芳基环己胺:修订间差异
删除的内容 添加的内容
| 第1行: | 第1行: | ||
[[Image:Phencyclidine structure.svg|right|thumb|160px|[[苯环己哌啶]],最常见的{{PAGENAME}}衍生物]] |
[[Image:Phencyclidine structure.svg|right|thumb|160px|[[苯环己哌啶]],最常见的{{PAGENAME}}衍生物]] |
||
'''{{PAGENAME}}衍生物'''({{lang-en|Arylcyclohexylamine}}、arylcyclohexamines或arylcyclohexanamines)是一类含氮[[有机化合物]],常用作[[药品]]和[[狡詐家藥物]]和[[试验药物]],其特点是[[环己烷]]的一个碳原子上连接了[[氨基|氨基的N原子]]和[[芳香族化合物|芳香基]],常见的有[[乙环利定]]、[[苯环己哌啶]]等<ref>{{cite web |title=4-(1-phenyl-cyclohexyl)-morpholine |url=https://www.chemsrc.com/cas/2201-40-3_1255636.html |website=CAS Number Search - chemsrc.com |publisher=chemsrc |access-date=15 March 2021}}</ref>。 |
'''{{PAGENAME}}衍生物'''({{lang-en|Arylcyclohexylamine}}、arylcyclohexamines或arylcyclohexanamines)是一类含氮[[有机化合物]],常用作[[药品]]和[[狡詐家藥物]]和[[试验药物]],其特点是[[环己烷]]的一个碳原子上连接了[[氨基|氨基的N原子]]和[[芳香族化合物|芳香基]],常见的有[[乙环利定]]、[[苯环己哌啶]]等<ref>{{cite web |title=4-(1-phenyl-cyclohexyl)-morpholine |url=https://www.chemsrc.com/cas/2201-40-3_1255636.html |website=CAS Number Search - chemsrc.com |publisher=chemsrc |access-date=15 March 2021}}</ref>。 |
||
[[Image:acha markush.png|120px|thumb|{{PAGENAME}}的各个取代基]] |
|||
==化合物== |
==化合物== |
||
{| class="wikitable sortable" |
|||
! '''Structures''' |
|||
! data-sort-type="text" | '''Compound''' |
|||
! data-sort-type="text" | '''Aryl Substituent''' |
|||
! data-sort-type="text" | '''N Group''' |
|||
! data-sort-type="text" | '''Cyclohexyl ring''' |
|||
! '''CAS number''' |
|||
|- |
|||
| [[File:PCA_structure.png|120px]] || PCA<ref name=Thurkauf>{{cite journal | vauthors = Thurkauf A, de Costa B, Yamaguchi S, Mattson MV, Jacobson AE, Rice KC, Rogawski MA | title = Synthesis and anticonvulsant activity of 1-phenylcyclohexylamine analogues | journal = Journal of Medicinal Chemistry | volume = 33 | issue = 5 | pages = 1452–8 | date = May 1990 | pmid = 2329567 | doi = 10.1021/jm00167a027 }}</ref> || Phenyl || NH<sub>2</sub> || - || 1934-71-0 |
|||
|- |
|||
| [[File:PCM_structure.png|120px]] || PCM<ref name=Thurkauf/> || Phenyl || Methylamino || - || 2201-16-3 |
|||
|- |
|||
| [[File:Eticyclidine.svg|120px]] || [[Eticyclidine]] || Phenyl || Ethylamino || - || 2201-15-2 |
|||
|- |
|||
| [[File:PCPr_structure.png|120px]] || [[PCPr]]<ref>{{cite journal | vauthors = Sauer C, Peters FT, Staack RF, Fritschi G, Maurer HH | title = Metabolism and toxicological detection of a new designer drug, N-(1-phenylcyclohexyl)propanamine, in rat urine using gas chromatography-mass spectrometry | journal = Journal of Chromatography A | volume = 1186 | issue = 1–2 | pages = 380–90 | date = April 2008 | pmid = 18035363 | doi = 10.1016/j.chroma.2007.11.002 }}</ref> || Phenyl || ''n''-Propylamino || - || 18949-81-0 |
|||
|- |
|||
| [[File:PCiP_structure.png|120px]] || PCiP || Phenyl || Isopropylamino || - || 1195-42-2 |
|||
|- |
|||
| [[File:PCAL_structure.png|120px]] || PCAL <ref>{{cite journal | vauthors = Kalir A, Teomy S, Amir A, Fuchs P, Lee SA, Holsztynska EJ, Rocki W, Domino EF | display-authors = 6 | title = N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties | journal = Journal of Medicinal Chemistry | volume = 27 | issue = 10 | pages = 1267–71 | date = October 1984 | pmid = 6481761 | doi = 10.1021/jm00376a006 }}</ref> || Phenyl || Allylamino || - || 2185-95-7 |
|||
|- |
|||
| [[File:PCBu_structure.png|120px]] || PCBu || Phenyl || ''n''-Butylamino || - || 73166-29-7 |
|||
|- |
|||
| [[File:PCEOH_structure.png|125px]] || PCEOH || Phenyl || Hydroxyethylamino || - || 2201-22-1 |
|||
|- |
|||
| [[File:PCMEA_structure.png|125px]] || PCMEA<ref name=Sauer>{{cite journal | vauthors = Sauer C, Peters FT, Schwaninger AE, Meyer MR, Maurer HH | title = Investigations on the cytochrome P450 (CYP) isoenzymes involved in the metabolism of the designer drugs N-(1-phenyl cyclohexyl)-2-ethoxyethanamine and N-(1-phenylcyclohexyl)-2-methoxyethanamine | journal = Biochemical Pharmacology | volume = 77 | issue = 3 | pages = 444–50 | date = February 2009 | pmid = 19022226 | doi = 10.1016/j.bcp.2008.10.024 }}</ref> || Phenyl || Methoxyethylamino || - || 2201-57-2 |
|||
|- || |
|||
| [[File:PCEEA_structure.png|125px]] || PCEEA || Phenyl || Ethoxyethylamino || - || 1072895-05-6 |
|||
|- |
|||
| [[File:PCMPA_structure.png|125px]] || PCMPA || Phenyl || Methoxypropylamino || - || 2201-58-3 |
|||
|- |
|||
| [[File:PCDM_structure.png|120px]] || PCDM<ref name=Thurkauf/> || Phenyl || Dimethylamino || - || 2201-17-4 |
|||
|- |
|||
