上扮演重要的腳色。天然酬賞(natural rewards)包含:美味的食物(palatable food)、性愛(sex)、運動(exercise)、......。 [102][114][120] 因為天然酬賞以及成癮性藥物皆會激發ΔFosB(這些酬賞讓大腦刺激ΔFosB的增加。原文:i.e., they cause the brain to produce more of it),長期過度地從事上述行為將可能導致類似的成癮之病理生理(pathological)。 [102][114]
ΔFosB是導致「安非他命成癮」、「安非他命引起的性成癮」中最關鍵的致癮因素。「安非他命引起的性成癮」為「安非他命使用」加上「過度的性活動」所引發的「衝動之下的性行為」。 [註 3][102][121][122] 這類的性成癮與多巴胺失調症候群相關,有時此症會出現在正在服用作用於多巴胺的藥物的人身上。 [註 4][102][120]
安非他命調節基因(gene regulation)的效果端視劑量與路徑(dose- and route-dependent)而定。 [115] 絕大多數主題為「基因調節(gene regulation)」和「成癮(addiction)」的研究都是透過動物試驗以及利用靜脈注射的方式對實驗動物注射超高劑量的安非他命來進行。 [115] 少數幾個透過人體試驗(依照體重來決定醫療用劑量)來進行的研究表明,口服醫療用劑量的安非他命並不會影響基因調節,即便有,也是極為輕微的。這表示安非他命用作醫療用途是十分安全的。 [115][115]
藥物治療(Pharmacological treatments)
截至2014年5月[update]並沒有能夠有效治療安非他命成癮的藥理療法 [123][124][125] 。 2015年到2016年間的論文回顧結果指出:選擇性TAAR1促進劑有非常大的可能在將來被用來治療中樞神經興奮劑的成癮; [46][126] 然而,截至2016年2月[update],已知可作為選擇性TAAR1促進劑的物質都屬於試驗性藥物。 [46][126] 安非他命成癮與伏隔核中的多巴胺接收器們以及位置相同(co-localized)的NMDA 接受器們的活化高度相關; [note 13] [98] 鎂離子藉由封鎖一個接受器-鈣離子通道,來阻斷 NMDA接受器們。 [98][127] 一篇論文回顧做成結論:根據動物試驗,因成癮而使用中樞神經刺激劑的人,可以發現過量的中樞神經興奮劑顯著降低腦細胞內部的鎂離子活動。 [註 5][98] 利用鎂元素補充劑,能降低安非他命使用者自我服用[d]的機會。然而這不被認為是有效治療安非他命成癮的單一療法(mono-therapy)。 [note 14][98]
行為治療(Behavioral treatments)
認知行為治療是當前治療中樞神經刺激劑成癮的療法。 [106] 除此之外,運動在生物神經元產生的效果的研究中表明維持每天從事有氧運動(例如:跑步等)的習慣,能避免藥物成癮纏身;本身也是一個對於治療安非他命成癮的有效附加療法。 [sources 5] 運動能讓所有疾病的預後都更加樂觀,特別是對於中樞神經刺激劑成癮。 [103][105][128] 值得一提的是,有氧運動能降低擅自服用中樞神經興奮劑的慾望[e],降低再次擅自服用中樞神經興奮劑的機率(reinstatment)(i.e., relapse)、降低「試圖取得藥物所做出的舉動(drug-seeking behavior)」、降低多巴胺接收器 D2在紋狀體中的密度。 [102][128] 它在病生理學中的腳色是相對於「興奮劑的使用」和「興奮劑的效果」,它會引起紋狀體中DRD2密度的減少。 [102] 一篇論文回顧提到,藉由改變紋狀體(striatum)中的ΔFosB、c-Fos immunoreactivity或部份的腦內回饋系統(reward system)來避免藥物成癮在一個人身上的發展。 [104]
| 神經可塑性和行為可塑性的形式 | 增強物的種類 | 來源 | |||||
|---|---|---|---|---|---|---|---|
| 鴉片類 | 中樞神經刺激劑 | 高脂肪或高糖食物 | 性交 | 運動與神經元關係 | 環境豐富化 | ||
| 伏隔核中D1-type中的ΔFosB表現 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [102] |
| 行為可塑性 | |||||||
| 攝取量的增加 | 有 | 有 | 有 | [102] | |||
| 中樞神經刺激劑跨越-敏化作用 | 有 | 不適用 | 有 | 有 | 削減 | 削減 | [102] |
| 未經過處方而自行私下攝取中樞神經刺激劑 | ↑ | ↑ | ↓ | ↓ | ↓ | [102] | |
| 強化「在特定地點攝取興奮劑的習慣」 | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [102] |
| 強化「試圖取得該致癮藥物的行為」 | ↑ | ↑ | ↓ | ↓ | [102] | ||
| 神經化學物質的可塑性 | |||||||
| 伏隔核中CREB磷酸化 | ↓ | ↓ | ↓ | ↓ | ↓ | [102] | |
| 伏隔核中對於多巴胺的過敏反應 | 沒有 | 有 | 沒有 | 有 | [102] | ||
| 經過變動的紋狀體多巴胺接收器的訊號發送 | ↓DRD2 , ↑DRD3 | ↑DRD1, ↓DRD2 , ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [102] | |
| 經過變動的紋狀體鴉片样肽受体的訊號發送 | 未改變,或 ↑μ-鴉片接收器 |
↑μ-鴉片接收器 ↑κ-鴉片接收器 |
↑μ-鴉片接收器 | ↑μ-鴉片接收器 | 未改變 | 未改變 | [102] |
| 發生於紋狀體鴉片肽的改變 | ↑強啡肽 腦啡肽未改變 |
↑強啡肽 | ↓腦啡肽 | ↑強啡肽 | ↑強啡肽 | [102] | |
| 多巴胺通道的神經突觸的可塑性 | |||||||
| 伏隔核中树突的數量 | ↓ | ↑ | ↑ | [102] | |||
| 伏隔核中樹突棘的密度 | ↓ | ↑ | ↑ | [102] | |||
註解:DRD2 = 多巴胺受體D2;↑ = 上升;↓ = 下降
依賴和戒斷症狀 Dependence and withdrawal
根據另一篇由考科藍協作組織所做的一篇論文回顧指出當一個長期嚴重攝取安非他命或甲基安非他命的藥物成癮者某天突然停止攝取安非他命或甲基安非他命,那麼根據許多成癮個案的報告顯示,具有時效性(time-limited)的戒斷症狀將在他們上一次攝取安非他命後的24小時內出現。 在成癮患者停用安非他命後,安非他命的戒斷症狀的出現率接近九成。這九成都出現至少六個定義在「《精神疾病診斷與統計手冊》安非他命戒斷症狀」中的症狀。年紀與劑量和戒斷症狀的嚴重度呈正相關。安非他命的戒斷症狀共有兩個階段且總共可能歷時三周或更多。第一階段(撞牆期 marked "crush" phase)約持續一周。 [129] [129] 安非他命的戒斷症狀可能包含:對於各種刺激極度敏感、躁動不安(irritability)、焦慮、對於安非他命有難以抑制的渴求、煩躁、疲倦、食慾放大、過動或行動遲緩、缺乏動機、嗜睡、和清醒夢(lucid dreams)。 [130] [129]
這些特徵及症狀必須非由其他疾病(包含心理疾病)引起,且無法歸因於其他物質的濫用。滿足上述條件,才符合「安非他命戒斷症狀」症候群的診斷標準。 [131] [129]
通過美國食品藥物管理局嚴格審核的安非他命藥品說明書上並未提到任何安非他命在醫療用劑量下突然停用會導致任何安非他命戒斷症狀的出現。由此可知,長期攝取醫療用劑量的安非他命並突然停用,並不會導致戒斷症狀出現。 [91][132][133][134]
DSM中,安非他命中毒及戒斷症狀之標準(DSM criteria for intoxication and withdrawal)
DSM-5中关于兴奋剂中毒的标准如下:
A.最近曾经服用过安非他命类的物质、可卡因或其他兴奋剂
B. 在服用兴奋剂时(或者服用后很快表现出)临床表现出显著问题行为或心理变化(如:欣快症或感情迟钝;群性、社交性的改变;过于警觉;人际交往敏感;焦虑、紧张或者恼怒;刻板行为;判断力受损)。
C.在服用兴奋剂时或服用后即刻表现出以下任意两种(或以上)症状:
- 心动过速或心动过缓
- 瞳孔扩散
- 血压升高或降低
- 出汗或发冷
- 感到恶心或呕吐
- 体重降低
- 精神运动性焦躁或精神运动性迟滞
- 肌肉无力、呼吸抑制、胸口疼痛或心律失常
- 精神错乱、癫痫、运动困难、肌张力障碍或昏迷
包括其他物质中毒的情况在内,其他身体情况不可能出现该发病迹象、症状,并且也无法理解为其他的精神问题。
DSM-5中关于兴奋剂戒断症状的标准如下:
A. 停止服用(或减少服用)长期的安非他命类物质、可卡因或其他兴奋剂。
B. 在A的情况发生后的几小时至几天内,出现烦躁的情绪并伴有以下任意两种(或以上)的心理变化:
- 疲劳
- 生动而不愉快的梦
- 失眠或嗜睡
- 食欲增大
- 精神运动性焦躁或精神运动性迟滞
B中的症状或迹象导致了临床显著的压力,或者在社会、工作等重要方面功能出现受损。
安非他命戒断症状的频率列表(Table of symptoms of amphetamine withdrawal by frequency)
| 症状 | 频率 |
|---|---|
| 无症状 | 14% |
| 易怒 | 78% |
| 疼痛和痛苦 | 58% |
| 感到沮丧 | 50% |
| 社交能力受损 | 46% |
| 发抖、出冷汗 | 36% |
| 难以入睡 | 32% |
| 虚脱 | 22% |
| 恶心、呕吐 | 16% |
| 头痛 | 14% |
| 难以保持清醒 | 12% |
| 食欲增大 | 12% |
| 便秘 | 10% |
| 食欲缩小 | 8% |
| 腹泻 | 6% |
| 原因 | 个人尝试戒毒 | 医学指导戒毒 |
|---|---|---|
| 对生活整体现状(犯罪、无聊、金钱)不满 | 42(89%) | 6(37%) |
| 对心理健康感到担忧(偏执、忧虑、依赖) | 25(53%) | 3(19%) |
| 家庭原因(父母或配偶的压力,子女出生) | 24(51%) | 5(31%) |
| 身体健康(动脉注射、血管萎陷、感染) | 17(36%) | 4(25%) |
| 避免入狱 | 0 | 2 (12%) |
| 其他原因 | 2(4%) | 0 |
| Header text | Self detoxication | Enforced detoxication |
|---|---|---|
| Increased consumption of other drugs | Example | Example |
| Cannabis | 22 (27%) | 10 (59%) |
| Temazepam | 21 (26%) | Example |
| Alcohol | 17 (21%) | 2 (12%) |
| Opiates | 12 (15%) | 1 (6%) |
| Diazepam | 4 (5%) | Example |
| Barbiturates | 3 (4%) | 1 (6%) |
| Psychosocial techniques | Example | Example |
| Keeping occupied with other things (working, watching television) | 35 (21%) | 1 (6%) |
| Cutting off contact with drug-using friends | 31 (19%) | Example |
| Gaining support from others (friends giving up, family, support groups) | 11 (7%) | Example |
| Throwing away drugs and needles | 5 (3%) | Example |
Toxicity and psychosis
In rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized by dopamine terminal degeneration and reduced transporter and receptor function.[136][137] There is no evidence that amphetamine is directly neurotoxic in humans.[138][139] However, large doses of amphetamine may indirectly cause dopaminergic neurotoxicity as a result of hyperpyrexia, the excessive formation of reactive oxygen species, and increased autoxidation of dopamine.[sources 8] Animal models of neurotoxicity from high-dose amphetamine exposure indicate that the occurrence of hyperpyrexia (i.e., core body temperature ≥ 40 °C) is necessary for the development of amphetamine-induced neurotoxicity.[137] Prolonged elevations of brain temperature above 40 °C likely promote the development of amphetamine-induced neurotoxicity in laboratory animals by facilitating the production of reactive oxygen species, disrupting cellular protein function, and transiently increasing blood–brain barrier permeability.[137]
A severe amphetamine overdose can result in a stimulant psychosis that may involve a variety of symptoms, such as delusions and paranoia.[35] A Cochrane Collaboration review on treatment for amphetamine, dextroamphetamine, and methamphetamine psychosis states that about 5–15% of users fail to recover completely.[35][142] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[35] Psychosis very rarely arises from therapeutic use.[36][89] }}
