JWH大麻素列表:修订间差异
外观
删除的内容 添加的内容
←建立内容为“克莱姆森大学的约翰·威廉·霍夫曼('''J'''ohn '''W'''illiam '''H'''uffman)研究小组合成了450多种大麻素。<ref>{{cite journal |vauthors=Manera C, Tuccinardi T, Martinelli A |year=2008 |title=Indoles and related compounds as cannabinoid ligands |journal=Mini Rev Med Chem |volume=8 |issue=4 |pages=370–87 |doi=10.2174/138955708783955935 |pmid=18473928}}</ref><ref>{{cite journal |vauthors=Wiley JL, Marusich JA, Huffm…”的新页面 |
(没有差异)
|
2023年6月5日 (一) 09:31的版本
克莱姆森大学的约翰·威廉·霍夫曼(John William Huffman)研究小组合成了450多种大麻素。[1][2][3][4]其中一些是:
| 名称 | C类别 | Ki/nM (CB1) | Ki/nM (CB2) | 选择性 | 结构 |
|---|---|---|---|---|---|
| JWH-004 | 萘甲酰基吲哚 | 48 ± 13 | 4 ± 1.5 | CB2 (12x) | 
|
| JWH-007[5] | 萘甲酰基吲哚 | 9.5 ± 4.5 | 2.9 ± 2.6 | CB2 (3.3x) | 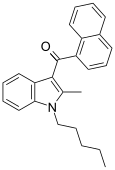
|
| JWH-009 | 萘甲酰基吲哚 | >10000 | 141 ± 14 | CB2 (>70x) | 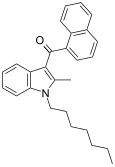
|
| JWH-011 | 萘甲酰基吲哚 | 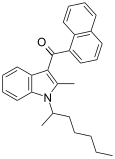
| |||
| JWH-015[5] | 萘甲酰基吲哚 | 164 ± 22 | 13.8 ± 4.6 | CB2 (12x) | 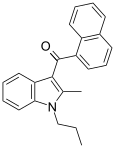
|
| JWH-016 | 萘甲酰基吲哚 | 22 ± 1.5 | 4.3 ± 1.6 | CB2 (5.1x) | 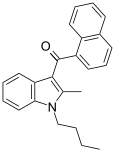
|
| JWH-018[5] | 萘甲酰基吲哚 | 9 ± 5 | 2.9 ± 2.6 | CB2 (3.1x) | 
|
| JWH-019 | 萘甲酰基吲哚 | 9.8 ± 2 | 5.55 ± 2 | CB2 (1.77x) | 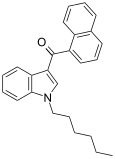
|
| JWH-020 | 萘甲酰基吲哚 | 128 ± 17 | 205 ± 20 | CB1 (1.6x) | 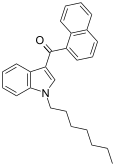
|
| JWH-030 | 萘甲酰基吡咯 | 87 ± 3 | 320 ± 127 | CB1 (3.7x) | 
|
| JWH-031 | 萘甲酰基吡咯 | 399 ± 109 | 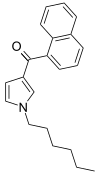
| ||
| JWH-032 | 萘甲酰基吡咯 | >10000 | >10000 | — | 
|
| JWH-033 | 萘甲酰基吡咯 | 666 ± 77 | 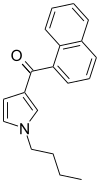
| ||
| JWH-036 | 萘甲酰基吡咯 | 309 ± 11 | 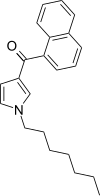
| ||
| JWH-042[6] | 萘甲酰基吲哚 | >10000 | 5050 ± 192 | CB2 | 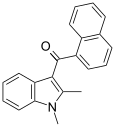
|
| JWH-043[6] | 萘甲酰基吲哚 | 1180 ± 44 | 964 ± 242 | CB2 (1.2x) | 
|
| JWH-044 | 萘甲酰基吡咯 | >10000 | >10000 | — | 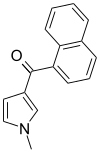
|
| JWH-045 | 萘甲酰基吡咯 | >10000 | >10000 | — | 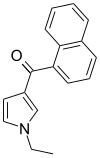
|
| JWH-046[6] | 萘甲酰基吲哚 | 343 ± 38 | 16.3 ± 4.9 | CB2 (21x) | 
|
| JWH-047[6] | 萘甲酰基吲哚 | 59 ± 3 | 3.47 ± 1.80 | CB2 (17x) | 
|
| JWH-048[6] | 萘甲酰基吲哚 | 10.7 ± 1.0 | 0.49 ± 0.13 | CB2 (22x) | 
|
| JWH-049[6] | 萘甲酰基吲哚 | 55.1 ± 17.0 | 32.3 ± 2.4 | CB2 (1.7x) | 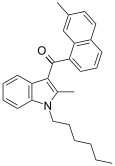
|
| JWH-050[6] | 萘甲酰基吲哚 | 342 ± 6 | 526 ± 133 | CB1 (1.5x) | 
|
| JWH-051 | 二苯并吡喃 | 1.20 | 0.03 | CB2 (40x) | 
|
| JWH-056[7] | 二苯并吡喃 | >10000 | 32 ± 9 | CB2 | 
|
| JWH-057[8] | 二苯并吡喃 | 23 ± 7 | 2.9 ± 1.6 | CB2 (8x) | 