| [[File:Dieticyclidine.svg|120px]] || [[Dieticyclidine]] || Phenyl || Diethylamino || - || 2201-19-6 |
|||
|- |
|||
| [[File:2-HO-PCP_structure.png|120px]] || 2-HO-PCP<ref name="pmid16229117"/> || Phenyl || Piperidine|| 2-Hydroxy || 94852-58-1 |
|||
|- |
|||
| [[File:2-Me-PCP_structure.png|120px]] || 2-Me-PCP<ref name="pmid1875352">{{cite journal | vauthors = Iorio MA, Tomassini L, Mattson MV, George C, Jacobson AE | title = Synthesis, stereochemistry, and biological activity of the 1-(1-phenyl-2-methylcyclohexyl)piperidines and the 1-(1-phenyl-4-methylcyclohexyl)piperidines. Absolute configuration of the potent trans-(-)-1-(1-phenyl-2-methylcyclohexyl)piperidine | journal = Journal of Medicinal Chemistry | volume = 34 | issue = 8 | pages = 2615–23 | date = August 1991 | pmid = 1875352 | doi = 10.1021/jm00112a041 }}</ref> || Phenyl || Piperidine || 2-Methyl || 59397-29-4 |
|||
|- |
|||
| [[File:2-MeO-PCP_structure.png|120px]] || 2-MeO-PCP<ref>{{cite journal | vauthors = Ahmadi A, Mahmoudi A | title = Synthesis with improved yield and study on the analgesic effect of 2-methoxyphencyclidine | journal = Arzneimittel-Forschung | volume = 56 | issue = 5 | pages = 346–50 | year = 2006 | pmid = 16821645 | doi = 10.1055/s-0031-1296732 | s2cid = 10370245 }}</ref>|| Phenyl || Piperidine || 2-Methoxy || 78636-34-7 |
|||
|- |
|||
| [[File:O-PCP_structure.png|120px]] || 2-Keto-PCP || Phenyl || Piperidine || 2-Keto || 101688-16-8 |
|||
|- |
|||
| [[File:O-PCE_structure.png|120px]] || [[Eticyclidone]] ("O-PCE") || Phenyl || Ethylamino || 2-Keto || 6740-82-5 |
|||
|- |
|||
| [[File:O-PCPr_structure.png|120px]] || 2-Keto-PCPr || Phenyl || ''n''-Propylamino || 2-Keto || |
|||
|- |
|||
| [[File:4-Me-PCP_structure.png|120px]] || 4-Methyl-PCP || Phenyl || Piperidine || 4-Methyl || 19420-52-1 |
|||
|- |
|||
| [[File:4-Keto-PCP_structure.png|120px]] || [[4-Keto-PCP]]<ref name="pmid33135332">{{cite journal | vauthors = Ortiz DM, Custodio RJ, Abiero A, Botanas CJ, Sayson LV, Kim M, Lee HJ, Kim HJ, Jeong Y, Yoon S, Lee YS, Cheong JH | display-authors = 6 | title = The dopaminergic alterations induced by 4-F-PCP and 4-Keto-PCP may enhance their drug-induced rewarding and reinforcing effects: Implications for abuse | journal = Addiction Biology | volume = 26 | issue = 4 | pages = e12981 | date = July 2021 | pmid = 33135332 | doi = 10.1111/adb.12981 | s2cid = 226234538 }}</ref> || Phenyl || Piperidine || 4-Keto || 65620-13-5 |
|||
|- |
|||
| [[File:2'-Cl-PCP_structure.png|120px]] || 2'-Cl-PCP || ''o''-Chlorophenyl || Piperidine || - || 2201-31-2 |
|||
|- |
|||
| [[File:3'-Cl-PCP_structure.png|120px]] || [[3-Chloro-PCP|3'-Cl-PCP]] || ''m''-Chlorophenyl || Piperidine || - || 2201-32-3 |
|||
|- |
|||
| [[File:2'-MeO-PCP_structure.png|120px]] || 2'-MeO-PCP || ''o''-Methoxyphenyl || Piperidine || - || 2201-34-5 |
|||
|- |
|||
| [[File:3'-F-PCP_structure.png|120px]] || [[3-Fluoro-PCP|3'-F-PCP]]<ref name=fluoro>{{Cite journal | doi = 10.1016/S0022-1139(01)00565-6| title = Syntheses of fluorinated phencyclidine analogs| journal = Journal of Fluorine Chemistry| volume = 114| pages = 39–42| year = 2002| vauthors = Ogunbadeniyi AM, Adejare A }}</ref> || ''m''-Fluorophenyl || Piperidine || - || 89156-99-0 |
|||
|- |
|||
| [[File:3'-Me-PCP_structure.png|120px]] || [[3-Methyl-PCP|3'-Me-PCP]]<ref name=pmid23554350>{{cite journal | vauthors = Wallach J, De Paoli G, Adejare A, Brandt SD | title = Preparation and analytical characterization of 1-(1-phenylcyclohexyl)piperidine (PCP) and 1-(1-phenylcyclohexyl)pyrrolidine (PCPy) analogues | journal = Drug Testing and Analysis | volume = 6 | issue = 7–8 | pages = 633–50 | year = 2013 | pmid = 23554350 | doi = 10.1002/dta.1468 }}</ref>|| ''m''-Tolyl || Piperidine || - || 2201-30-1 |
|||
|- |
|||
| [[File:3'-Me-PCPy_structure.png|120px]] || [[3-Methyl-PCPy|3'-Me-PCPy]] || ''m''-Tolyl || Pyrrolidine || - || 1622348-63-3 |
|||
|- |
|||
| [[File:3'-NH2-PCP_structure.png|120px]] || 3'-NH<sub>2</sub>-PCP || ''m''-Aminophenyl || Piperidine || - || 72242-00-3 |
|||
|- |
|||
| [[File:3-HO-PCP.png|120px]] || [[3-HO-PCP|3'-HO-PCP]] || ''m''-Hydroxyphenyl || Piperidine || - || 79787-43-2 |
|||
|- |
|||
| [[File:3-MeO-PCP structure.svg|120px]] || [[3-MeO-PCP|3'-MeO-PCP]] || ''m''-Methoxyphenyl || Piperidine || - || 72242-03-6 |
|||
|- |
|||
| [[File:MDPCP_structure.png|125px]] || [[Methylenedioxyphencyclidine|3',4'-MD-PCP]] || 3,4-Methylenedioxyphenyl || Piperidine || - || |