交互作用(Interactions)
Many types of substances are known to interact with amphetamine, resulting in altered drug action or metabolism of amphetamine, the interacting substance, or both.[4][143] Inhibitors of the enzymes that metabolize amphetamine (e.g., CYP2D6 and FMO3) will prolong its elimination half-life, meaning that its effects will last longer.[16][143] Amphetamine also interacts with MAOIs, particularly monoamine oxidase A inhibitors, since both MAOIs and amphetamine increase plasma catecholamines (i.e., norepinephrine and dopamine);[143] therefore, concurrent use of both is dangerous.[143] Amphetamine modulates the activity of most psychoactive drugs. In particular, amphetamine may decrease the effects of sedatives and depressants and increase the effects of stimulants and antidepressants.[143] Amphetamine may also decrease the effects of antihypertensives and antipsychotics due to its effects on blood pressure and dopamine respectively.[143] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 15][147]
In general, there is no significant interaction when consuming amphetamine with food, but the pH of gastrointestinal content and urine affects the absorption and excretion of amphetamine, respectively.[143] Acidic substances reduce the absorption of amphetamine and increase urinary excretion, and alkaline substances do the opposite.[143] Due to the effect pH has on absorption, amphetamine also interacts with gastric acid reducers such as proton pump inhibitors and H2 antihistamines, which increase gastrointestinal pH (i.e., make it less acidic).[143]
藥學(Pharmacology)
藥效動力學(Pharmacodynamics)
苯丙胺在多巴胺能神經元的藥物效應動力學
|
Amphetamine exerts its behavioral effects by altering the use of monoamines as neuronal signals in the brain, primarily in catecholamine neurons in the reward and executive function pathways of the brain.[45][60] The concentrations of the main neurotransmitters involved in reward circuitry and executive functioning, dopamine and norepinephrine, increase dramatically in a dose-dependent manner by amphetamine due to its effects on monoamine transporters.[45][60][148] The reinforcing and motivational salience-promoting effects of amphetamine are mostly due to enhanced dopaminergic activity in the mesolimbic pathway.[29] The euphoric and locomotor-stimulating effects of amphetamine are dependent upon the magnitude and speed by which it increases synaptic dopamine and norepinephrine concentrations in the striatum.[1]
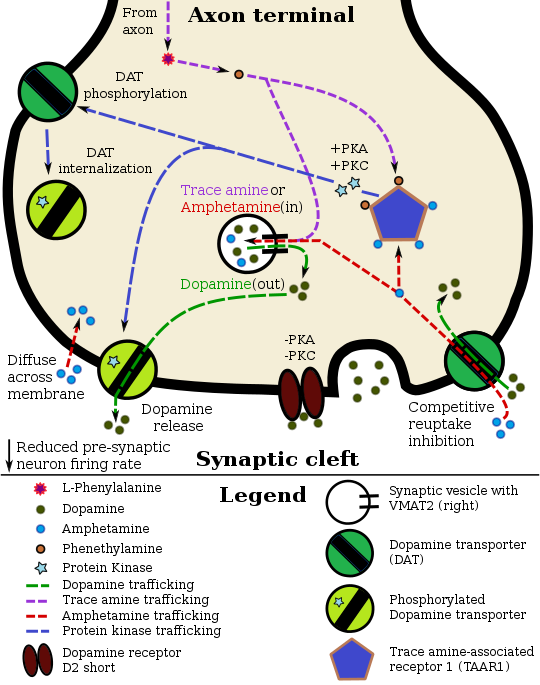
Amphetamine has been identified as a potent full agonist of trace amine-associated receptor 1 (TAAR1), a Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) discovered in 2001, which is important for regulation of brain monoamines.[45][149] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits monoamine transporter function.[45][150] Monoamine autoreceptors (e.g., D2 short, presynaptic α2, and presynaptic 5-HT1A) have the opposite effect of TAAR1, and together these receptors provide a regulatory system for monoamines.[45][46] Notably, amphetamine and trace amines bind to TAAR1, but not monoamine autoreceptors.[45][46] Imaging studies indicate that monoamine reuptake inhibition by amphetamine and trace amines is site specific and depends upon the presence of TAAR1 co-localization in the associated monoamine neurons.[45] 截至2010年[update] co-localization of TAAR1 and the dopamine transporter (DAT) has been visualized in rhesus monkeys, but co-localization of TAAR1 with the norepinephrine transporter (NET) and the serotonin transporter (SERT) has only been evidenced by messenger RNA (mRNA) expression.[45]
In addition to the neuronal monoamine transporters, amphetamine also inhibits both vesicular monoamine transporters, VMAT1 and VMAT2, as well as SLC1A1, SLC22A3, and SLC22A5.[sources 9] SLC1A1 is excitatory amino acid transporter 3 (EAAT3), a glutamate transporter located in neurons, SLC22A3 is an extraneuronal monoamine transporter that is present in astrocytes, and SLC22A5 is a high-affinity carnitine transporter.[sources 9] Amphetamine is known to strongly induce cocaine- and amphetamine-regulated transcript (CART) gene expression,[157][158] a neuropeptide involved in feeding behavior, stress, and reward, which induces observable increases in neuronal development and survival in vitro.[158][159][160] The CART receptor has yet to be identified, but there is significant evidence that CART binds to a unique Gi/Go-coupled GPCR.[160][161] Amphetamine also inhibits monoamine oxidase at very high doses, resulting in less dopamine and phenethylamine metabolism and consequently higher concentrations of synaptic monoamines.[18][162] In humans, the only post-synaptic receptor at which amphetamine is known to bind is the 5-HT1A receptor, where it acts as an agonist with micromolar affinity.[163][164]
The full profile of amphetamine's short-term drug effects in humans is mostly derived through increased cellular communication or neurotransmission of dopamine,[45] serotonin,[45] norepinephrine,[45] epinephrine,[148] histamine,[148] CART peptides,[157][158] endogenous opioids,[165][166][167] adrenocorticotropic hormone,[168][169] corticosteroids,[168][169] and glutamate,[151][153] which it effects through interactions with CART, 5-HT1A, EAAT3, TAAR1, VMAT1, VMAT2, and possibly other biological targets.[sources 10]
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[170] Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[34][170]
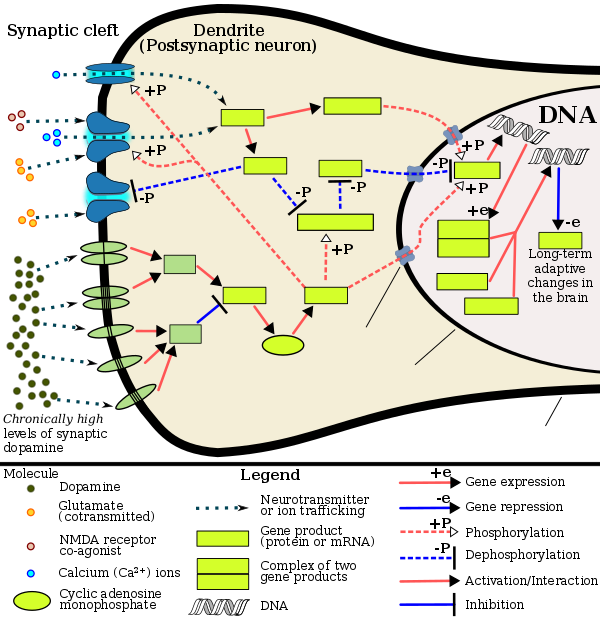
多巴胺
In certain brain regions, amphetamine increases the concentration of dopamine in the synaptic cleft.[45] Amphetamine can enter the presynaptic neuron either through DAT or by diffusing across the neuronal membrane directly.[45] As a consequence of DAT uptake, amphetamine produces competitive reuptake inhibition at the transporter.[45] Upon entering the presynaptic neuron, amphetamine activates TAAR1 which, through protein kinase A (PKA) and protein kinase C (PKC) signaling, causes DAT phosphorylation.[45] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces the reversal of dopamine transport through DAT (i.e., dopamine efflux).[45][171] Amphetamine is also known to increase intracellular calcium, an effect which is associated with DAT phosphorylation through an unidentified Ca2+/calmodulin-dependent protein kinase (CAMK)-dependent pathway, in turn producing dopamine efflux.[149][151][172] Through direct activation of G protein-coupled inwardly-rectifying potassium channels, TAAR1 reduces the firing rate of dopamine neurons, preventing a hyper-dopaminergic state.[173][174][175]