|
| JWH-065[7] | 二苯并吡喃 | 399 ± 76 | 10 ± 2 | CB2 (40x) | 
|
| JWH-070[6] | 萘甲酰基吲哚 | >10000 | >10000 | 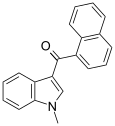
| |
| JWH-071[6] | 萘甲酰基吲哚 | 1340 ± 123 | 2940 ± 852 | CB1 (2.2x) | 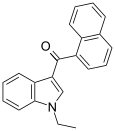
|
| JWH-072 | 萘甲酰基吲哚 | 1050 ± 5.5 | 170 ± 54 | CB2 (6x) | 
|
| JWH-073 | 萘甲酰基吲哚 | 8.9 ± 1.8 | 27 ± 12 | CB1 (3x) | 
|
| JWH-076[5] | 萘甲酰基吲哚 | 214 ± 11 | 106 ± 46 | CB2 (2x) | 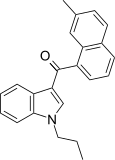
|
| JWH-077[6] | 萘甲酰基吲哚 | >10000 | >10000 | 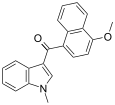
| |
| JWH-078[6] | 萘甲酰基吲哚 | 817 ± 60 | 633 ± 116 | CB2 (1.3x) | 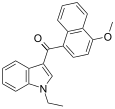
|
| JWH-079[6] | 萘甲酰基吲哚 | 63.0 ± 3.0 | 32.0 ± 6.0 | CB2 (2x) | 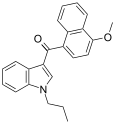
|
| JWH-080[6] | 萘甲酰基吲哚 | 8.9 ± 1.8 | 2.21 ± 1.30 | CB2 (4x) | 
|
| JWH-081[6] | 萘甲酰基吲哚 | 1.2 ± 0.03 | 12.4 ± 2.2 | CB1 (10x) | 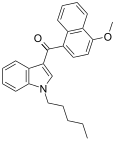
|
| JWH-082[6] | 萘甲酰基吲哚 | 5.3 ± 0.8 | 6.40 ± 0.94 | CB1 (1.2x) | 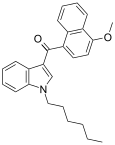
|
| JWH-083[6] | 萘甲酰基吲哚 | 106 ± 12 | 102 ± 50 | — | 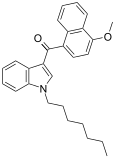
|
| JWH-091[9] (Δ8-THCP) | 二苯并吡喃 | 22.0 ± 3.9 | 
| ||
| JWH-093[6] | 萘甲酰基吲哚 | 40.7 ± 2.8 | 59.1 ± 10.5 | CB1 (1.45x) | 
|
| JWH-094[6] | 萘甲酰基吲哚 | 476 ± 67 | 97.3 ± 2.7 | CB2 (4.9x) | 
|
| JWH-095[6] | 萘甲酰基吲哚 | 140 ± 4.3 | 312 ± 83 | CB1 (2.2x) | 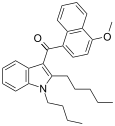
|
| JWH-096[6] | 萘甲酰基吲哚 | 33.7 ± 2.9 | 13.3 ± 5.6 | CB2 (2.5x) | 
|
| JWH-097[6] | 萘甲酰基吲哚 | 455 ± 28 | 121 ± 15 | CB2 (3.8x) | 
|
| JWH-098[6] | 萘甲酰基吲哚 | 4.5 ± 0.1 | 1.9 ± 0.3 | CB2 (2.4x) | 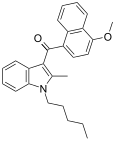
|
| JWH-099[6] | 萘甲酰基吲哚 | 35.3 ± 9.0 | 17.8 ± 2.9 | CB2 (2x) | 
|
| JWH-100[6] | 萘甲酰基吲哚 | 381 ± 102 | 155 ± 74 | CB2 (2.5x) | 
|
| JWH-102[7] | 二苯并吡喃 | 7.9 ± 0.9 | 5.2 ± 2.0 | CB2 (1.5x) | 
|
| JWH-103[7] | 二苯并吡喃 | 28 ± 3 | 23 ± 7 | CB2 (1.2x) | 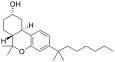
|
| JWH-116[10] | 萘甲酰基吲哚 | 52 ± 5 | 
| ||
| JWH-120[5] | 萘甲酰基吲哚 | 1054 ± 31 | 6.1 ± 0.7 | CB2 (173x) | 
|
| JWH-122[10] | 萘甲酰基吲哚 | 0.69 ± 0.05 | 1.2 ± 1.2 | — | 
|
| JWH-124 (Δ8-Parahexyl) | 二苯并吡喃 | 41.0 ± 3.8 | 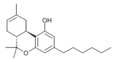
| ||
| JWH-130 (Δ8-THCB) | 二苯并吡喃 | 65.0 ± 13 | 
| ||
| JWH-133[7] | 二苯并吡喃 | 677 ± 132 | 3.4 ± 1.0 | CB2 (200x) | 
|
| JWH-138[11] | 二苯并吡喃 | 8.5 ± 1.4 | 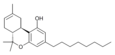
| ||
| JWH-139[12] | 二苯并吡喃 | 2290 ± 505 | 14 ± 10 | CB2 (164x) | 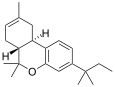
|