|||
|- |
|||
| [[File:3-MeO-PCE.svg|120px]] || [[3-MeO-PCE|3'-MeO-PCE]] || ''m''-Methoxyphenyl || Ethylamino || - || 1364933-80-1 |
|||
|- |
|||
| [[File:3'-OH-PCE_structure.png|120px]] || 3'-HO-PCE || ''m''-Hydroxyphenyl || Ethylamino || - || |
|||
|- |
|||
| [[File:3'-MeO-PCPr_structure.png|120px]] || 3'-MeO-PCPr || ''m''-Methoxyphenyl || ''n''-Propylamino || - || 1364933-81-2 |
|||
|- |
|||
| [[File:3'-OH-PCPr_structure.png|120px]] || 3'-HO-PCPr || ''m''-Hydroxyphenyl || ''n''-Propylamino || - || |
|||
|- |
|||
| [[File:MDPCPr_structure.png|125px]] || 3',4'-MD-PCPr || 3,4-Methylenedioxyphenyl || ''n''-Propylamino || - || |
|||
|- |
|||
| [[File:3'-MeO-PCPy_structure.png|120px]] || 3'-MeO-PCPy<ref name=pmid23554350/>|| ''m''-Methoxyphenyl || Pyrrolidine || - || 1364933-79-8 |
|||
|- |
|||
| [[File:4'-HO-PCP_structure.png|120px]] || 4'-HO-PCP || ''p''-Hydroxyphenyl || Piperidine || - || 66568-88-5 |
|||
|- |
|||
| [[File:4-methoxyphencyclidine.png|120px]] || [[Methoxydine]] (4'-MeO-PCP) || ''p''-Methoxyphenyl || Piperidine || - || 2201-35-6 |
|||
|- |
|||
| [[File:4'-MeO-PCE_structure.png|120px]] || 4'-MeO-PCE|| ''p''-Methoxyphenyl || Ethylamino || - || |
|||
|- |
|||
| [[File:4'-F-PCP_structure.png|120px]] || 4'-F-PCP<ref name=fluoro/> || ''p''-Fluorophenyl || Piperidine || - || 22904-99-0 |
|||
|- |
|||
| [[File:4'-F-PCPy_structure.png|120px]] || 4'-F-PCPy || ''p''-Fluorophenyl || Pyrrolidine || - || |
|||
|- |
|||
| [[File:Arketamine structure.svg|120px]] || [[Arketamine]] || ''o''-Chlorophenyl || Methylamino || 2-Keto || 33643-49-1 |
|||
|- |
|||
| [[File:Deschloroketamine.png|120px]] || [[Deschloroketamine]] || Phenyl || Methylamino || 2-Keto || 7063-30-1 |
|||
|- |
|||
| [[File:Esketamine2DCSD.svg|120px]] || [[Esketamine]] || ''o''-Chlorophenyl || Methylamino || 2-Keto || 33643-46-8 |
|||
|- |
|||
| [[File:Ketamine2DCSD.svg|120px]] || [[Ketamine]] || ''o''-Chlorophenyl || Methylamino || 2-Keto || 6740-88-1 |
|||
|- |
|||
| [[File:(2R,6R)-Hydroxynorketamine Formula V1.svg|120px]] || [[Hydroxynorketamine]] || ''o''-Chlorophenyl || NH<sub>2</sub> || 2-Keto, 6-Hydroxy || 81395-70-2 |
|||
|- |
|||
| [[File:N-Ethylnorketamine_structure.png|120px]] || [[N-Ethylnorketamine|Ethketamine]] || ''o''-Chlorophenyl || Ethylamino || 2-Keto || 1354634-10-8 |
|||
|- |
|||
| [[File:NPNK_structure.png|125px]] || NPNK || ''o''-Chlorophenyl || ''n''-Propylamino || 2-Keto || 2749326-65-4 |
|||
|- |
|||
| [[File:Methoxyketamine.svg|120px]] || [[Methoxyketamine]] || ''o''-Methoxyphenyl || Methylamino || 2-Keto || 7063-51-6 |
|||
|- |
|||
| [[File:2-MeO-NEK_structure.png|120px]] || 2-MeO-NEK<ref name="pmid30891619">{{cite journal | vauthors = Sayson LV, Botanas CJ, Custodio RJ, Abiero A, Kim M, Lee HJ, Kim HJ, Yoo SY, Lee KW, Ryu HW, Acharya S, Kim KM, Lee YS, Cheong JH | display-authors = 6 | title = The novel methoxetamine analogs N-ethylnorketamine hydrochloride (NENK), 2-MeO-N-ethylketamine hydrochloride (2-MeO-NEK), and 4-MeO-N-ethylketamine hydrochloride (4-MeO-NEK) elicit rapid antidepressant effects via activation of AMPA and 5-HT2 receptors | journal = Psychopharmacology | volume = 236 | issue = 7 | pages = 2201–2210 | date = July 2019 | pmid = 30891619 | doi = 10.1007/s00213-019-05219-x | s2cid = 83463722 }}</ref> || ''o''-Methoxyphenyl || Ethylamino || 2-Keto || |
|||
|- |
|||
| [[File:OMDCK_structure.png|120px]] || oMDCK<ref>{{cite patent | country = WO | number = 2021134086| url = https://patents.google.com/patent/WO2021134086A1 | inventor = Kruegel AC, Sames D, Hashimoto K | assign1 = Gilgamesh Pharmaceuticals, Inc. | assign2 = The Trustees Of Columbia University In The City Of New York | title = Arylcyclohexylamine derivatives and their use in the treatment of psychiatric disorders | pubdate = 1 July 2021 | postscript = . }}</ref> || ''o''-Tolyl || Methylamino || 2-Keto || 7063-37-8 |
|||
|- |
|||
| [[File:MMDCK_structure.png|120px]] || mMDCK || ''m''-Tolyl || Methylamino || 2-Keto || |
|||
|- |
|||
| [[File:Meta-ketamine_structure.png|120px]] || ''meta''-Ketamine || ''m''-Chlorophenyl || Methylamino || 2-Keto || 7063-53-8 |
|||
|- |
|||
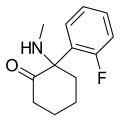
| [[File:Isoketamine_structure.png|120px]] || ''iso''-Ketamine || ''o''-Chlorophenyl || Methylamino || 4-Keto || |
|||
|- |
|||
| [[File:2-Fluorodeschloroketamine.svg|120px]] || [[2-Fluorodeschloroketamine]]|| ''o''-Fluorophenyl || Methylamino || 2-Keto || 111982-50-4 |