Amphetamine is also a substrate for the presynaptic vesicular monoamine transporter, VMAT2.[148][176] Following amphetamine uptake at VMAT2, amphetamine induces the collapse of the vesicular pH gradient, which results in the release of dopamine molecules from synaptic vesicles into the cytosol via dopamine efflux through VMAT2.[148][176] Subsequently, the cytosolic dopamine molecules are released from the presynaptic neuron into the synaptic cleft via reverse transport at DAT.[45][148][176]
去甲肾上腺素
Similar to dopamine, amphetamine dose-dependently increases the level of synaptic norepinephrine, the direct precursor of epinephrine.[47][60] Based upon neuronal TAAR1 mRNA expression, amphetamine is thought to affect norepinephrine analogously to dopamine.[45][148][171] In other words, amphetamine induces TAAR1-mediated efflux and non-competitive reuptake inhibition at phosphorylated NET, competitive NET reuptake inhibition, and norepinephrine release from VMAT2.[45][148]
血清素
Amphetamine exerts analogous, yet less pronounced, effects on serotonin as on dopamine and norepinephrine.[45][60] Amphetamine affects serotonin via VMAT2 and, like norepinephrine, is thought to phosphorylate SERT via TAAR1.[45][148] Like dopamine, amphetamine has low, micromolar affinity at the human 5-HT1A receptor.[163][164]
Other neurotransmitters, peptides, and hormones
Acute amphetamine administration in humans increases endogenous opioid release in several brain structures in the reward system.[165][166][167] Extracellular levels of glutamate, the primary excitatory neurotransmitter in the brain, have been shown to increase in the striatum following exposure to amphetamine.[151] This increase in extracellular glutamate presumably occurs via the amphetamine-induced internalization of EAAT3, a glutamate reuptake transporter, in dopamine neurons.[151][153] Amphetamine also induces the selective release of histamine from mast cells and efflux from histaminergic neurons through VMAT2.[148] Acute amphetamine administration can also increase adrenocorticotropic hormone and corticosteroid levels in blood plasma by stimulating the hypothalamic–pituitary–adrenal axis.[43][168][169]
藥物代謝動力學
安非他命的口服生物體可利用率[參 21]與腸胃的pH值連動; [143] 安非他命非常容易在腸道被吸收,dextroampetamine的生體可利用率在多數的情況下高於75%。 [2] 安非他命呈弱鹼性,其pKa值介於9–10之間;[4] 因此,當pH值呈鹼性時,多數的安非他命會以其易溶於脂類的純胺類型態形式存在。在此情況下,身體會通過腸道上皮組織富含脂類的細胞膜[參 22]來吸收安非他命。 [4] [143] 相反地,酸性的pH值表示安非他命主要以易溶於水的離子[參 23](鹽)形式存在,因此較少能被吸收。 [4] 大約15–40%循環於血管中的安非他命與血漿蛋白[參 24]相連接。 [3] 安非他命的對映異構物的半衰期會隨著尿液的pH值而有所不同。 [4] 當尿液的酸鹼值落在正常範圍中,dextroamphetamine和levoamphetamine的半衰期分別為9–11 小時及 11–14 小時。 [4] 酸性飲食會導致安非他命的對映異構物的半衰期降低至8–11 小時;鹼性飲食則會使安非他命的對映異構物的半衰期增加到16–31 小時。 [5][11]
成分為安非他命或其衍生物的短效藥品大約在口服後三小時在體內達到最高血漿濃度;而成分為安非他命或其衍生物的長效藥品則在口服後大約七小時在體內達到最高血漿濃度。 [4]
安非他命主要透過腎臟來代謝,大約30–40%的藥物以藥物本身原始的型態從酸鹼度正常的尿液中排出。 [4] 當尿液是鹼性時,安非他命傾向以其純胺類型態存在,因此較少被排泄。 [4]
當尿液的pH值失常時,各種安非他命的分解物在尿液中重新結合的程度將從最低1%到最高75%。該程度的高低大多取決於於尿液的酸鹼值,尿液越酸,結合率越高;尿液愈鹼,結合率越低。 [4] 安非他命通常於口服後兩天內自體內完全代謝完畢。 [5] 安非他命確切的半衰期及藥效作用期隨著(小於兩天的)重複服用導致的血漿內安非他命濃度(plasma concentration of amphetamine)的增加而延長。[177]
對人體無藥效的前驅藥物體(prodrug):lisdexamfetamine並不若安非他命一樣容易受腸胃道環境的pH值影響; [178] lisdexamfetamine在腸道被吸收進入血管的血液後很快就會透過水解(hydrolysis)的方式轉化為dextroamphetamine。而參與這水解反應的酶(enzymes)與紅血球有關。 [178]
Lisdexamfetamine的半衰期通常小於一個小時。 [178]
細胞色素 P450 2D6(Cytochrome P450 2D6、或CYP2D6)、多巴胺β羥化酶(Dopamine β-hydroxylase、或DBH)、flavin-containing monooxygenase 3、butyrate-CoA ligase、和 glycine N-acyltransferase為已知在人體中參與[註 6]「安非他命」及「安非他命代謝後之產物」的代謝反應的酶(enzyme)。 [sources 11]
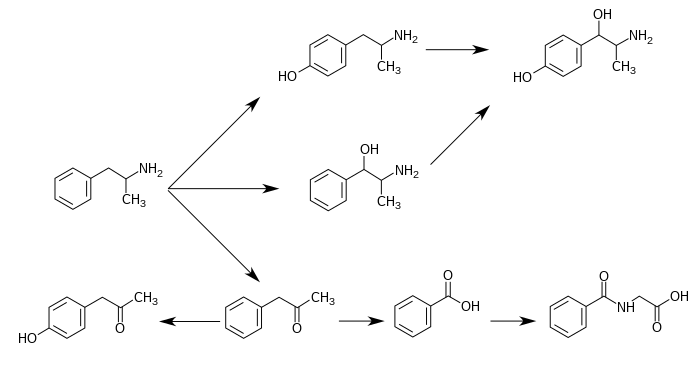
「安非他命代謝後之產物」包含:4-hydroxyamphetamine、4-hydroxynorephedrine、4-hydroxyphenylacetone、苯甲酸(benzoic acid)、馬尿酸(hippuric acid)、苯丙醇胺(norephedrine)、苯基丙酮(phenylacetone)[註 7] [4] [5] [6]。
在這些「安非他命代謝後之產物」之中,有實際藥效的產物(sympathomimetics)為:4‑hydroxyamphetamine[181]、4‑hydroxynorephedrine[182]、和norephedrine[183]。
安非他命的主要代謝途徑包含:aromatic para-hydroxylation、aliphatic alpha- 、beta-hydroxylation、N-oxidation、N-dealkylation、和 deamination。 [4][5]
下圖為已知的「安非他命」代謝途徑和「安非他命代謝後之產物」: [4][16][6]
苯丙胺的代謝途徑
從酸鹼度正常的尿液中可發現,大約30–40%的「安非他命」以本身原始的型態排出;大約50%的安非他命以不具藥效的「安非他命代謝後之產物」(即為圖片中最下列的產物)的型態排出。 [4] 剩下的10–20%則為「安非他命代謝後之產物」之中,有實際藥效的產物。 [4] 苯甲酸(Benzoic acid)被butyrate-CoA連接酶(butyrate-CoA ligase)代謝後成為一個中介物質/中間產物(intermediate product):benzoyl-CoA [179] 隨後透過glycine N-acyltransferase代謝並轉化為馬尿酸(hippuric acid)。[180] |
相關的內部生成化合物/混和物(endogenous compound)
歷史、社會與文化
合法狀態與條件
藥品
備註A
- ^ 别名有:1-phenylpropan-2-amine (IUPAC name), α-methylbenzeneethanamine, α-methylphenethylamine, amfetamine (International Nonproprietary Name [INN]), β-phenylisopropylamine, desoxynorephedrine, and speed.[18][21][22]
- ^ 對映異構體指的是兩個形狀相同但方向相反的兩個分子,他們又稱為彼此的鏡中影像。[23] Levoamphetamine 和 dextroamphetamine 分別被簡稱為 L-amph 或 levamfetamine (INN) 和 D-amph 或 dexamfetamine (INN) [18]
- ^
"Adderall"是一個品牌名稱。因為以下幾個安非他命的異構物的英文名稱太長了:("dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate, and amphetamine aspartate"),因此本文單獨以此名稱來表示此安非他命的此種混合物。
原文對照:"Adderall" is a brand name as opposed to a nonproprietary name; because the latter ("dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate, and amphetamine aspartate" [44]) is excessively long, this article exclusively refers to this amphetamine mixture by the brand name. - ^
「安非他命」一詞也意指一個化學分類,但與「替代性安非他命」這個化學分類不同的是,「安非他命」類在學術上並無標準的定義。
[13][25]
有一個「安非他命」類的定義嚴格限定分類中僅有:安非他命的racemate and enantiomers 和 甲基安非他命methamphetamine的racemate and enantiomers。
[25]
大多數「安非他命」類的定義為那些在藥理學上以及結構上與安非他命相關的化合物。
[25]
為避免讓amphetamine 和 amphetamines 把讀者給弄糊塗了,本條目中僅會使用amphetamine、amphetamines來表示racemic amphetamine, levoamphetamine, and dextroamphetamine;‘替代性安非他命(substituted amphetamines)’來表示安非他命的結構分類。
原文對照: Due to confusion that may arise from use of the plural form, this article will only use the terms "amphetamine" and "amphetamines" to refer to racemic amphetamine, levoamphetamine, and dextroamphetamine and reserve the term "substituted amphetamines" for its structural class. - ^
研究證實,長期以中樞神經興奮劑治療ADHD能在下列這些方面產生大幅的進步:學業、駕駛、降低藥物濫用、降低肥胖、自尊、和社交功能等。
[57]
在上述領域中,最為突出的領域為: 學業(例如:GPA分數 grade point average、成果測驗分數 achievement test scores、受教育的時間長度 length of education、和教育程度 education level) 、自尊(例如:自尊心測驗分數 self-esteem questionnaire assessments、嘗試自殺的次數、自殺率等) 和社交功能(例如:peer nomination scores、社交技巧、家庭關係 quality of family、同儕關係 quality of peer、和浪漫關係/情侶關係 romantic relationships) [57]
長期以「藥物治療合併行為治療」的模式來治療ADHD,能夠比單獨以藥物治療,產生更全面且更長足的進步。 [57] - ^ 考科藍協作組織對於歷年眾多的「隨機對照試驗」的系統性回顧、數據統整分析後所得出的總結,基本上都是非常有水準且深具參考價值的。 [64]
- ^ 美國食品藥物管理局核准的藥品使用指引及醫療上的禁忌(放在藥盒中的仿單/說明書)並非為了限制醫師的決策而是為了避免藥商恣意宣稱藥物的作用。醫師可以此為參考,並依照每位病人的實際情況做出獨立的判斷。 [88]
- ^ 然而根據一篇回顧性論文,安非他命可以處方給曾有藥物濫用歷史的人,不過需要有對患者適度的藥品控管,例如:每天由醫護人員配給處方劑量。[1]
- ^ 曾受此副作用的用藥者,身高及體重在在短暫停藥後恢復至應有水準是可以被預期的。[56][58][94] 根據追蹤,持續三年過程不停歇的安非他命治療(沒有合併任何積極減少安非他命副作用的療法的情況下)平均會減少 2公分的最終身高。 [94]
- ^ 「95% 信賴區間」指的是:有95%的機率,真實的死亡人數介於3,425 和 4,145 之間。
- ^
轉錄因子是一種可以增加或降低一個特定基因的基因表現的蛋白。
