1,4-二溴丁-2-烯
外观
(重定向自C4H6Br2)
| 1,4-二溴丁-2-烯 | |
|---|---|
| |
| 识别 | |
| CAS号 | 6974-12-5 821-06-7(E) 18866-73-4(Z) |
| 性质 | |
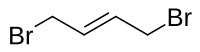
| 化学式 | C4H6Br2 |
| 摩尔质量 | 213.9 g·mol−1 |
| 外观 | 固体(E) 油状物(Z) |
| 熔点 | 50~53 °C(trans)[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
1,4-二溴丁-2-烯是一种有机化合物,化学式为C4H6Br2,它存在一对顺反异构体。它可由1,3-丁二烯和溴反应制得。[2]顺式的异构体可由(Z)-2-丁烯-1,4-二醇、三苯基膦和溴反应制得。[3]它和丙二酸二乙酯在碱存在下反应,可以得到2-乙烯基环丙烷-1,1-二甲酸二乙酯。[4][5]
参考文献
[编辑]- ^ Stereospecific synthesis of optically active succinic-d2 acid. J. Org. Chem. 1981. 46. doi:10.1021/jo00330a042.
- ^ Shantz, Edgar M. Synthesis of compounds related to vitamin A from hydroxymethylene-β-ionone. J. Am. Chem. Soc. 1946. 68. doi:10.1021/ja01216a038.
- ^ Synthesis and Characterization of Cyanobutadiene Isomers—Molecules of Astrochemical Significance. J. Org. Chem. 2020, 85, 9, 5787–5798. doi:10.1021/acs.joc.9b03388.
- ^ Ring-Opening of Vinylcyclopropane-1,1-dicarboxylates by Boronic Acids under Ligandless Palladium Catalysis in Neat Water. J. Org. Chem. 2015, 80, 13, 6529–6536. doi:10.1021/acs.joc.5b00672.
- ^ Photoredox Generation of Isothiouronyl Radical Cations: A New Platform in Covalent Radical Catalysis. Angew Chem Int Ed, 2022. 61 (32). doi:10.1002/anie.202205596.