|||
|- |
|||
| [[File:3FDCK_structure.png|120px]] || [[3-Fluorodeschloroketamine]] || ''m''-Fluorophenyl || Methylamino || 2-Keto || 2657761-23-2 |
|||
|- |
|||
| [[File:Bromoketamine_structure.png|120px]] || [[Bromoketamine]] || ''o''-Bromophenyl || Methylamino || 2-Keto || 120807-70-7 |
|||
|- |
|||
| [[File:TFMDCK_structure.png|120px]] || [[Trifluoromethyldeschloroketamine|TFMDCK]] || ''o''-Trifluoromethylphenyl || Methylamino || 2-Keto || 1782149-73-8 |
|||
|- |
|||
| [[File:SN35210_structure.png|120px]] || [[SN 35210]]<ref>{{cite journal | vauthors = Harvey M, Sleigh J, Voss L, Pruijn F, Jose J, Gamage S, Denny W | title = Determination of the Hypnotic Potency in Rats of the Novel Ketamine Ester Analogue SN 35210 | journal = Pharmacology | volume = 96 | issue = 5–6 | pages = 226–32 | year = 2015 | pmid = 26352278 | doi = 10.1159/000439598 | s2cid = 36017002 }}</ref> || ''o''-Chlorophenyl || Carbomethoxybutylamino || 2-Keto || 1450615-41-4 |
|||
|- |
|||
| [[File:Methoxetamine2DCSD.svg|120px]] || [[Methoxetamine]] || ''m''-Methoxyphenyl || Ethylamino || 2-Keto || 1239943-76-0 |
|||
|- |
|||
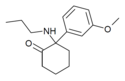
| [[File:Methoxmetamine.png|120px]] || [[Methoxmetamine]] || ''m''-Methoxyphenyl || Methylamino || 2-Keto || 1781829-56-8 |
|||
|- |
|||
| [[File:MXPr_structure.png|125px]] || [[Methoxpropamine]] || ''m''-Methoxyphenyl || ''n''-Propylamino || 2-Keto || 2504100-71-2 |
|||
|- |
|||
| [[File:MXiPr_structure.png|125px]] || [[MXiPr]] || ''m''-Methoxyphenyl || ''i''-Propylamino || 2-Keto || |
|||
|- |
|||
| [[File:Ethoxetamine_structure.png|125px]] || Ethoxetamine || ''m''-Ethoxyphenyl || Ethylamino || 2-Keto || |
|||
|- |
|||
| [[File:DMXE_structure.svg|120px]] || [[Deoxymethoxetamine]] (3-Me-2'-Oxo-PCE) || ''m''-Tolyl || Ethylamino || 2-Keto || 2666932-45-0 |
|||
|- |
|||
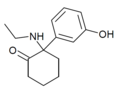
| [[File:Br-MXE_structure.png|120px]] || Br-MXE || 2-bromo-5-methoxyphenyl || Ethylamino || 2-Keto || |
|||
|- |
|||
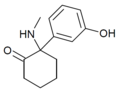
| [[File:HXE_structure.png|120px]] || [[Hydroxetamine]] (HXE) || ''m''-Hydroxyphenyl || Ethylamino || 2-Keto || 1620054-73-0 |
|||
|- |
|||
| [[File:HXM_structure.png|120px]] || HXM || ''m''-Hydroxyphenyl || Methylamino || 2-Keto || |
|||
|- |
|||
| [[File:2F-NENDCK_structure.png|120px]] || [[2F-NENDCK]] || ''o''-Fluorophenyl || Ethylamino || 2-Keto || |
|||
|- |
|||
| [[File:FXE_structure.png|120px]] || [[Fluorexetamine]] (FXE) || ''m''-Fluorophenyl || Ethylamino || 2-Keto || |
|||
|- |
|||
| [[File:Phencyclidine structure.svg|120px]] || [[Phencyclidine]] (PCP) || Phenyl || Piperidine || - || 77-10-1 |
|||
|- |
|||
| [[File:PC3MP_structure.png|120px]] || PC3MP || Phenyl || 3-Methylpiperidine || - || 2201-41-4 |
|||
|- |
|||
| [[File:PC4MP_structure.png|120px]] || PC4MP || Phenyl || 4-Methylpiperidine || - || 2201-42-5 |
|||
|- |
|||
| [[File:Rolicyclidine.svg|120px]] || [[Rolicyclidine]] (PCPy) || Phenyl || Pyrrolidine || - || 2201-39-0 |
|||
|- |
|||
| [[File:PCDMPy_structure.png|120px]] || PCDMPy || Phenyl || 3,3-Dimethylpyrrolidine || - || |
|||
|- |
|||
| [[File:PCMo_structure.png|120px]] || PCMo || Phenyl || Morpholine || - || 2201-40-3 |
|||
|- |
|||
| [[File:2'-MeO-PCMo_structure.png|120px]] || Methoxy-PCM<ref name="pmid21215770"/> (2'-MeO-PCMo) || ''o''-Methoxyphenyl || Morpholine || - || 1314323-88-0 |
|||
|- |
|||
| [[File:3-MeO-PCMo.svg|120px]] || [[3-MeO-PCMo|3'-MeO-PCMo]] || ''m''-Methoxyphenyl || Morpholine || - || 138873-80-0 |
|||
|- |
|||
| [[File:4'-MeO-PCMo_structure.png|120px]] || 4'-MeO-PCMo || ''p''-Methoxyphenyl || Morpholine || - || |
|||
|- |
|||
| [[File:4'-Me-PCMo_structure.png|120px]] || Methyl-PCM<ref name="pmid21428243">{{cite journal | vauthors = Ahmadi A, Khalili M, Hajikhani R, Naserbakht M | title = Synthesis and determination of acute and chronic pain activities of 1-[1-(4-methylphenyl) (cyclohexyl)] morpholine as a new phencyclidine derivative in rats | journal = Arzneimittel-Forschung | volume = 61 | issue = 2 | pages = 92–7 | year = 2011 | pmid = 21428243 | doi = 10.1055/s-0031-1296173 | s2cid = 8094521 }}</ref> (4'-Me-PCMo) || ''p''-Tolyl || Morpholine || - || 120803-52-3 |
|||
|- |
|||
| [[File:2'-Me-4'-HO-PCMo_structure.png|120px]] || Hydroxy-methyl-PCM || 2-Methyl-4-hydroxyphenyl || Morpholine || - || 1314323-89-1 |
|||
|- |
|||