| JWH-142[7] | 二苯并吡喃 | 529 ± 49 | 35 ± 14 | CB2 (15x) | 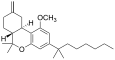
|
| JWH-143[7] | 二苯并吡喃 | 924 ± 104 | 65 ± 8 | CB2 (14x) | 
|
| JWH-145[13] | 萘甲酰基吡咯 | 14 ± 2 | 6.4 ± 0.4 | CB2 (2.2x) | 
|
| JWH-146[13] | 萘甲酰基吡咯 | 21 ± 2 | 62 ± 5 | CB2 (3.0x) | 
|
| JWH-147[13] | 萘甲酰基吡咯 | 11 ± 1 | 7.1 ± 0.2 | CB2 (1.5x) | 
|
| JWH-148[5] | 萘甲酰基吲哚 | 123 ± 8 | 14.0 ± 1.0 | CB2 (8x) | 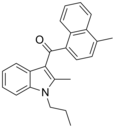
|
| JWH-149[5] | 萘甲酰基吲哚 | 5.0 ± 2.1 | 0.73 ± 0.03 | CB2 (6.8x) | 
|
| JWH-150[13] | 萘甲酰基吡咯 | 60 ± 1 | 15 ± 2 | CB2 (4x) | 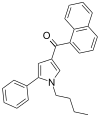
|
| JWH-151[5] | 萘甲酰基吲哚 | >10000 | 30 ± 1.1 | CB2 (>333x) | 
|
| JWH-153[5] | 萘甲酰基吲哚 | 250 ± 24 | 11 ± 0.5 | CB2 (23x) | 
|
| JWH-156[13] | 萘甲酰基吡咯 | 404 ± 18 | 104 ± 18 | CB2 (4x) | 
|
| JWH-159[5] | 萘甲酰基吲哚 | 45 ± 1 | 10.4 ± 1.4 | CB2 (4.3x) | 
|
| JWH-160[5] | 萘甲酰基吲哚 | 1568 ± 201 | 441 ± 110 | CB2 (3.6x) | 
|
| JWH-161 | 二苯并吡喃合体 | 19.0 | 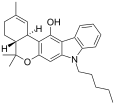
| ||
| JWH-163[5] | 萘甲酰基吲哚 | 2358 ± 215 | 138 ± 12 | CB2 (17x) | 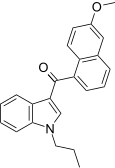
|
| JWH-164[5] | 萘甲酰基吲哚 | 6.6 ± 0.7 | 6.9 ± 0.2 | — | 
|
| JWH-165[5] | 萘甲酰基吲哚 | 204 ± 26 | 71 ± 8 | CB2 (2.9x) | 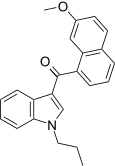
|
| JWH-166[5] | 萘甲酰基吲哚 | 44 ± 10 | 1.9 ± 0.08 | CB2 (23x) | 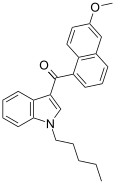
|
| JWH-167 | 苯乙酰吲哚 | 90 ± 17 | 159 ± 14 | CB1 (1.77x) | 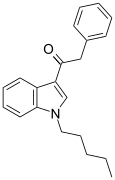
|
| JWH-171 | 碳氢 | 51 | 
| ||
| JWH-175[10] | 萘甲基吲哚 | 22 ± 2 | 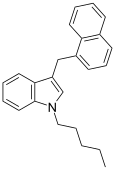
| ||
| JWH-176[10] | 碳氢 | 26 ± 4 | 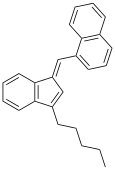
| ||
| JWH-180[5] | 萘甲酰基吲哚 | 26 ± 2 | 9.6 ± 2.0 | CB2 (2.7x) | 
|
| JWH-181[5] | 萘甲酰基吲哚 | 1.3 ± 0.1 | 0.62 ± 0.04 | CB2 (2.1x) | 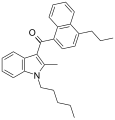
|
| JWH-182[5] | 萘甲酰基吲哚 | 0.65 ± 0.03 | 1.1 ± 0.1 | CB1 (1.7x) | 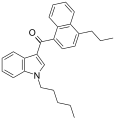
|
| JWH-184[10] | 萘甲基吲哚 | 23 ± 6 | 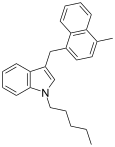
| ||
| JWH-185[10] | 萘甲基吲哚 | 17 ± 3 | 
| ||
| JWH-186[14] | 二苯并吡喃 | 187 ± 23 | 5.6 ± 1.7 | CB2 (33x) | 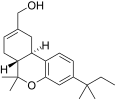
|
| JWH-187[14] | 二苯并吡喃 | 84 ± 16 | 3.4 ± 0.5 | CB2 (25x) | 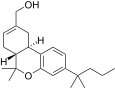
|
| JWH-188[14] | 二苯并吡喃 | 270 ± 58 | 18 ± 2 | CB2 (15x) | 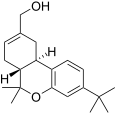
|
| JWH-189[5] | 萘甲酰基吲哚 | 52 ± 2 | 12 ± 0.8 | CB2 (4.3x) | 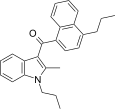
|
| JWH-190[14] | 二苯并吡喃 | 8.8 ± 1.4 | 1.6 ± 0.03 | CB2 (5.5x) | 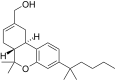
|
| JWH-191[14] | 二苯并吡喃 | 1.8 ± 0.3 | 0.52 ± 0.03 | CB2 (3.5x) | 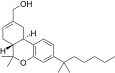