原文:Transcription factors are proteins that increase or decrease the expression of specific genes.[116] - ^ 簡單來說,這裡的「充分且必要(necessary and sufficient)」關係指的是「ΔFosB在伏隔核中的破表(over-expression)」與「成癮衍生的行為」及「神經元為了適應新常態所做的調適」永遠都是一起發生。
- ^
NMDA接受器們為與電壓相關的ligand-gated ion channels。ligand-gated ion channels這個通道需要glutamate 以及一個共同促進劑(co-agonist ):(D-serine 或 大豆屬glycine)的同時連接才能被開啟。
原文: NMDA receptors are voltage-dependent ligand-gated ion channels that requires simultaneous binding of glutamate and a co-agonist (D-serine or glycine) to open the ion channel.[127] - ^
該篇回顧表示magnesium L-aspartate 及 氯化鎂(magnesium chloride)能大幅改善成癮行為。
原文: The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[98] other forms of magnesium were not mentioned. - ^ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[144][145][146] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[146]
備註B
- ^ 智力測驗結果與專注力有關,詳見注意力不足過動症#智力
- ^ 因成癮所致的行為
- ^ 原文:Consequently, ΔFosB is the most significant factor involved in both amphetamine addiction and amphetamine-induced sex addictions, which are compulsive sexual behaviors that result from excessive sexual activity and amphetamine use.
- ^ 原文:These sex addictions are associated with a 多巴胺失調症候群 which occurs in some patients taking 作用於多巴胺的藥物.
- ^ 原文:One review suggested that, based upon animal testing, pathological (addiction-inducing) psychostimulant use significantly reduces the level of intracellular magnesium throughout the brain.
- ^ 酶做為反應的催化劑catalyst,並不實際參與反應。
- ^ 不是苯丙酮
備註C
注释
英文名稱對照
- ^ 英文名稱為:delusions
- ^ 英文名稱為:paranoia
- ^ 英文名稱為:Prescription drug
- ^ 英文名稱為:Pharmaceutical amphetamine
- ^ 英文名稱為:racemic amphetamine
- ^ 英文名稱為:Prodrug
- ^ 英文名稱為:substituted amphetamine
- ^ 英文名稱為:Bupropion
- ^ 英文名稱為:meth-amphetamine
- ^ 英文名稱為:Randomized controlled trials
- ^ 英文名稱為:follow-up studies
- ^ 英文名稱為:neurotransmitter systems
- ^ 英文名稱為:dopamine
- ^ 英文名稱為:locus coeruleus
- ^ 英文名稱為:prefrontal cortex
- ^ 英文名稱為:nor-epinephrine或nor-adrenaline
- ^ 英文名稱為:Cochrane Collaboration
- ^ 英文名稱為:systematic review
- ^ 英文名稱為:meta-analysis
- ^ 英文名稱為:臨床試驗
- ^ 英文名稱為:bioavailability
- ^ 英文名稱為:cell membrane
- ^ 英文名稱為:cation
- ^ 英文名稱為:plasma protein
引用
- ^ [10][29][30][31][32][33][34][35][36][37][38][39]
- ^ [1][25] [29] [30] [31] [40] [41] [42] [32] [26] [24][43]
- ^ [1] [10] [29] [40] [43] [45] [46]
- ^ [47] [48] [49]
- ^ 5.0 5.1 [102][103][104][105][128]
- ^ [22][31][34][95][107]
- ^ [99][102][114][117][118]
- ^ [51][137][140][141]
- ^ 9.0 9.1 [148][151][152][153][154][155][156]
- ^ [45][148][152][153][157][163]
- ^ [4][13] [14] [15] [16] [17] [179] [180]
來源
- ^ 1.0 1.1 1.2 1.3 1.4 1.5 引用错误:没有为名为
Amph Uses的参考文献提供内容 - ^ 2.0 2.1 Dextroamphetamine. DrugBank. University of Alberta. 2013-02-08.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 3.0 3.1
Amphetamine. DrugBank. University of Alberta. 2013-02-08.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 12–13. December 2013 [2013-12-30].
- ^ 5.0 5.1 5.2 5.3 5.4
Amphetamine. Pubchem Compound. National Center for Biotechnology Information.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 6.0 6.1 6.2 6.3 Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G. Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection. J. Pharm. Biomed. Anal. September 2002, 30 (2): 247–255. PMID 12191709. doi:10.1016/S0731-7085(02)00330-8.
- ^ amphetamine/dextroamphetamine. Medscape. WebMD.
Onset of action: 30–60 min
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 8.0 8.1 8.2
Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (编). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 112. ISBN 9781441913968.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ 9.0 9.1 Brams M, Mao AR, Doyle RL. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad. Med. September 2008, 120 (3): 69–88. PMID 18824827. doi:10.3810/pgm.2008.09.1909.
- ^ 10.0 10.1 10.2 10.3 10.4 Adderall IR Prescribing Information (PDF). United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc.: 1–6. October 2015 [2016-05-18].
- ^ 11.0 11.1
AMPHETAMINE. United States National Library of Medicine – Toxnet. Hazardous Substances Data Bank.
Concentrations of (14)C-amphetamine declined less rapidly in the plasma of human subjects maintained on an alkaline diet (urinary pH > 7.5) than those on an acid diet (urinary pH < 6). Plasma half-lives of amphetamine ranged between 16-31 hr & 8-11 hr, respectively, & the excretion of (14)C in 24 hr urine was 45 & 70%.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 12.0 12.1
Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. October 2012, 9 (4): 739–752. PMC 3480574
 . PMID 23065655. doi:10.1007/s13311-012-0150-9.
. PMID 23065655. doi:10.1007/s13311-012-0150-9.
- ^ 13.0 13.1 13.2 13.3
Glennon RA. Phenylisopropylamine stimulants: amphetamine-related agents. Lemke TL, Williams DA, Roche VF, Zito W (编). Foye's principles of medicinal chemistry 7th. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013: 646–648 [2015-09-11]. ISBN 9781609133450.
The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ 14.0 14.1
Taylor KB. Dopamine-beta-hydroxylase. Stereochemical course of the reaction (PDF). J. Biol. Chem. January 1974, 249 (2): 454–458 [2014-11-06]. PMID 4809526.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ 15.0 15.1
Horwitz D, Alexander RW, Lovenberg W, Keiser HR. Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity. Circ. Res. May 1973, 32 (5): 594–599. PMID 4713201. doi:10.1161/01.RES.32.5.594.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ^ 16.0 16.1 16.2 16.3
Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. June 2005, 106 (3): 357–387. PMC 1828602
 . PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
. PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
"Table 5: N-containing drugs and xenobiotics oxygenated by FMO" - ^ 17.0 17.1 Cashman JR, Xiong YN, Xu L, Janowsky A. N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication. J. Pharmacol. Exp. Ther. March 1999, 288 (3): 1251–1260. PMID 10027866.
- ^ 18.0 18.1 18.2 18.3 Amphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 2015-04-11.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 引用错误:没有为名为
Properties的参考文献提供内容 - ^ Amphetamine. Chemspider.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 21.0 21.1 引用错误:没有为名为
DrugBank1的参考文献提供内容 - ^ 22.0 22.1 Greene SL, Kerr F, Braitberg G. Review article: amphetamines and related drugs of abuse. Emerg. Med. Australas. October 2008, 20 (5): 391–402. PMID 18973636. doi:10.1111/j.1742-6723.2008.01114.x.
- ^
Enantiomer. IUPAC Goldbook. International Union of Pure and Applied Chemistry. [2014-03-14]. doi:10.1351/goldbook.E02069. (原始内容存档于2013-03-17).
One of a pair of molecular entities which are mirror images of each other and non-superposable.