| [[File:PYCP_structure.png|120px]] || PYCP <ref>{{cite journal | vauthors = Zarantonello P, Bettini E, Paio A, Simoncelli C, Terreni S, Cardullo F | title = Novel analogues of ketamine and phencyclidine as NMDA receptor antagonists | journal = Bioorganic & Medicinal Chemistry Letters | volume = 21 | issue = 7 | pages = 2059–63 | date = April 2011 | pmid = 21334205 | doi = 10.1016/j.bmcl.2011.02.009 }}</ref> || 2-Pyridinyl || Piperidine || - || |
|||
|- |
|||
| [[File:TCM_structure.png|120px]] || TCM || 2-Thienyl || Methylamino || - || 139401-07-3 |
|||
|- |
|||
| [[File:TCE_structure.png|120px]] || TCE || 2-Thienyl || Ethylamino || - || 101589-62-2 |
|||
|- |
|||
| [[File:TCPr_structure.png|120px]] || TCPr <ref>{{cite journal | vauthors = Wallach J, Colestock T, Cicali B, Elliott SP, Kavanagh PV, Adejare A, Dempster NM, Brandt SD | display-authors = 6 | title = Syntheses and analytical characterizations of N-alkyl-arylcyclohexylamines | journal = Drug Testing and Analysis | volume = 8 | issue = 8 | pages = 801–15 | date = August 2016 | pmid = 26360516 | doi = 10.1002/dta.1861 | s2cid = 1599386 | url = http://researchonline.ljmu.ac.uk/id/eprint/3240/1/%EF%BF%BC%EF%BF%BC%EF%BF%BCDTA-15-0185.R1.pdf }}</ref> || 2-Thienyl || Propylamino || - || |
|||
|- |
|||
| [[File:Tenocyclidine.svg|120px]] || [[Tenocyclidine]] (TCP) || 2-Thienyl || Piperidine || - || 21500-98-1 |
|||
|- |
|||
| [[File:T3CP_structure.png|120px]] || T3CP || 3-Thienyl || Piperidine || - || 19420-50-9 |
|||
|- |
|||
| [[File:TCPy_structure.png|120px]] || TCPy || 2-Thienyl || Pyrrolidine || - || 22912-13-6 |
|||
|- |
|||
| [[File:Tiletamine.svg|120px]] || [[Tiletamine]] || 2-Thienyl || Ethylamino || 2-Keto || 14176-49-9 |
|||
|- |
|||
| [[File:MXTE_structure.png|120px]] || MXTE || 4-Methoxy-2-thienyl || Ethylamino || 2-Keto || |
|||
|- |
|||
| [[File:Gacyclidine.png|120px]] || [[Gacyclidine]] || [[Thiophene|2-Thienyl]] || Piperidine || 2-Methyl || 68134-81-6 |
|||
|- |
|||
| [[File:Bromadol_Skeletal.png|130px]] || [[Bromadol|BDPC]] || ''p''-Bromophenyl || Dimethylamino || 4-Phenethyl-4-hydroxy || 77239-98-6 |
|||
|- |
|||
| [[File:C-8813.svg|130px]] || [[C-8813]] || ''p''-Bromophenyl || Dimethylamino || 4-(thiophen-2-yl)ethyl-4-hydroxy || 616898-54-5 |
|||
|- |
|||
| [[File:Dimetamine_structure.png|120px]] || [[4-Dimethylamino-4-(p-tolyl)cyclohexanone|Dimetamine]]<ref>{{cite journal | vauthors = Lednicer D, VonVoigtlander PF, Emmert DE | title = 4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring | journal = Journal of Medicinal Chemistry | volume = 23 | issue = 4 | pages = 424–30 | date = April 1980 | pmid = 7381841 | doi = 10.1021/jm00178a014 }}</ref> || ''p''-Tolyl || Dimethylamino || 4-Keto || 65619-06-9 |
|||
|- |
|||
| [[File:Ahmadi pcp 2010.svg|125px]] || <nowiki>3''-OH-2'-Me-PCP</nowiki> <ref>{{cite journal | vauthors = Ahmadi A, Solati J, Hajikhani R, Onagh M, Javadi M | title = Synthesis and analgesic effects of 1-[1-(2-methylphenyl)(cyclohexyl)]-3-piperidinol as a new derivative of phencyclidine in mice | journal = Arzneimittel-Forschung | volume = 60 | issue = 8 | pages = 492–6 | year = 2010 | pmid = 20863005 | doi = 10.1055/s-0031-1296317 | s2cid = 24803623 }}</ref> || ''o''-Tolyl || 3-Hydroxypiperidine || - || |
|||
|- |
|||
| [[File:1-(1-PhCHX)-4-Ph-4-OH-piperidine_structure.png|130px]] || <nowiki>4''-Ph-4''-OH-PCP</nowiki> <ref>{{cite journal | vauthors = Itzhak Y, Kalir A, Weissman BA, Cohen S | title = New analgesic drugs derived from phencyclidine | journal = Journal of Medicinal Chemistry | volume = 24 | issue = 5 | pages = 496–9 | date = May 1981 | pmid = 7241506 | doi = 10.1021/jm00137a004 }}</ref> || Phenyl || 4-Phenyl-4-hydroxypiperidine || - || 77179-39-6 |
|||
|- |
|||
| [[File:BTCP.svg|120px]] || [[Benocyclidine|BTCP]]<ref name="pmid3384005">{{cite journal | vauthors = Vignon J, Pinet V, Cerruti C, Kamenka JM, Chicheportiche R | title = [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): a new phencyclidine analog selective for the dopamine uptake complex | journal = European Journal of Pharmacology | volume = 148 | issue = 3 | pages = 427–36 | date = April 1988 | pmid = 3384005 | doi = 10.1016/0014-2999(88)90122-7 }}</ref>|| Benzothiophen-2-yl || Piperidine || - || 112726-66-6 |
|||
|- |
|||
| [[File:BTCPy_structure.png|120px]] || BTCPy<ref name="pmid8098066"/> || Benzothiophen-2-yl || Pyrrolidine || - || |
|||
|- |
|||