|
| JWH-192[10] | 萘甲基吲哚 | 41 ± 13 | 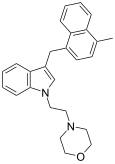
| ||
| JWH-193[10] | 萘甲酰基吲哚 | 6 ± 1 | 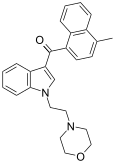
| ||
| JWH-194[10] | 萘甲基吲哚 | 127 ± 19 | 
| ||
| JWH-195[10] | 萘甲基吲哚 | 113 ± 28 | 
| ||
| JWH-196[10] | 萘甲基吲哚 | 151 ± 18 | 
| ||
| JWH-197[10] | 萘甲基吲哚 | 323 ± 98 | 
| ||
| JWH-198[10] | 萘甲酰基吲哚 | 10 ± 2 | 
| ||
| JWH-199[10] | 萘甲基吲哚 | 20 ± 2 | 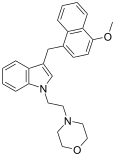
| ||
| JWH-200[10] | 萘甲酰基吲哚 | 42 ± 5 | 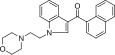
| ||
| JWH-201[15] | 苯乙酰吲哚 | 1064 ± 21 | 444 ± 14 | CB2 (2.4x) | 
|
| JWH-202[15] | 苯乙酰吲哚 | 1678 ± 63 | 645 ± 6 | CB2 (2.6x) | 
|
| JWH-203[15] | 苯乙酰吲哚 | 8.0 ± 0.9 | 7.0 ± 1.3 | — | 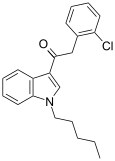
|
| JWH-204[15] | 苯乙酰吲哚 | 13 ± 1 | 25 ± 1 | CB1 (1.9x) | 
|
| JWH-205[15] | 苯乙酰吲哚 | 124 ± 23 | 180 ± 9 | CB1 (1.45x) | 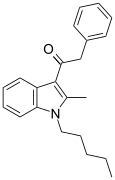
|
| JWH-206[15] | 苯乙酰吲哚 | 389 ± 25 | 498 ± 37 | CB1 (1.28x) | 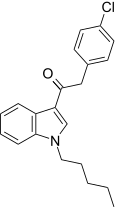
|
| JWH-207[15] | 苯乙酰吲哚 | 1598 ± 134 | 3723 ± 10 | CB1 (2.33x) | 
|
| JWH-208[15] | 苯乙酰吲哚 | 179 ± 7 | 570 ± 127 | CB1 (3.18x) | 
|
| JWH-209[15] | 苯乙酰吲哚 | 746 ± 49 | 1353 ± 270 | CB1 (1.81x) | 
|
| JWH-210[5] | 萘甲酰基吲哚 | 0.46 ± 0.03 | 0.69 ± 0.01 | CB1 (1.5x) | 
|
| JWH-211[5] | 萘甲酰基吲哚 | 70 ± 0.8 | 12 ± 0.8 | CB2 (5.8x) | 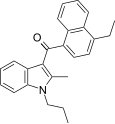
|
| JWH-212[5] | 萘甲酰基吲哚 | 33 ± 0.9 | 10 ± 1.2 | CB2 (3.3x) | 
|
| JWH-213[5] | 萘甲酰基吲哚 | 1.5 ± 0.2 | 0.42 ± 0.05 | CB2 (3.6x) | 
|
| JWH-215[14] | 二苯并吡喃 | 1008 ± 117 | 85 ± 21 | CB2 (12x) | 
|
| JWH-216[14] | 二苯并吡喃 | 1856 ± 148 | 333 ± 104 | CB2 (5.6x) | 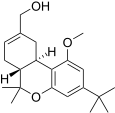
|
| JWH-217[14] | 二苯并吡喃 | >10000 | 1404 ± 66 | CB2 (>7x) | 
|
| JWH-220 | 碳氢 | 19 | 
| ||
| JWH-224[14] | 二苯并吡喃 | 347 ± 34 | 28 ± 1 | CB2 (12.3x) | 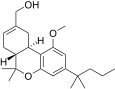
|
| JWH-225[14] | 二苯并吡喃 | >10000 | 325 ± 70 | CB2 (>31x) | 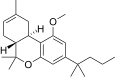
|
| JWH-226[14] | 二苯并吡喃 | 4001 ± 282 | 43 ± 3 | CB2 (93x) | 
|
| JWH-227[14] | 二苯并吡喃 | 40 ± 6 | 4.4 ± 0.3 | CB2 (9x) | 
|
| JWH-229[16] | 二苯并吡喃 | 3134 ± 110 | 18 ± 2 | CB2 (174x) | 
|
| JWH-230[14] | 二苯并吡喃 | 15 ± 3 | 1.4 ± 0.12 | CB2 (10.7x) | 
|
| JWH-233[14] | 二苯并吡喃 | 14 ± 3 | 1.0 ± 0.3 | CB2 (14x) | 
|
| JWH-234[5] | 萘甲酰基吲哚 | 8.4 ± 1.8 | 3.8 ± 0.6 | CB2 (2.2x) | 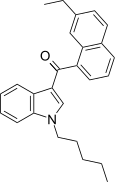
|
| JWH-235[5] | 萘甲酰基吲哚 | 338 ± 34 | 123 ± 34 | CB2 (2.7x) | 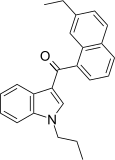
|
| JWH-236[5] | 萘甲酰基吲哚 | 1351 ± 204 | 240 ± 63 | CB2 (5.6x) | 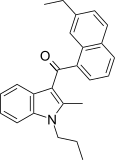