- ^ 24.0 24.1
Guidelines on the Use of International Nonproprietary Names (INNS) for Pharmaceutical Substances. World Health Organization. 1997 [2014-12-01].
In principle, INNs are selected only for the active part of the molecule which is usually the base, acid or alcohol. In some cases, however, the active molecules need to be expanded for various reasons, such as formulation purposes, bioavailability or absorption rate. In 1975 the experts designated for the selection of INN decided to adopt a new policy for naming such molecules. In future, names for different salts or esters of the same active substance should differ only with regard to the inactive moiety of the molecule. ... The latter are called modified INNs (INNMs).
- ^ 25.0 25.1 25.2 25.3 25.4 25.5
Yoshida T. Chapter 1: Use and Misuse of Amphetamines: An International Overview. Klee H (编). Amphetamine Misuse: International Perspectives on Current Trends. Amsterdam, Netherlands: Harwood Academic Publishers. 1997: 2 [2014-12-01]. ISBN 9789057020810.
Amphetamine, in the singular form, properly applies to the racemate of 2-amino-1-phenylpropane. ... In its broadest context, however, the term [amphetamines] can even embrace a large number of structurally and pharmacologically related substances.
- ^ 26.0 26.1 Amphetamine. Medical Subject Headings. United States National Library of Medicine. [2013-12-16].
- ^ 27.0 27.1 27.2 Spencer RC, Devilbiss DM, Berridge CW. The Cognition-Enhancing Effects of Psychostimulants Involve Direct Action in the Prefrontal Cortex. Biol. Psychiatry. June 2015, 77 (11): 940–950. PMID 25499957. doi:10.1016/j.biopsych.2014.09.013.
The procognitive actions of psychostimulants are only associated with low doses. Surprisingly, despite nearly 80 years of clinical use, the neurobiology of the procognitive actions of psychostimulants has only recently been systematically investigated. Findings from this research unambiguously demonstrate that the cognition-enhancing effects of psychostimulants involve the preferential elevation of catecholamines in the PFC and the subsequent activation of norepinephrine α2 and dopamine D1 receptors. ... This differential modulation of PFC-dependent processes across dose appears to be associated with the differential involvement of noradrenergic α2 versus α1 receptors. Collectively, this evidence indicates that at low, clinically relevant doses, psychostimulants are devoid of the behavioral and neurochemical actions that define this class of drugs and instead act largely as cognitive enhancers (improving PFC-dependent function). This information has potentially important clinical implications as well as relevance for public health policy regarding the widespread clinical use of psychostimulants and for the development of novel pharmacologic treatments for attention-deficit/hyperactivity disorder and other conditions associated with PFC dysregulation. ... In particular, in both animals and humans, lower doses maximally improve performance in tests of working memory and response inhibition, whereas maximal suppression of overt behavior and facilitation of attentional processes occurs at higher doses.
引用错误:带有name属性“Unambiguous PFC D1 A2”的<ref>标签用不同内容定义了多次 - ^ 28.0 28.1 Ilieva IP, Hook CJ, Farah MJ. Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis. J. Cogn. Neurosci. January 2015: 1–21. PMID 25591060. doi:10.1162/jocn_a_00776.
Specifically, in a set of experiments limited to high-quality designs, we found significant enhancement of several cognitive abilities. ... The results of this meta-analysis ... do confirm the reality of cognitive enhancing effects for normal healthy adults in general, while also indicating that these effects are modest in size.
引用错误:带有name属性“Cognitive and motivational effects”的<ref>标签用不同内容定义了多次 - ^ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09
Malenka RC, Nestler EJ, Hyman SE. Chapter 13: Higher Cognitive Function and Behavioral Control. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 318, 321. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in normal subjects and those with ADHD. ... stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks ... through indirect stimulation of dopamine and norepinephrine receptors. ...
Beyond these general permissive effects, dopamine (acting via D1 receptors) and norepinephrine (acting at several receptors) can, at optimal levels, enhance working memory and aspects of attention. - ^ 30.0 30.1 30.2 30.3 30.4
Liddle DG, Connor DJ. Nutritional supplements and ergogenic AIDS. Prim. Care. June 2013, 40 (2): 487–505. PMID 23668655. doi:10.1016/j.pop.2013.02.009.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
· Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
· Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
· Improved reaction time
· Increased muscle strength and delayed muscle fatigue
· Increased acceleration
· Increased alertness and attention to task - ^ 31.0 31.1 31.2 31.3 31.4 31.5 引用错误:没有为名为
FDA Abuse & OD的参考文献提供内容 - ^ 32.0 32.1 引用错误:没有为名为
Libido的参考文献提供内容 - ^ 33.0 33.1 引用错误:没有为名为
FDA Effects的参考文献提供内容 - ^ 34.0 34.1 34.2 34.3 34.4 34.5 34.6 引用错误:没有为名为
Westfall的参考文献提供内容 - ^ 35.0 35.1 35.2 35.3 Shoptaw SJ, Kao U, Ling W. Shoptaw SJ, Ali R , 编. Treatment for amphetamine psychosis. Cochrane Database Syst. Rev. January 2009, (1): CD003026. PMID 19160215. doi:10.1002/14651858.CD003026.pub3.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - ^ 36.0 36.1 引用错误:没有为名为
Stimulant Misuse的参考文献提供内容 - ^ 37.0 37.1 Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and Addictive Disorders. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 368. ISBN 9780071481274.
Such agents also have important therapeutic uses; cocaine, for example, is used as a local anesthetic (Chapter 2), and amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction, especially when they are rapidly administered or when high-potency forms are given.
- ^ 38.0 38.1 Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr. Med. Res. Opin. May 2008, 24 (5): 1345–1357. PMID 18384709. doi:10.1185/030079908X280707.
When oral formulations of psychostimulants are used at recommended doses and frequencies, they are unlikely to yield effects consistent with abuse potential in patients with ADHD.
- ^ 39.0 39.1 Stolerman IP. Stolerman IP , 编. Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. 2010: 78. ISBN 9783540686989.
- ^ 40.0 40.1 引用错误:没有为名为
Benzedrine的参考文献提供内容 - ^ 引用错误:没有为名为
UN Convention的参考文献提供内容 - ^ 引用错误:没有为名为
Nonmedical的参考文献提供内容 - ^ 43.0 43.1 43.2 43.3 引用错误:没有为名为
Evekeo的参考文献提供内容 - ^ National Drug Code Amphetamine Search Results. National Drug Code Directory. United States Food and Drug Administration. [2013-12-16]. (原始内容存档于2013-12-16).
- ^ 45.00 45.01 45.02 45.03 45.04 45.05 45.06 45.07 45.08 45.09 45.10 45.11 45.12 45.13 45.14 45.15 45.16 45.17 45.18 45.19 45.20 45.21 45.22 Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. January 2011, 116 (2): 164–176. PMC 3005101
 . PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
. PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
- ^ 46.0 46.1 46.2 46.3 46.4 Grandy DK, Miller GM, Li JX. "TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug Alcohol Depend. February 2016, 159: 9–16. PMID 26644139. doi:10.1016/j.drugalcdep.2015.11.014.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ 47.0 47.1 引用错误:没有为名为
Trace Amines的参考文献提供内容 - ^ Amphetamine. European Monitoring Centre for Drugs and Drug Addiction. [2013-10-19].
- ^ 引用错误:没有为名为
Amphetamine - a substituted amphetamine的参考文献提供内容 - ^ Obsessive compulsive disorder (OCD). NHS Choice. 2016-09-28 [2017-04-04].
- ^ 51.0 51.1 Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L. Toxicity of amphetamines: an update. Arch. Toxicol. August 2012, 86 (8): 1167–1231. PMID 22392347. doi:10.1007/s00204-012-0815-5.
- ^ Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann. N. Y. Acad. Sci. October 2008, 1141: 195–220. PMC 2769923
 . PMID 18991959. doi:10.1196/annals.1441.031.
. PMID 18991959. doi:10.1196/annals.1441.031.
- ^ 53.0 53.1 Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. February 2013, 70 (2): 185–198. PMID 23247506. doi:10.1001/jamapsychiatry.2013.277.
- ^ 54.0 54.1
Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J. Clin. Psychiatry. September 2013, 74 (9): 902–917. PMC 3801446
 . PMID 24107764. doi:10.4088/JCP.12r08287.
. PMID 24107764. doi:10.4088/JCP.12r08287.
- ^ 55.0 55.1
Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects.. Acta psychiatrica Scand. February 2012, 125 (2): 114–126. PMID 22118249. doi:10.1111/j.1600-0447.2011.01786.x.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- ^ 56.0 56.1 56.2 56.3
Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (编). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 121–123, 125–127. ISBN 9781441913968.
Ongoing research has provided answers to many of the parents’ concerns, and has confirmed the effectiveness and safety of the long-term use of medication.
- ^ 57.0 57.1 57.2 57.3 57.4 Arnold LE, Hodgkins P, Caci H, Kahle J, Young S. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS ONE. February 2015, 10 (2): e0116407. PMC 4340791
 . PMID 25714373. doi:10.1371/journal.pone.0116407.
. PMID 25714373. doi:10.1371/journal.pone.0116407. The highest proportion of improved outcomes was reported with combination treatment (83% of outcomes). Among significantly improved outcomes, the largest effect sizes were found for combination treatment. The greatest improvements were associated with academic, self-esteem, or social function outcomes.
Figure 3: Treatment benefit by treatment type and outcome group - ^ 58.0 58.1 58.2 58.3 58.4 Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. July 2011, 25 (7): 539–554. PMID 21699268. doi:10.2165/11589380-000000000-00000.
Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. ... In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ^ 59.0 59.1 59.2 Malenka RC, Nestler EJ, Hyman SE. Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 154–157. ISBN 9780071481274.
- ^ 60.0 60.1 60.2 60.3 60.4 Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol. Biochem. Behav. August 2011, 99 (2): 262–274. PMC 3353150
 . PMID 21596055. doi:10.1016/j.pbb.2011.05.002.
. PMID 21596055. doi:10.1016/j.pbb.2011.05.002.
- ^
Parker J, Wales G, Chalhoub N, Harpin V. The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials. Psychol. Res. Behav. Manag. (systematic review (secondary source)). September 2013, 6: 87–99. PMC 3785407
 . PMID 24082796. doi:10.2147/PRBM.S49114.