| [[File:GK-189_structure.png|120px]] || GK-189<ref>[https://patents.google.com/patent/US5248686A Kamenka JM, et al. Substituted cyclic amines and pharmaceutical composition containing them. Patent US5248686, 28 September 1993]</ref> || Naphthalen-2-yl || Piperidine || - || 81490-58-6 |
|||
|- |
|||
|} |
|||
==参考文献== |
|||
{{reflist}} |
|||
2024年2月23日 (五) 04:33的版本
芳基环己胺衍生物(英語:Arylcyclohexylamine、arylcyclohexamines或arylcyclohexanamines)是一类含氮有机化合物,常用作药品和狡詐家藥物和试验药物,其特点是环己烷的一个碳原子上连接了氨基的N原子和芳香基,常见的有乙环利定、苯环己哌啶等[1]。

化合物
| Structures | Compound | Aryl Substituent | N Group | Cyclohexyl ring | CAS number |
|---|---|---|---|---|---|
 |
PCA[2] | Phenyl | NH2 | - | 1934-71-0 |
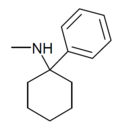 |
PCM[2] | Phenyl | Methylamino | - | 2201-16-3 |
 |
Eticyclidine | Phenyl | Ethylamino | - | 2201-15-2 |
 |
PCPr[3] | Phenyl | n-Propylamino | - | 18949-81-0 |
 |
PCiP | Phenyl | Isopropylamino | - | 1195-42-2 |
 |
PCAL [4] | Phenyl | Allylamino | - | 2185-95-7 |
 |
PCBu | Phenyl | n-Butylamino | - | 73166-29-7 |
 |
PCEOH | Phenyl | Hydroxyethylamino | - | 2201-22-1 |
 |
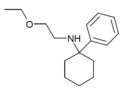
PCMEA[5] | Phenyl | Methoxyethylamino | - | 2201-57-2 |
 |
PCEEA | Phenyl | Ethoxyethylamino | - | 1072895-05-6 |
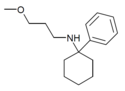 |
PCMPA | Phenyl | Methoxypropylamino | - | 2201-58-3 |
 |
PCDM[2] | Phenyl | Dimethylamino | - | 2201-17-4 |
 |
Dieticyclidine | Phenyl | Diethylamino | - | 2201-19-6 |
 |
2-HO-PCP[6] | Phenyl | Piperidine | 2-Hydroxy | 94852-58-1 |
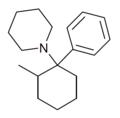 |
2-Me-PCP[7] | Phenyl | Piperidine | 2-Methyl | 59397-29-4 |
 |
2-MeO-PCP[8] | Phenyl | Piperidine | 2-Methoxy | 78636-34-7 |
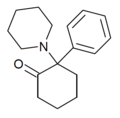 |
2-Keto-PCP | Phenyl | Piperidine | 2-Keto | 101688-16-8 |
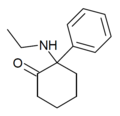 |
Eticyclidone ("O-PCE") | Phenyl | Ethylamino | 2-Keto | 6740-82-5 |
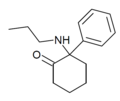 |
2-Keto-PCPr | Phenyl | n-Propylamino | 2-Keto | |
 |
4-Methyl-PCP | Phenyl | Piperidine | 4-Methyl | 19420-52-1 |
 |
4-Keto-PCP[9] | Phenyl | Piperidine | 4-Keto | 65620-13-5 |
 |
2'-Cl-PCP | o-Chlorophenyl | Piperidine | - | 2201-31-2 |
 |
3'-Cl-PCP | m-Chlorophenyl | Piperidine | - | 2201-32-3 |
 |
2'-MeO-PCP | o-Methoxyphenyl | Piperidine | - | 2201-34-5 |
 |
3'-F-PCP[10] | m-Fluorophenyl | Piperidine | - | 89156-99-0 |
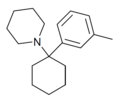 |
3'-Me-PCP[11] | m-Tolyl | Piperidine | - | 2201-30-1 |
 |
3'-Me-PCPy | m-Tolyl | Pyrrolidine | - | 1622348-63-3 |
 |
3'-NH2-PCP | m-Aminophenyl | Piperidine | - | 72242-00-3 |
 |
3'-HO-PCP | m-Hydroxyphenyl | Piperidine | - | 79787-43-2 |
 |
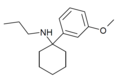
3'-MeO-PCP | m-Methoxyphenyl | Piperidine | - | 72242-03-6 |
 |
3',4'-MD-PCP | 3,4-Methylenedioxyphenyl | Piperidine | - | |
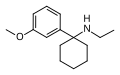 |
3'-MeO-PCE | m-Methoxyphenyl | Ethylamino | - | 1364933-80-1 |
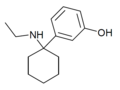 |
3'-HO-PCE | m-Hydroxyphenyl | Ethylamino | - | |
 |
3'-MeO-PCPr | m-Methoxyphenyl | n-Propylamino | - | 1364933-81-2 |
 |
3'-HO-PCPr | m-Hydroxyphenyl | n-Propylamino | - | |
 |
3',4'-MD-PCPr | 3,4-Methylenedioxyphenyl | n-Propylamino | - | |
 |
3'-MeO-PCPy[11] | m-Methoxyphenyl | Pyrrolidine | - | 1364933-79-8 |
 |
4'-HO-PCP | p-Hydroxyphenyl | Piperidine | - | 66568-88-5 |
 |
Methoxydine (4'-MeO-PCP) | p-Methoxyphenyl | Piperidine | - | 2201-35-6 |
 |
4'-MeO-PCE | p-Methoxyphenyl | Ethylamino | - | |
 |
4'-F-PCP[10] | p-Fluorophenyl | Piperidine | - | 22904-99-0 |
 |
4'-F-PCPy | p-Fluorophenyl | Pyrrolidine | - | |
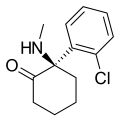 |
Arketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-49-1 |
 |
Deschloroketamine | Phenyl | Methylamino | 2-Keto | 7063-30-1 |
 |
Esketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-46-8 |
 |
Ketamine | o-Chlorophenyl | Methylamino | 2-Keto | 6740-88-1 |
 |
Hydroxynorketamine | o-Chlorophenyl | NH2 | 2-Keto, 6-Hydroxy | 81395-70-2 |
 |
Ethketamine | o-Chlorophenyl | Ethylamino | 2-Keto | 1354634-10-8 |
 |
NPNK | o-Chlorophenyl | n-Propylamino | 2-Keto | 2749326-65-4 |
 |
Methoxyketamine | o-Methoxyphenyl | Methylamino | 2-Keto | 7063-51-6 |
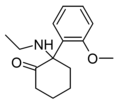 |
2-MeO-NEK[12] | o-Methoxyphenyl | Ethylamino | 2-Keto | |
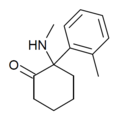 |
oMDCK[13] | o-Tolyl | Methylamino | 2-Keto | 7063-37-8 |
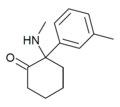 |
mMDCK | m-Tolyl | Methylamino | 2-Keto | |
 |