|
| JWH-237[15] | 苯乙酰吲哚 | 38 ± 10 | 106 ± 2 | CB1 (2.8x) | 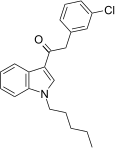
|
| JWH-239[5] | 萘甲酰基吲哚 | 342 ± 20 | 52 ± 6 | CB2 (6.6x) | 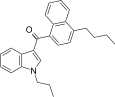
|
| JWH-240[5] | 萘甲酰基吲哚 | 14 ± 1 | 7.2 ± 1.3 | CB2 (1.9x) | 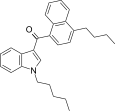
|
| JWH-241[5] | 萘甲酰基吲哚 | 147 ± 20 | 49 ± 7 | CB2 (3.0x) | 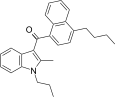
|
| JWH-242[5] | 萘甲酰基吲哚 | 42 ± 9 | 6.5 ± 0.3 | CB2 (6.5x) | 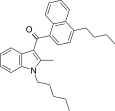
|
| JWH-243[13] | 萘甲酰基吡咯 | 285 ± 40 | 41 ± 3 | CB2 (6.95x) | 
|
| JWH-244[13] | 萘甲酰基吡咯 | 130 ± 6 | 18 ± 1 | CB2 (7.22x) | 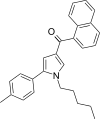
|
| JWH-245[13] | 萘甲酰基吡咯 | 276 ± 4 | 25 ± 2 | CB2 (11x) | 
|
| JWH-246[13] | 萘甲酰基吡咯 | 70 ± 4 | 16 ± 1 | CB2 (4.38x) | 
|
| JWH-247[14] | 二苯并吡喃 | 427 ± 31 | 99 ± 4 | CB2 (4.3x) | 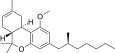
|
| JWH-248[15] | 苯乙酰吲哚 | 1028 ± 39 | 657 ± 19 | CB2 (1.56x) | 
|
| JWH-249[15] | 苯乙酰吲哚 | 8.4 ± 1.8 | 20 ± 2 | CB1 (2.38x) | 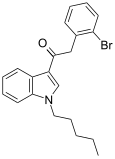
|
| JWH-250[15] | 苯乙酰吲哚 | 11 ± 2 | 33 ± 2 | CB1 (3x) | 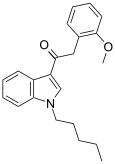
|
| JWH-251[15] | 苯乙酰吲哚 | 29 ± 3 | 146 ± 36 | CB2 (5x) | 
|
| JWH-252[15] | 苯乙酰吲哚 | 23 ± 3 | 19 ± 1 | CB2 (1.2x) | 
|
| JWH-253[15] | 苯乙酰吲哚 | 62 ± 10 | 84 ± 12 | CB1 (1.35x) | 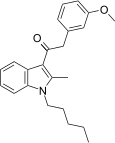
|
| JWH-254[14] | 二苯并吡喃 | 4724 ± 509 | 319 ± 16 | CB2 (14.8x) | 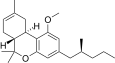
|
| JWH-256[14] | 二苯并吡喃 | 4300 ± 888 | 97 ± 18 | CB2 (44x) | 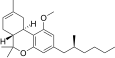
|
| JWH-258[5] | 萘甲酰基吲哚 | 4.6 ± 0.6 | 10.5 ± 1.3 | CB1 (2.3x) | 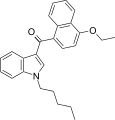
|
| JWH-259[5] | 萘甲酰基吲哚 | 220 ± 29 | 74 ± 7 | CB2 (3.0x) | 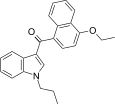
|
| JWH-260[5] | 萘甲酰基吲哚 | 29 ± 0.4 | 25 ± 1.9 | CB2 (1.2x) | 
|
| JWH-261[5] | 萘甲酰基吲哚 | 767 ± 105 | 221 ± 14 | CB2 (3.5x) | 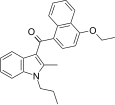
|
| JWH-262[5] | 萘甲酰基吲哚 | 28 ± 3 | 5.6 ± 0.7 | CB2 (5.0x) | 
|
| JWH-265[5] | 萘甲酰基吲哚 | 3788 ± 323 | 80 ± 13 | CB2 (47x) | 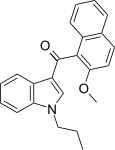
|
| JWH-266[5] | 萘甲酰基吲哚 | >10000 | 455 ± 55 | CB2 (>22x) | 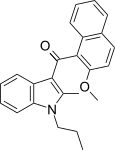
|
| JWH-267[5] | 萘甲酰基吲哚 | 381 ± 16 | 7.2 ± 0.14 | CB2 (53x) | 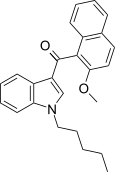
|
| JWH-268[5] | 萘甲酰基吲哚 | 1379 ± 193 | 40 ± 0.6 | CB2 (34x) | 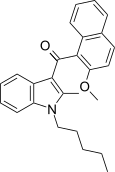
|
| JWH-277[14] | 二苯并吡喃 | 3905 ± 91 | 589 ± 65 | CB2 (6.6x) | 
|
| JWH-278[14] | 二苯并吡喃 | 906 ± 80 | 69 ± 6 | CB2 (13x) | 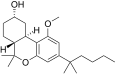