. PMID 24082796. doi:10.2147/PRBM.S49114. Only one paper53 examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- ^ Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (编). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 111–113. ISBN 9781441913968.
- ^ Stimulants for Attention Deficit Hyperactivity Disorder. WebMD. Healthwise. 2010-04-12 [2013-11-12].
- ^ Scholten RJ, Clarke M, Hetherington J. The Cochrane Collaboration. Eur. J. Clin. Nutr. August 2005,. 59 Suppl 1: S147–S149; discussion S195–S196. PMID 16052183. doi:10.1038/sj.ejcn.1602188.
- ^ Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Castells X , 编. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst. Rev. June 2011, (6): CD007813. PMID 21678370. doi:10.1002/14651858.CD007813.pub2.
- ^ Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. February 2016, 2: CD009996. PMID 26844979. doi:10.1002/14651858.CD009996.pub2.
- ^ Pringsheim T, Steeves T. Pringsheim T , 编. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst. Rev. April 2011, (4): CD007990. PMID 21491404. doi:10.1002/14651858.CD007990.pub2.
- ^ 引用错误:没有为名为
medlineplus1的参考文献提供内容 - ^ 69.0 69.1 69.2 Abuse, National Institute on Drug. Stimulant ADHD Medications: Methylphenidate and Amphetamines.
- ^ Choices, N. H. S. What is a controlled medicine (drug)? - Health questions - NHS Choices. 2016-12-12.
- ^ Methylphenidate. Home of MedlinePlus → Drugs, Herbs and Supplements → Methylphenidate Methylphenidate pronounced as (meth il fen i date). 2016-02-15 [February twenty seventh, 2017].
- ^ Combining medications could offer better results for ADHD patients. Science News. Elsevier. 2016-08-01 [January 2017]. (原始内容存档于August 2016).
"Three studies to be published in the August 2016 issue of the Journal of the American Academy of Child and Adolescent Psychiatry (JAACAP) report that combining two standard medications could lead to greater clinical improvements for children with attention-deficit/hyperactivity disorder (ADHD) than either ADHD therapy alone.", August, 2016
- ^ Adults with ADHD. MedlinePlus the Magazine 9. 8600 Rockville Pike • Bethesda, MD 20894, United States of America: NATIONAL LIBRARY OF MEDICINE at the NATIONAL INSTITUTES OF HEALTH. Spring 2014: 19. ISSN 1937-4712 (美国英语).
- ^ Attention deficit hyperactivity disorder. Home → Medical Encyclopedia → Attention deficit hyperactivity disorder. NATIONAL LIBRARY OF MEDICINE at the NATIONAL INSTITUTES OF HEALTH. 2016-05-25 [February twenty seventh, 2017.].
- ^ All Disorders. National Institute of Neurological Disorders and Stroke. [February twenty seventh, 2017].
- ^
Bagot KS, Kaminer Y. Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review. Addiction. April 2014, 109 (4): 547–557. PMC 4471173
 . PMID 24749160. doi:10.1111/add.12460.
. PMID 24749160. doi:10.1111/add.12460. Amphetamine has been shown to improve consolidation of information (0.02 ≥ P ≤ 0.05), leading to improved recall.
- ^ Devous MD, Trivedi MH, Rush AJ. Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers. J. Nucl. Med. April 2001, 42 (4): 535–542. PMID 11337538.
- ^
Malenka RC, Nestler EJ, Hyman SE. Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ^ 79.0 79.1 79.2 Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. January 2014, 66 (1): 193–221. PMID 24344115. doi:10.1124/pr.112.007054.
- ^ Twohey M. Pills become an addictive study aid. JS Online. 2006-03-26 [2007-12-02]. (原始内容存档于2007-08-15).
- ^ 81.0 81.1 81.2 81.3
Parr JW. Attention-deficit hyperactivity disorder and the athlete: new advances and understanding. Clin. Sports Med. July 2011, 30 (3): 591–610. PMID 21658550. doi:10.1016/j.csm.2011.03.007.
In 1980, Chandler and Blair47 showed significant increases in knee extension strength, acceleration, anaerobic capacity, time to exhaustion during exercise, pre-exercise and maximum heart rates, and time to exhaustion during maximal oxygen consumption (VO2 max) testing after administration of 15 mg of dextroamphetamine versus placebo. Most of the information to answer this question has been obtained in the past decade through studies of fatigue rather than an attempt to systematically investigate the effect of ADHD drugs on exercise.
- ^ 82.0 82.1 82.2
Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R. Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing. Sports Med. May 2013, 43 (5): 301–311. PMID 23456493. doi:10.1007/s40279-013-0030-4.
In high-ambient temperatures, dopaminergic manipulations clearly improve performance. The distribution of the power output reveals that after dopamine reuptake inhibition, subjects are able to maintain a higher power output compared with placebo. ... Dopaminergic drugs appear to override a safety switch and allow athletes to use a reserve capacity that is ‘off-limits’ in a normal (placebo) situation.
- ^ Bracken NM. National Study of Substance Use Trends Among NCAA College Student-Athletes (PDF). NCAA Publications. National Collegiate Athletic Association. January 2012 [2013-10-08].
- ^
Docherty JR. Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Br. J. Pharmacol. June 2008, 154 (3): 606–622. PMC 2439527
 . PMID 18500382. doi:10.1038/bjp.2008.124.
. PMID 18500382. doi:10.1038/bjp.2008.124.
- ^
Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson's disease and timing deficits. Front. Integr. Neurosci. October 2013, 7: 75. PMC 3813949
 . PMID 24198770. doi:10.3389/fnint.2013.00075.
. PMID 24198770. doi:10.3389/fnint.2013.00075. Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or “clock,” activity. For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft advances the start of responding during interval timing, whereas antagonists of D2 type dopamine receptors typically slow timing;... Depletion of dopamine in healthy volunteers impairs timing, while amphetamine releases synaptic dopamine and speeds up timing.
- ^
Rattray B, Argus C, Martin K, Northey J, Driller M. Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance?. Front. Physiol. March 2015, 6: 79. PMC 4362407
 . PMID 25852568. doi:10.3389/fphys.2015.00079.
. PMID 25852568. doi:10.3389/fphys.2015.00079. Aside from accounting for the reduced performance of mentally fatigued participants, this model rationalizes the reduced RPE and hence improved cycling time trial performance of athletes using a glucose mouthwash (Chambers et al., 2009) and the greater power output during a RPE matched cycling time trial following amphetamine ingestion (Swart, 2009). ... Dopamine stimulating drugs are known to enhance aspects of exercise performance (Roelands et al., 2008)
- ^
Roelands B, De Pauw K, Meeusen R. Neurophysiological effects of exercise in the heat. Scand. J. Med. Sci. Sports. June 2015,. 25 Suppl 1: 65–78. PMID 25943657. doi:10.1111/sms.12350.
This indicates that subjects did not feel they were producing more power and consequently more heat. The authors concluded that the “safety switch” or the mechanisms existing in the body to prevent harmful effects are overridden by the drug administration (Roelands et al., 2008b). Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort.
- ^
Kessler S. Drug therapy in attention-deficit hyperactivity disorder. South. Med. J. January 1996, 89 (1): 33–38. PMID 8545689. doi:10.1097/00007611-199601000-00005.
statements on package inserts are not intended to limit medical practice. Rather they are intended to limit claims by pharmaceutical companies. ... the FDA asserts explicitly, and the courts have upheld that clinical decisions are to be made by physicians and patients in individual situations.
- ^ 89.0 89.1 89.2 89.3 89.4 89.5 89.6 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 4–6. December 2013 [2013-12-30].
- ^ 90.00 90.01 90.02 90.03 90.04 90.05 90.06 90.07 90.08 90.09 Heedes G, Ailakis J. Amphetamine (PIM 934). INCHEM. International Programme on Chemical Safety. [2014-06-24].
- ^ 91.0 91.1 Dexedrine Prescribing Information (PDF). United States Food and Drug Administration. Amedra Pharmaceuticals LLC. October 2013 [2013-11-04].
- ^
Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J. Clin. Psychiatry. November 2004, 65 (11): 1520–1524. \ PMID 15554766 \ 请检查
|pmid=值 (帮助). doi:10.4088/jcp.v65n1113. - ^ Stewart JW, Deliyannides DA, McGrath PJ. How treatable is refractory depression?. J. Affect. Disord. June 2014, 167: 148–152. PMID 24972362. doi:10.1016/j.jad.2014.05.047.
- ^ 94.0 94.1 引用错误:没有为名为
pmid18295156的参考文献提供内容 - ^ 95.0 95.1 Spiller HA, Hays HL, Aleguas A. Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs. June 2013, 27 (7): 531–543. PMID 23757186. doi:10.1007/s40263-013-0084-8.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- ^ Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013 (PDF). Lancet. 2015, 385 (9963): 117–171 [2015-03-03]. PMC 4340604
 . PMID 25530442. doi:10.1016/S0140-6736(14)61682-2.
. PMID 25530442. doi:10.1016/S0140-6736(14)61682-2. Amphetamine use disorders ... 3,788 (3,425–4,145)
- ^ Kanehisa Laboratories. Amphetamine – Homo sapiens (human). KEGG Pathway. 2014-10-10 [2014-10-31].
- ^ 98.0 98.1 98.2 98.3 98.4 98.5 Nechifor M. Magnesium in drug dependences. Magnes. Res. March 2008, 21 (1): 5–15. PMID 18557129.
- ^ 99.0 99.1 99.2 99.3 99.4 Ruffle JK. Molecular neurobiology of addiction: what's all the (Δ)FosB about?. Am. J. Drug Alcohol Abuse. November 2014, 40 (6): 428–437. PMID 25083822. doi:10.3109/00952990.2014.933840.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure.
- ^ 100.0 100.1 100.2 100.3 100.4 Nestler, Eric J. Cellular basis of memory for addiction. Dialogues in Clinical Neuroscience. 2013-12, 15 (4): 431–443. ISSN 1294-8322. PMC 3898681
 . PMID 24459410. doi:10.31887/DCNS.2013.15.4/enestler.