meta-Ketamine | m-Chlorophenyl | Methylamino | 2-Keto | 7063-53-8 |
 |
iso-Ketamine | o-Chlorophenyl | Methylamino | 4-Keto | |
 |
2-Fluorodeschloroketamine | o-Fluorophenyl | Methylamino | 2-Keto | 111982-50-4 |
 |
3-Fluorodeschloroketamine | m-Fluorophenyl | Methylamino | 2-Keto | 2657761-23-2 |
 |
Bromoketamine | o-Bromophenyl | Methylamino | 2-Keto | 120807-70-7 |
 |
TFMDCK | o-Trifluoromethylphenyl | Methylamino | 2-Keto | 1782149-73-8 |
 |
SN 35210[14] | o-Chlorophenyl | Carbomethoxybutylamino | 2-Keto | 1450615-41-4 |
 |
Methoxetamine | m-Methoxyphenyl | Ethylamino | 2-Keto | 1239943-76-0 |
 |
Methoxmetamine | m-Methoxyphenyl | Methylamino | 2-Keto | 1781829-56-8 |
 |
Methoxpropamine | m-Methoxyphenyl | n-Propylamino | 2-Keto | 2504100-71-2 |
 |
MXiPr | m-Methoxyphenyl | i-Propylamino | 2-Keto | |
 |
Ethoxetamine | m-Ethoxyphenyl | Ethylamino | 2-Keto | |
 |
Deoxymethoxetamine (3-Me-2'-Oxo-PCE) | m-Tolyl | Ethylamino | 2-Keto | 2666932-45-0 |
 |
Br-MXE | 2-bromo-5-methoxyphenyl | Ethylamino | 2-Keto | |
 |
Hydroxetamine (HXE) | m-Hydroxyphenyl | Ethylamino | 2-Keto | 1620054-73-0 |
 |
HXM | m-Hydroxyphenyl | Methylamino | 2-Keto | |
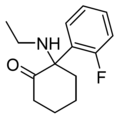 |
2F-NENDCK | o-Fluorophenyl | Ethylamino | 2-Keto | |
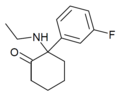 |
Fluorexetamine (FXE) | m-Fluorophenyl | Ethylamino | 2-Keto | |
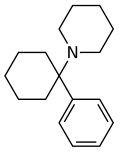 |
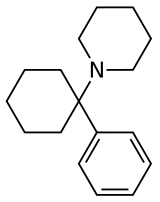
Phencyclidine (PCP) | Phenyl | Piperidine | - | 77-10-1 |
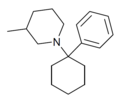 |
PC3MP | Phenyl | 3-Methylpiperidine | - | 2201-41-4 |
 |
PC4MP | Phenyl | 4-Methylpiperidine | - | 2201-42-5 |
 |
Rolicyclidine (PCPy) | Phenyl | Pyrrolidine | - | 2201-39-0 |
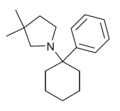 |
PCDMPy | Phenyl | 3,3-Dimethylpyrrolidine | - | |
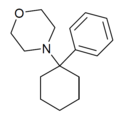 |
PCMo | Phenyl | Morpholine | - | 2201-40-3 |
 |
Methoxy-PCM[15] (2'-MeO-PCMo) | o-Methoxyphenyl | Morpholine | - | 1314323-88-0 |
 |
3'-MeO-PCMo | m-Methoxyphenyl | Morpholine | - | 138873-80-0 |
 |
4'-MeO-PCMo | p-Methoxyphenyl | Morpholine | - | |
 |
Methyl-PCM[16] (4'-Me-PCMo) | p-Tolyl | Morpholine | - | 120803-52-3 |
 |
Hydroxy-methyl-PCM | 2-Methyl-4-hydroxyphenyl | Morpholine | - | 1314323-89-1 |
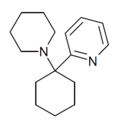 |
PYCP [17] | 2-Pyridinyl | Piperidine | - | |
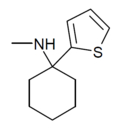 |
TCM | 2-Thienyl | Methylamino | - | 139401-07-3 |
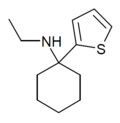 |
TCE | 2-Thienyl | Ethylamino | - | 101589-62-2 |
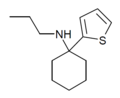 |
TCPr [18] | 2-Thienyl | Propylamino | - | |
 |
Tenocyclidine (TCP) | 2-Thienyl | Piperidine | - | 21500-98-1 |
 |
T3CP | 3-Thienyl | Piperidine | - | 19420-50-9 |
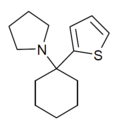 |
TCPy | 2-Thienyl | Pyrrolidine | - | 22912-13-6 |
 |
Tiletamine | 2-Thienyl | Ethylamino | 2-Keto | 14176-49-9 |
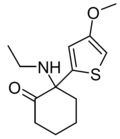 |
MXTE | 4-Methoxy-2-thienyl | Ethylamino | 2-Keto | |
 |
Gacyclidine | 2-Thienyl | Piperidine | 2-Methyl | 68134-81-6 |
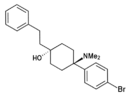 |
BDPC | p-Bromophenyl | Dimethylamino | 4-Phenethyl-4-hydroxy | 77239-98-6 |
 |
C-8813 | p-Bromophenyl | Dimethylamino | 4-(thiophen-2-yl)ethyl-4-hydroxy | 616898-54-5 |
 |
Dimetamine[19] | p-Tolyl | Dimethylamino | 4-Keto | 65619-06-9 |
 |
3''-OH-2'-Me-PCP [20] | o-Tolyl | 3-Hydroxypiperidine | - | |
 |
4''-Ph-4''-OH-PCP [21] | Phenyl | 4-Phenyl-4-hydroxypiperidine | - | 77179-39-6 |
 |
BTCP[22] | Benzothiophen-2-yl | Piperidine | - | 112726-66-6 |
 |
BTCPy[23] | Benzothiophen-2-yl | Pyrrolidine | - | |
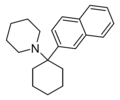 |
GK-189[24] | Naphthalen-2-yl | Piperidine | - | 81490-58-6 |
参考文献
- ^ 4-(1-phenyl-cyclohexyl)-morpholine. CAS Number Search - chemsrc.com. chemsrc. [15 March 2021].
- ^ 2.0 2.1 2.2 Thurkauf A, de Costa B, Yamaguchi S, Mattson MV, Jacobson AE, Rice KC, Rogawski MA. Synthesis and anticonvulsant activity of 1-phenylcyclohexylamine analogues. Journal of Medicinal Chemistry. May 1990, 33 (5): 1452–8. PMID 2329567. doi:10.1021/jm00167a027.
- ^ Sauer C, Peters FT, Staack RF, Fritschi G, Maurer HH. Metabolism and toxicological detection of a new designer drug, N-(1-phenylcyclohexyl)propanamine, in rat urine using gas chromatography-mass spectrometry. Journal of Chromatography A. April 2008, 1186 (1–2): 380–90. PMID 18035363. doi:10.1016/j.chroma.2007.11.002.