|
| JWH-292[13] | 萘甲酰基吡咯 | 29 ± 1 | 20 ± 1 | CB2 (1.45x) | 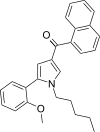
|
| JWH-293[13] | 萘甲酰基吡咯 | 100 ± 5 | 41 ± 4 | CB2 (2.44x) | 
|
| JWH-298[14] | 二苯并吡喃 | 812 ± 67 | 198 ± 23 | CB2 (4.1x) | 
|
| JWH-299[14] | 二苯并吡喃 | 415 ± 50 | 30 ± 2 | CB2 (13.8x) | 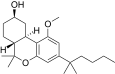
|
| JWH-300[12] | 二苯并吡喃 | 118 ± 16 | 5.3 ± 0.1 | CB2 (22x) | 
|
| JWH-301[14] | 二苯并吡喃 | 295 ± 64 | 48 ± 4 | CB2 (6.1x) | 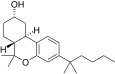
|
| JWH-302[15] | 苯乙酰吲哚 | 17 ± 2 | 89 ± 15 | CB1 (5.26x) | 
|
| JWH-303[15] | 苯乙酰吲哚 | 117 ± 10 | 138 ± 12 | CB1 (1.18x) | 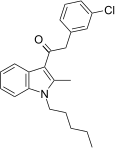
|
| JWH-304[15] | 苯乙酰吲哚 | 3363 ± 332 | 2679 ± 688 | CB2 (1.26x) | 
|
| JWH-305[15] | 苯乙酰吲哚 | 15 ± 1.8 | 29 ± 5 | CB1 (1.93x) | 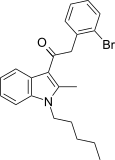
|
| JWH-306[15] | 苯乙酰吲哚 | 25 ± 1 | 82 ± 11 | CB1 (3.28x) | 
|
| JWH-307[13] | 萘甲酰基吡咯 | 7.7 ± 1.8 | 3.3 ± 0.2 | CB2 (2.33x) | 
|
| JWH-308[13] | 萘甲酰基吡咯 | 41 ± 1 | 33 ± 2 | CB2 (1.24x) | 
|
| JWH-309[13] | 萘甲酰基吡咯 | 41 ± 3 | 49 ± 7 | CB1 (1.20x) | 
|
| JWH-310[14] | 二苯并吡喃 | 1059 ± 51 | 36 ± 3 | CB2 (29x) | 
|
| JWH-311[15] | 苯乙酰吲哚 | 23 ± 2 | 39 ± 3 | CB1 (1.70x) | 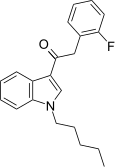
|
| JWH-312[15] | 苯乙酰吲哚 | 72 ± 7 | 91 ± 20 | CB1 (1.26x) | 
|
| JWH-313[15] | 苯乙酰吲哚 | 422 ± 19 | 365 ± 92 | CB2 (1.16x) | 
|
| JWH-314[15] | 苯乙酰吲哚 | 39 ± 2 | 76 ± 4 | CB1 (1.95x) | 
|
| JWH-315[15] | 苯乙酰吲哚 | 430 ± 24 | 182 ± 23 | CB2 (3.36x) | 
|
| JWH-316[15] | 苯乙酰吲哚 | 2862 ± 670 | 781 ± 105 | CB2 (3.66x) | 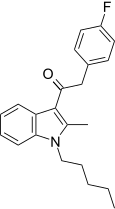
|
| JWH-336[12] | 二苯并吡喃 | 4589 ± 367 | 153 ± 15 | CB2 (30x) | 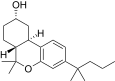
|
| JWH-338[14] | 二苯并吡喃 | >10000 | 111 ± 16 | CB2 (>90x) | 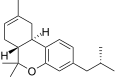
|
| JWH-339[14] | 二苯并吡喃 | >10000 | 2317 ± 93 | CB2 (>4.3x) | 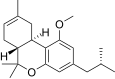
|
| JWH-340[14] | 二苯并吡喃 | 135 ± 6 | 30 ± 1 | CB2 (4.5x) | 
|
| JWH-341[14] | 二苯并吡喃 | 100 ± 8 | 10 ± 0.1 | CB2 (10x) | 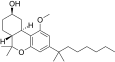
|
| JWH-346[13] | 萘甲酰基吡咯 | 67 ± 6 | 39 ± 2 | CB2 (1.72x) | 
|
| JWH-347[13] | 萘甲酰基吡咯 | 333 ± 17 | 169 ± 17 | CB2 (1.97x) | 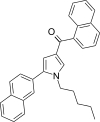
|
| JWH-348[13] | 萘甲酰基吡咯 | 218 ± 19 | 53 ± 1 | CB2 (4.11x) | 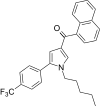
|
| JWH-349[14] | 二苯并吡喃 | 376 ± 1 | 38 ± 4 | CB2 (9.9x) | 
|
| JWH-350[12] | 二苯并吡喃 | 395 ± 50 | 12 ± 1 | CB2 (33x) | 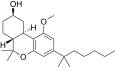
|
| JWH-351[14] | 二苯并吡喃 | >10000 | 295 ± 3 | CB2 (>34x) | 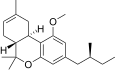