. PMID 24459410. doi:10.31887/DCNS.2013.15.4/enestler.
- ^ Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. November 2011, 12 (11): 623–637. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111. ΔFosB serves as one of the master control proteins governing this structural plasticity.
- ^ 102.00 102.01 102.02 102.03 102.04 102.05 102.06 102.07 102.08 102.09 102.10 102.11 102.12 102.13 102.14 102.15 102.16 102.17 102.18 102.19 102.20 102.21 Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. December 2011, 61 (7): 1109–1122. PMC 3139704
 . PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010.
. PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010. Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- ^ 103.0 103.1 103.2 103.3 Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci. Biobehav. Rev. September 2013, 37 (8): 1622–1644. PMC 3788047
 . PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011.
. PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011. These findings suggest that exercise may “magnitude”-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuroadaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ^ 104.0 104.1 104.2 Zhou Y, Zhao M, Zhou C, Li R. Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front. Neuroendocrinol. July 2015, 40: 24–41. PMID 26182835. doi:10.1016/j.yfrne.2015.07.001.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation.
- ^ 105.0 105.1 105.2 Linke SE, Ussher M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am. J. Drug Alcohol Abuse. January 2015, 41 (1): 7–15. PMC 4831948
 . PMID 25397661. doi:10.3109/00952990.2014.976708.
. PMID 25397661. doi:10.3109/00952990.2014.976708. The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- ^ 106.0 106.1 Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and Addictive Disorders. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 386. ISBN 9780071481274.
Currently, cognitive–behavioral therapies are the most successful treatment available for preventing the relapse of psychostimulant use.
- ^ Albertson TE. Amphetamines. Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AH (编). Poisoning & Drug Overdose 6th. New York: McGraw-Hill Medical. 2011: 77–79. ISBN 9780071668330.
- ^ Nestler, Eric J.; Malenka, Robert C. Chapter 15: Reinforcement and Addictive Disorders. Molecular neuropharmacology : a foundation for clinical neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 364–375. ISBN 978-0-07-164119-7. OCLC 273018757.
- ^ Glossary. Icahn School of Medicine. [2021-04-29].
- ^ Volkow, Nora D.; Koob, George F.; McLellan, A. Thomas. Longo, Dan L. , 编. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine. 2016-01-28, 374 (4): 363–371. ISSN 0028-4793. PMC 6135257
 . PMID 26816013. doi:10.1056/NEJMra1511480 (英语).
. PMID 26816013. doi:10.1056/NEJMra1511480 (英语).
- ^ Amphetamines: Drug Use and Abuse. Merck Manual Home Edition. Merck. February 2003 [2007-02-28]. (原始内容存档于2007-02-17).
- ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M. Pérez-Mañá C , 编. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev. September 2013, 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
- ^ Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. July 2006, 29: 565–598. PMID 16776597. doi:10.1146/annurev.neuro.29.051605.113009.
- ^ 114.0 114.1 114.2 114.3 114.4 114.5 114.6 114.7 Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. November 2011, 12 (11): 623–637. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111.
- ^ 115.0 115.1 115.2 115.3 115.4 Steiner H, Van Waes V. Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. January 2013, 100: 60–80. PMC 3525776
 . PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
. PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 4: Signal Transduction in the Brain. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 94. ISBN 9780071481274.
- ^ Kanehisa Laboratories. Alcoholism – Homo sapiens (human). KEGG Pathway. 2014-10-29 [2014-10-31].
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. February 2009, 106 (8): 2915–2920. PMC 2650365
 . PMID 19202072. doi:10.1073/pnas.0813179106.
. PMID 19202072. doi:10.1073/pnas.0813179106.
- ^ Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. January 2014,. 76 Pt B: 259–268. PMC 3766384
 . PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004.
. PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004.
- ^ 120.0 120.1 Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M. Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. J. Psychoactive Drugs. March 2012, 44 (1): 38–55. PMC 4040958
 . PMID 22641964. doi:10.1080/02791072.2012.662112.
. PMID 22641964. doi:10.1080/02791072.2012.662112.
- ^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J. Neurosci. February 2013, 33 (8): 3434–3442. PMC 3865508
 . PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
. PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
- ^ Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM. Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats. Neuropharmacology. February 2016, 101: 154–164. PMID 26391065. doi:10.1016/j.neuropharm.2015.09.023.
- ^ Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. May 2014, 7 (3): 363–374. PMC 4017926
 . PMID 24716825. doi:10.1586/17512433.2014.909283.
. PMID 24716825. doi:10.1586/17512433.2014.909283. Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev. September 2013, 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. ... Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy
- ^ Forray A, Sofuoglu M. Future pharmacological treatments for substance use disorders. Br. J. Clin. Pharmacol. February 2014, 77 (2): 382–400. PMC 4014020
 . PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x.
. PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x.
- ^ 126.0 126.1 Jing L, Li JX. Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction. Eur. J. Pharmacol. August 2015, 761: 345–352. PMC 4532615
 . PMID 26092759. doi:10.1016/j.ejphar.2015.06.019.
. PMID 26092759. doi:10.1016/j.ejphar.2015.06.019. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction.
- ^ 127.0 127.1 Malenka RC, Nestler EJ, Hyman SE. Chapter 5: Excitatory and Inhibitory Amino Acids. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 124–125. ISBN 9780071481274.
- ^ 128.0 128.1 128.2 Carroll ME, Smethells JR. Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments. Front. Psychiatry. February 2016, 6: 175. PMC 4745113
 . PMID 26903885. doi:10.3389/fpsyt.2015.00175.
. PMID 26903885. doi:10.3389/fpsyt.2015.00175. Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. - ^ 129.0 129.1 129.2 129.3 Shoptaw SJ, Kao U, Heinzerling K, Ling W. Shoptaw SJ , 编. Treatment for amphetamine withdrawal. Cochrane Database Syst. Rev. April 2009, (2): CD003021. PMID 19370579. doi:10.1002/14651858.CD003021.pub2.
The prevalence of this withdrawal syndrome is extremely common (Cantwell 1998; Gossop 1982) with 87.6% of 647 individuals with amphetamine dependence reporting six or more signs of amphetamine withdrawal listed in the DSM when the drug is not available (Schuckit 1999) ... The severity of withdrawal symptoms is greater in amphetamine dependent individuals who are older and who have more extensive amphetamine use disorders (McGregor 2005). Withdrawal symptoms typically present within 24 hours of the last use of amphetamine, with a withdrawal syndrome involving two general phases that can last 3 weeks or more. The first phase of this syndrome is the initial "crash" that resolves within about a week (Gossop 1982;McGregor 2005) ...
- ^ Cantwell, Bernadette; McBride, Andrew J. Self detoxication by amphetamine dependent patients: a pilot study. Drug and Alcohol Dependence (Elsevier BV). 1998, 49 (2): 157–163 [2017-05-09]. doi:10.1016/s0376-8716(97)00160-9.
- ^ Amphetamine-Related Psychiatric Disorders Clinical Presentation: History, Physical, Causes. Medscape Reference. 2015-12-03 [2017-05-09].
- ^ Adderall IR Prescribing Information (PDF). United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc. October 2015 [2016-05-18].
- ^ Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc. December 2013 [2013-12-30].
- ^ The amphetamine withdrawal syndrome. Department of Health. [2017-05-09].
- ^ 135.0 135.1 135.2 Self detoxication by amphetamine dependent patients: a pilot study. ScienceDirect.com. 2016-01-01 [2017-05-09].
- ^ Advokat C. Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD. J. Atten. Disord. July 2007, 11 (1): 8–16. PMID 17606768. doi:10.1177/1087054706295605.
- ^ 137.0 137.1 137.2 137.3 Bowyer JF, Hanig JP. Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity. Temperature (Austin). November 2014, 1 (3): 172–182. PMC 5008711
 . PMID 27626044. doi:10.4161/23328940.2014.982049.
. PMID 27626044. doi:10.4161/23328940.2014.982049. Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40°C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. ... The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. ... In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus.
- ^ Amphetamine. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. [2014-02-26].
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and addictive disorders. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- ^ Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox. Res. February 2000, 1 (3): 181–195. PMID 12835101. doi:10.1007/BF03033289.
- ^ Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself (PDF). Acta Med. Okayama. June 2008, 62 (3): 141–150. PMID 18596830.
- ^ Hofmann FG. A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects 2nd. New York, USA: Oxford University Press. 1983: 329. ISBN 9780195030570.
- ^ 143.00 143.01 143.02 143.03 143.04 143.05 143.06 143.07 143.08 143.09 143.10 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 8–10. December 2013 [2013-12-30].
- ^ Krause J. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Rev. Neurother. April 2008, 8 (4): 611–625. PMID 18416663. doi:10.1586/14737175.8.4.611.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ^ Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. February 2011, 69 (4): 628–649. PMC 3065181
 . PMID 21338876. doi:10.1016/j.neuron.2011.02.010.
. PMID 21338876. doi:10.1016/j.neuron.2011.02.010. They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- ^ 146.0 146.1 Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH. The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. June 2002, 277 (24): 21505–21513. PMID 11940571. doi:10.1074/jbc.M112265200.
The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET).
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry. October 2012, 51 (10): 1003–1019.e20. PMID 23021477. doi:10.1016/j.jaac.2012.08.015.
With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110
- ^ 148.00 148.01 148.02 148.03 148.04 148.05 148.06 148.07 148.08 148.09 148.10 148.11 Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. January 2011, 1216: 86–98. PMC 4183197
 . PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x.
. PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x. VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). ... AMPH release of DA from synapses requires both an action at VMAT2 to release DA to the cytoplasm and a concerted release of DA from the cytoplasm via "reverse transport" through DAT.
- ^ 149.0 149.1 Maguire JJ, Davenport AP. TA1 receptor. IUPHAR database. International Union of Basic and Clinical Pharmacology. 2014-12-02 [2014-12-08].
- ^ Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. July 2001, 98 (16): 8966–8971. PMC 55357
 . PMID 11459929. doi:10.1073/pnas.151105198.
. PMID 11459929. doi:10.1073/pnas.151105198.
- ^ 151.0 151.1 151.2 151.3 151.4 Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. July 2014, 83 (2): 404–416. PMC 4159050
 . PMID 25033183. doi:10.1016/j.neuron.2014.05.043.