- ^ Kalir A, Teomy S, Amir A, Fuchs P, Lee SA, Holsztynska EJ, et al. N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties. Journal of Medicinal Chemistry. October 1984, 27 (10): 1267–71. PMID 6481761. doi:10.1021/jm00376a006.
- ^ Sauer C, Peters FT, Schwaninger AE, Meyer MR, Maurer HH. Investigations on the cytochrome P450 (CYP) isoenzymes involved in the metabolism of the designer drugs N-(1-phenyl cyclohexyl)-2-ethoxyethanamine and N-(1-phenylcyclohexyl)-2-methoxyethanamine. Biochemical Pharmacology. February 2009, 77 (3): 444–50. PMID 19022226. doi:10.1016/j.bcp.2008.10.024.
- ^ 引证错误:没有为名为
pmid16229117的参考文献提供内容 - ^ Iorio MA, Tomassini L, Mattson MV, George C, Jacobson AE. Synthesis, stereochemistry, and biological activity of the 1-(1-phenyl-2-methylcyclohexyl)piperidines and the 1-(1-phenyl-4-methylcyclohexyl)piperidines. Absolute configuration of the potent trans-(-)-1-(1-phenyl-2-methylcyclohexyl)piperidine. Journal of Medicinal Chemistry. August 1991, 34 (8): 2615–23. PMID 1875352. doi:10.1021/jm00112a041.
- ^ Ahmadi A, Mahmoudi A. Synthesis with improved yield and study on the analgesic effect of 2-methoxyphencyclidine. Arzneimittel-Forschung. 2006, 56 (5): 346–50. PMID 16821645. S2CID 10370245. doi:10.1055/s-0031-1296732.
- ^ Ortiz DM, Custodio RJ, Abiero A, Botanas CJ, Sayson LV, Kim M, et al. The dopaminergic alterations induced by 4-F-PCP and 4-Keto-PCP may enhance their drug-induced rewarding and reinforcing effects: Implications for abuse. Addiction Biology. July 2021, 26 (4): e12981. PMID 33135332. S2CID 226234538. doi:10.1111/adb.12981.
- ^ 10.0 10.1 Ogunbadeniyi AM, Adejare A. Syntheses of fluorinated phencyclidine analogs. Journal of Fluorine Chemistry. 2002, 114: 39–42. doi:10.1016/S0022-1139(01)00565-6.
- ^ 11.0 11.1 Wallach J, De Paoli G, Adejare A, Brandt SD. Preparation and analytical characterization of 1-(1-phenylcyclohexyl)piperidine (PCP) and 1-(1-phenylcyclohexyl)pyrrolidine (PCPy) analogues. Drug Testing and Analysis. 2013, 6 (7–8): 633–50. PMID 23554350. doi:10.1002/dta.1468.
- ^ Sayson LV, Botanas CJ, Custodio RJ, Abiero A, Kim M, Lee HJ, et al. The novel methoxetamine analogs N-ethylnorketamine hydrochloride (NENK), 2-MeO-N-ethylketamine hydrochloride (2-MeO-NEK), and 4-MeO-N-ethylketamine hydrochloride (4-MeO-NEK) elicit rapid antidepressant effects via activation of AMPA and 5-HT2 receptors. Psychopharmacology. July 2019, 236 (7): 2201–2210. PMID 30891619. S2CID 83463722. doi:10.1007/s00213-019-05219-x.
- ^ WO 2021134086,Kruegel AC, Sames D, Hashimoto K,「Arylcyclohexylamine derivatives and their use in the treatment of psychiatric disorders」,发表于1 July 2021,指定于Gilgamesh Pharmaceuticals, Inc.和The Trustees Of Columbia University In The City Of New York.
- ^ Harvey M, Sleigh J, Voss L, Pruijn F, Jose J, Gamage S, Denny W. Determination of the Hypnotic Potency in Rats of the Novel Ketamine Ester Analogue SN 35210. Pharmacology. 2015, 96 (5–6): 226–32. PMID 26352278. S2CID 36017002. doi:10.1159/000439598.
- ^ 引证错误:没有为名为
pmid21215770的参考文献提供内容 - ^ Ahmadi A, Khalili M, Hajikhani R, Naserbakht M. Synthesis and determination of acute and chronic pain activities of 1-[1-(4-methylphenyl) (cyclohexyl)] morpholine as a new phencyclidine derivative in rats. Arzneimittel-Forschung. 2011, 61 (2): 92–7. PMID 21428243. S2CID 8094521. doi:10.1055/s-0031-1296173.
- ^ Zarantonello P, Bettini E, Paio A, Simoncelli C, Terreni S, Cardullo F. Novel analogues of ketamine and phencyclidine as NMDA receptor antagonists. Bioorganic & Medicinal Chemistry Letters. April 2011, 21 (7): 2059–63. PMID 21334205. doi:10.1016/j.bmcl.2011.02.009.
- ^ Wallach J, Colestock T, Cicali B, Elliott SP, Kavanagh PV, Adejare A, et al. Syntheses and analytical characterizations of N-alkyl-arylcyclohexylamines (PDF). Drug Testing and Analysis. August 2016, 8 (8): 801–15. PMID 26360516. S2CID 1599386. doi:10.1002/dta.1861.
- ^ Lednicer D, VonVoigtlander PF, Emmert DE. 4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring. Journal of Medicinal Chemistry. April 1980, 23 (4): 424–30. PMID 7381841. doi:10.1021/jm00178a014.
- ^ Ahmadi A, Solati J, Hajikhani R, Onagh M, Javadi M. Synthesis and analgesic effects of 1-[1-(2-methylphenyl)(cyclohexyl)]-3-piperidinol as a new derivative of phencyclidine in mice. Arzneimittel-Forschung. 2010, 60 (8): 492–6. PMID 20863005. S2CID 24803623. doi:10.1055/s-0031-1296317.
- ^ Itzhak Y, Kalir A, Weissman BA, Cohen S. New analgesic drugs derived from phencyclidine. Journal of Medicinal Chemistry. May 1981, 24 (5): 496–9. PMID 7241506. doi:10.1021/jm00137a004.
- ^ Vignon J, Pinet V, Cerruti C, Kamenka JM, Chicheportiche R. [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): a new phencyclidine analog selective for the dopamine uptake complex. European Journal of Pharmacology. April 1988, 148 (3): 427–36. PMID 3384005. doi:10.1016/0014-2999(88)90122-7.
- ^ 引证错误:没有为名为
pmid8098066的参考文献提供内容 - ^ Kamenka JM, et al. Substituted cyclic amines and pharmaceutical composition containing them. Patent US5248686, 28 September 1993