|
| JWH-352[14] | 二苯并吡喃 | >10000 | 47 ± 2 | CB2 (>213x) | 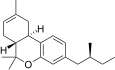
|
| JWH-353[14] | 二苯并吡喃 | 1493 ± 10 | 31 ± 1 | CB2 (48x) | 
|
| JWH-354[14] | 二苯并吡喃 | 1961 ± 21 | 241 ± 14 | CB2 (8.1x) | 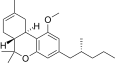
|
| JWH-355[14] | 二苯并吡喃 | 2162 ± 220 | 108 ± 17 | CB2 (20x) | 
|
| JWH-356[14] | 二苯并吡喃 | 5837 ± 701 | 108 ± 17 | CB2 (54x) | 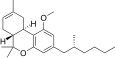
|
| JWH-357[14] | 二苯并吡喃 | 647 ± 78 | 185 ± 4 | CB2 (3.5x) | 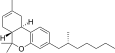
|
| JWH-358[14] | 二苯并吡喃 | 1243 ± 266 | 52 ± 3 | CB2 (24x) | 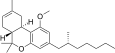
|
| JWH-359 | 二苯并吡喃 | 2918 ± 450 | 13.0 ± 0.2 | CB2 (220x) | 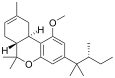
|
| JWH-360[14] | 二苯并吡喃 | 2449 ± 606 | 160 ± 8 | CB2 (15x) | 
|
| JWH-361[14] | 二苯并吡喃 | 63 ± 3 | 2.7 ± 0.1 | CB2 (23x) | 
|
| JWH-362[14] | 二苯并吡喃 | 127 ± 8 | 34 ± 5 | CB2 (3.7x) | 
|
| JWH-363[13] | 萘甲酰基吡咯 | 245 ± 5 | 71 ± 1 | CB2 (3.45x) | 
|
| JWH-364[13] | 萘甲酰基吡咯 | 34 ± 3 | 29 ± 1 | CB2 (1.17x) | 
|
| JWH-365[13] | 萘甲酰基吡咯 | 17 ± 1 | 3.4 ± 0.2 | CB2 (5.0x) | 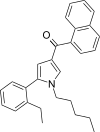
|
| JWH-366[13] | 萘甲酰基吡咯 | 191 ± 12 | 24 ± 1 | CB2 (7.96x) | 
|
| JWH-367[13] | 萘甲酰基吡咯 | 53 ± 2 | 23 ± 1 | CB2 (2.30x) | 
|
| JWH-368[13] | 萘甲酰基吡咯 | 16 ± 1 | 9.1 ± 0.7 | CB2 (1.76x) | 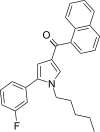
|
| JWH-369[13] | 萘甲酰基吡咯 | 7.9 ± 0.4 | 5.2 ± 0.3 | CB2 (1.52x) | 
|
| JWH-370[13] | 萘甲酰基吡咯 | 5.6 ± 0.4 | 4.0 ± 0.5 | CB2 (1.40x) | 
|
| JWH-371[13] | 萘甲酰基吡咯 | 42 ± 1 | 64 ± 2 | CB1 (1.52x) | 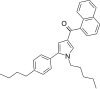
|
| JWH-372[13] | 萘甲酰基吡咯 | 77 ± 2 | 8.2 ± 0.2 | CB1 (9.39x) | 
|
| JWH-373[13] | 萘甲酰基吡咯 | 60 ± 3 | 69 ± 2 | CB1 (1.15x) | 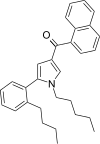
|
| JWH-387[17] | 萘甲酰基吲哚 | 1.2 ± 0.1 | 1.1 ± 0.1 | — | 
|
| JWH-398[18] | 萘甲酰基吲哚 | 2.3 ± 0.1 | 2.8 ± 0.2 | CB1 (1.22x) | |
| JWH-416[17] | 萘甲酰基吲哚 | 73 ± 10 | 3.3 ± 0.1 | CB2 (22x) | 
|
| JWH-417[17] | 萘甲酰基吲哚 | 522 ± 58 | 13 ± 0.2 | CB2 (40x) | 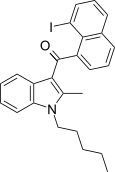
|
| JWH-422[17] | 萘甲酰基吲哚 | 501 ± 48 | 20 ± 0.4 | CB2 (25x) | 
|
| JWH-423[17] | 萘甲酰基吲哚 | 140 ± 10 | 6.6 ± 0.2 | CB2 (21x) | 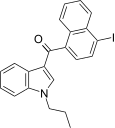
|
| JWH-424[17] | 萘甲酰基吲哚 | 21 ± 3.4 | 5.4 ± 0.2 | CB2 (3.9x) | 
|
| JWH-425[17] | 萘甲酰基吲哚 | 54 ± 11 | 10 ± 0.4 | CB2 (5.4x) | 
|
参见
注释
参考资料
- ^ Manera C, Tuccinardi T, Martinelli A. Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem. 2008, 8 (4): 370–87. PMID 18473928. doi:10.2174/138955708783955935.