. PMID 25033183. doi:10.1016/j.neuron.2014.05.043. AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
- ^ 152.0 152.1 SLC18 family of vesicular amine transporters. IUPHAR database. International Union of Basic and Clinical Pharmacology. [2015-11-13].
- ^ 153.0 153.1 153.2 153.3 SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 [ Homo sapiens (human) ]. NCBI Gene. United States National Library of Medicine – National Center for Biotechnology Information. [2014-11-11].
Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. ... internalization of EAAT3 triggered by amphetamine increases glutamatergic signaling and thus contributes to the effects of amphetamine on neurotransmission.
- ^ Zhu HJ, Appel DI, Gründemann D, Markowitz JS. Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J. Neurochem. July 2010, 114 (1): 142–149. PMC 3775896
 . PMID 20402963. doi:10.1111/j.1471-4159.2010.06738.x.
. PMID 20402963. doi:10.1111/j.1471-4159.2010.06738.x.
- ^ Rytting E, Audus KL. Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells. J. Pharmacol. Exp. Ther. January 2005, 312 (1): 192–198. PMID 15316089. doi:10.1124/jpet.104.072363.
- ^ Inazu M, Takeda H, Matsumiya T. [The role of glial monoamine transporters in the central nervous system]. Nihon Shinkei Seishin Yakurigaku Zasshi. August 2003, 23 (4): 171–178. PMID 13677912 (Japanese).
- ^ 157.0 157.1 157.2 Vicentic A, Jones DC. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. February 2007, 320 (2): 499–506. PMID 16840648. doi:10.1124/jpet.105.091512.
The physiological importance of CART was further substantiated in numerous human studies demonstrating a role of CART in both feeding and psychostimulant addiction. ... Colocalization studies also support a role for CART in the actions of psychostimulants. ... CART and DA receptor transcripts colocalize (Beaudry et al., 2004). Second, dopaminergic nerve terminals in the NAc synapse on CART-containing neurons (Koylu et al., 1999), hence providing the proximity required for neurotransmitter signaling. These studies suggest that DA plays a role in regulating CART gene expression possibly via the activation of CREB.
- ^ 158.0 158.1 158.2 Amphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ Zhang M, Han L, Xu Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin. Exp. Pharmacol. Physiol. June 2012, 39 (6): 586–592. PMID 22077697. doi:10.1111/j.1440-1681.2011.05642.x.
Recently, it was demonstrated that CART, as a neurotrophic peptide, had a cerebroprotective against focal ischaemic stroke and inhibited the neurotoxicity of β-amyloid protein, which focused attention on the role of CART in the central nervous system (CNS) and neurological diseases. ... The literature indicates that there are many factors, such as regulation of the immunological system and protection against energy failure, that may be involved in the cerebroprotection afforded by CART
- ^ 160.0 160.1 Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat. Rev. Neurosci. October 2008, 9 (10): 747–758. PMC 4418456
 . PMID 18802445. doi:10.1038/nrn2493.
. PMID 18802445. doi:10.1038/nrn2493. Several studies on CART (cocaine- and amphetamine-regulated transcript)-peptide-induced cell signalling have demonstrated that CART peptides activate at least three signalling mechanisms. First, CART 55–102 inhibited voltage-gated L-type Ca2+ channels ...
- ^ Lin Y, Hall RA, Kuhar MJ. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6–38 as a CART receptor antagonist. Neuropeptides. October 2011, 45 (5): 351–358. PMC 3170513
 . PMID 21855138. doi:10.1016/j.npep.2011.07.006.
. PMID 21855138. doi:10.1016/j.npep.2011.07.006.
- ^ Monoamine oxidase (Homo sapiens). BRENDA. Technische Universität Braunschweig. 2014-01-01 [2014-05-04].
- ^ 163.0 163.1 163.2 Amphetamine. T3DB. University of Alberta. [2015-02-24].
|section=被忽略 (帮助) - ^ 164.0 164.1 Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res. Monogr. March 1998, 178: 440–466. PMID 9686407.
- ^ 165.0 165.1 Finnema SJ, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, Sallinen J, Wong E, Farde L, Halldin C, Grimwood S. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology (Berl.). November 2015, 232 (21–22): 4129–4157. PMC 4600473
 . PMID 25921033. doi:10.1007/s00213-015-3938-6.
. PMID 25921033. doi:10.1007/s00213-015-3938-6. More recently, Colasanti and colleagues reported that a pharmacologically induced elevation in endogenous opioid release reduced [11C]carfentanil binding in several regions of the human brain, including the basal ganglia, frontal cortex, and thalamus (Colasanti et al. 2012). Oral administration of d-amphetamine, 0.5 mg/kg, 3 h before [11C]carfentanil injection, reduced BPND values by 2–10 %. The results were confirmed in another group of subjects (Mick et al. 2014). However, Guterstam and colleagues observed no change in [11C]carfentanil binding when d-amphetamine, 0.3 mg/kg, was administered intravenously directly before injection of [11C]carfentanil (Guterstam et al. 2013). It has been hypothesized that this discrepancy may be related to delayed increases in extracellular opioid peptide concentrations following amphetamine-evoked monoamine release (Colasanti et al. 2012; Mick et al. 2014).
- ^ 166.0 166.1 Loseth GE, Ellingsen DM, Leknes S. State-dependent μ-opioid modulation of social motivation. Front. Behav. Neurosci. December 2014, 8: 1–15. PMC 4264475
 . PMID 25565999. doi:10.3389/fnbeh.2014.00430.
. PMID 25565999. doi:10.3389/fnbeh.2014.00430. Similar MOR activation patterns were reported during positive mood induced by an amusing video clip (Koepp et al., 2009) and following amphetamine administration in humans (Colasanti et al., 2012).
- ^ 167.0 167.1 Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, Waldman AD, Schruers KR, Matthews PM, Gunn RN, Nutt DJ, Rabiner EA. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol. Psychiatry. September 2012, 72 (5): 371–377. PMID 22386378. doi:10.1016/j.biopsych.2012.01.027.
- ^ 168.0 168.1 168.2 Gunne LM. Effects of Amphetamines in Humans. Drug Addiction II: Amphetamine, Psychotogen, and Marihuana Dependence. Berlin, Germany; Heidelberg, Germany: Springer. 2013: 247–260 [2015-12-04]. ISBN 9783642667091.
- ^ 169.0 169.1 169.2 Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. April 2005, 30 (4): 821–832. PMID 15702139. doi:10.1038/sj.npp.1300667.
Findings from several prior investigations have shown that plasma levels of glucocorticoids and ACTH are increased by acute administration of AMPH in both rodents and humans
- ^ 170.0 170.1 Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg. Med. Chem. December 2011, 19 (23): 7044–7048. PMC 3236098
 . PMID 22037049. doi:10.1016/j.bmc.2011.10.007.
. PMID 22037049. doi:10.1016/j.bmc.2011.10.007.
- ^ 171.0 171.1 Maguire JJ, Parker WA, Foord SM, Bonner TI, Neubig RR, Davenport AP. International Union of Pharmacology. LXXII. Recommendations for trace amine receptor nomenclature. Pharmacol. Rev. March 2009, 61 (1): 1–8. PMC 2830119
 . PMID 19325074. doi:10.1124/pr.109.001107.
. PMID 19325074. doi:10.1124/pr.109.001107.
- ^ Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol. Sci. September 2013, 34 (9): 489–496. PMC 3831354
 . PMID 23968642. doi:10.1016/j.tips.2013.07.005.
. PMID 23968642. doi:10.1016/j.tips.2013.07.005.
- ^ Ledonne A, Berretta N, Davoli A, Rizzo GR, Bernardi G, Mercuri NB. Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons. Front. Syst. Neurosci. July 2011, 5: 56. PMC 3131148
 . PMID 21772817. doi:10.3389/fnsys.2011.00056.
. PMID 21772817. doi:10.3389/fnsys.2011.00056.
- ^ mct. TAAR1. GenAtlas. University of Paris. 2012-01-28 [2014-05-29].
· tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA) - ^ Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, Durkin S, Zbinden KG, Norcross R, Meyer CA, Metzler V, Chaboz S, Ozmen L, Trube G, Pouzet B, Bettler B, Caron MG, Wettstein JG, Hoener MC. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc. Natl. Acad. Sci. U.S.A. May 2011, 108 (20): 8485–8490. PMC 3101002
 . PMID 21525407. doi:10.1073/pnas.1103029108.
. PMID 21525407. doi:10.1073/pnas.1103029108.
- ^ 176.0 176.1 176.2 Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. August 2016, 6 (3): 123–148. PMC 4850498
 . PMID 27141430. doi:10.1016/j.baga.2016.02.001.
. PMID 27141430. doi:10.1016/j.baga.2016.02.001. Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.
- ^ Richard RA. Chapter 5—Medical Aspects of Stimulant Use Disorders. National Center for Biotechnology Information Bookshelf. Treatment Improvement Protocol 33. Substance Abuse and Mental Health Services Administration. 1999.
|section-url=被忽略 (帮助);|section=被忽略 (帮助) - ^ 178.0 178.1 178.2 Vyvanse Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 12–16. January 2015 [2015-02-24].
- ^ 179.0 179.1 butyrate-CoA ligase. BRENDA. Technische Universität Braunschweig.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 180.0 180.1
glycine N-acyltransferase. BRENDA. Technische Universität Braunschweig.
|section-url=被忽略 (帮助);|section=被忽略 (帮助); - ^ 引用错误:没有为名为
sympathomimetics的参考文献提供内容 - ^ 引用错误:没有为名为
metabolites的参考文献提供内容 - ^ 引用错误:没有为名为
norephedrine的参考文献提供内容
參見
外部連結
- CID 3007 from PubChem – Amphetamine
- CID 5826 from PubChem – Dextroamphetamine
- CID 32893 from PubChem – Levoamphetamine
- Comparative Toxicogenomics Database entry: Amphetamine
- Comparative Toxicogenomics Database entry: CARTPT
- Drug abuse and drug addictions
- Mechanism of Drug Addiction in the Brain, Animation.
- Drug dependence