- ^ Wiley JL, Marusich JA, Huffman JW. Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014, 97 (1): 55–63. PMC 3944940
 . PMID 24071522. doi:10.1016/j.lfs.2013.09.011.
. PMID 24071522. doi:10.1016/j.lfs.2013.09.011.
- ^ Wiley JL, Marusich JA, Thomas BF. Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids. Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 2017, 32: 231–248. ISBN 978-3-319-52442-9. PMID 27753007. doi:10.1007/7854_2016_17
 .
.
- ^ Banister SD, Connor M. The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins. Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 2018, 252: 165–190. ISBN 978-3-030-10560-0. PMID 29980914. doi:10.1007/164_2018_143.
- ^ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 5.28 5.29 5.30 5.31 5.32 5.33 5.34 5.35 5.36 5.37 5.38 Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorganic & Medicinal Chemistry. January 2005, 13 (1): 89–112. PMID 15582455. doi:10.1016/j.bmc.2004.09.050.
- ^ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug and Alcohol Dependence. August 2000, 60 (2): 133–40. PMID 10940540. doi:10.1016/S0376-8716(99)00152-0.
- ^ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(1',1'-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorganic & Medicinal Chemistry. December 1999, 7 (12): 2905–14. PMID 10658595. doi:10.1016/s0968-0896(99)00219-9.
- ^ Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, Martin BR, Bramblett RD, Reggio PH. Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor. Journal of Medicinal Chemistry. September 1996, 39 (20): 3875–7. PMID 8831752. doi:10.1021/JM960394Y.
- ^ Bow EW, Rimoldi JM. The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect Medicin Chem. 2016 Jun 28;8:17-39. doi:10.4137/PMC.S32171 PMID 27398024
- ^ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 10.14 Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR. 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor. Bioorganic & Medicinal Chemistry. February 2003, 11 (4): 539–49. PMID 12538019. S2CID 29107765. doi:10.1016/S0968-0896(02)00451-0.
- ^ Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, Saha B, Razdan RK. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J Pharmacol Exp Ther. 1999 Sep;290(3):1065-79. PMID 10454479
- ^ 12.0 12.1 12.2 12.3 Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews. June 2002, 54 (2): 161–202. PMID 12037135. S2CID 8259002. doi:10.1124/pr.54.2.161.
- ^ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 13.14 13.15 13.16 13.17 13.18 13.19 13.20 13.21 13.22 13.23 13.24 13.25 13.26 13.27 Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR. 1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: new high affinity ligands for the cannabinoid CB1 and CB2 receptors. Bioorganic & Medicinal Chemistry Letters. October 2006, 16 (20): 5432–5. PMID 16889960. doi:10.1016/j.bmcl.2006.07.051.
- ^ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 14.11 14.12 14.13 14.14 14.15 14.16 14.17 14.18 14.19 14.20 14.21 14.22 14.23 14.24 14.25 14.26 14.27 14.28 14.29 14.30 14.31 14.32 14.33 14.34 14.35 14.36 14.37 14.38 Marriott KS, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor. Current Topics in Medicinal Chemistry. 2008, 8 (3): 187–204. PMID 18289088. doi:10.2174/156802608783498014.
- ^ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 15.10 15.11 15.12 15.13 15.14 15.15 15.16 15.17 15.18 15.19 15.20 15.21 15.22 15.23 15.24 15.25 15.26 Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorganic & Medicinal Chemistry Letters. September 2005, 15 (18): 4110–3. PMID 16005223. doi:10.1016/j.bmcl.2005.06.008.
- ^ Huffman JW, Bushell SM, Miller JR, Wiley JL, Martin BR. 1-Methoxy-, 1-deoxy-11-hydroxy- and 11-hydroxy-1-methoxy-Delta(8)-tetrahydrocannabinols: new selective ligands for the CB2 receptor. Bioorganic & Medicinal Chemistry. December 2002, 10 (12): 4119–29. PMID 12413866. doi:10.1016/s0968-0896(02)00331-0.
- ^ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 Wiley JL, Smith VJ, Chen J, Martin BR, Huffman JW. Synthesis and pharmacology of 1-alkyl-3-(1-naphthoyl)indoles: Steric and electronic effects of 4- and 8-halogenated naphthoyl substituents. Bioorganic & Medicinal Chemistry. 2012, 20 (6): 2067–2081. PMC 3298571
 . PMID 22341572. doi:10.1016/j.bmc.2012.01.038.
. PMID 22341572. doi:10.1016/j.bmc.2012.01.038.
- ^ The Cannabinoid Receptors. The Receptors. 2009. ISBN 978-1-58829-712-9. doi:10.1007/978-1-59745-503-9.
