3-溴甲苯
外观
(重定向自间溴甲苯)
| 3-溴甲苯 | |
|---|---|
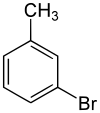
| |
| 别名 | 间溴甲苯 3-甲基溴苯 |
| 识别 | |
| CAS号 | 591-17-3 |
| PubChem | 11560 |
| SMILES |
|
| 性质 | |
| 化学式 | C7H7Br |
| 摩尔质量 | 171.03 g·mol−1 |
| 外观 | 无色液体 |
| 密度 | 1.4022 g·cm−3(25 °C)[1] |
| 熔点 | −39.8 °C(233.3 K)[2] |
| 沸点 | 183.7 °C(456.8 K)[2] |
| 溶解性(水) | 难溶 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
3-溴甲苯是一种有机化合物,化学式为C7H7Br,是溴甲苯的同分异构体之一。
合成
[编辑]3-溴甲苯可由3-溴苯甲醛在钯碳催化剂存在下被氢气还原得到,[3]或通过3-甲基苯胺的重氮化-溴化反应制得。[4]3-甲基苯肼盐酸盐和三溴化硼在二甲基亚砜中反应,也可以得到3-溴甲苯。[5]
反应
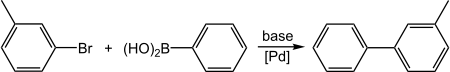
[编辑]3-溴甲苯和苯硼酸可以发生铃木偶联反应(C–C偶联),生成3-甲基联苯:[6]
类似地,在三(二亚苄基丙酮)二钯催化下,它和格氏试剂可以发生熊田偶联反应,如和4-甲氧基苯基溴化镁反应,得到3-甲基-4'-甲氧基联苯。[7]该催化剂也可催化3-溴甲苯与胺的反应,如3-溴甲苯和哌啶在碱的存在下反应,可以得到C–C偶联产物1-(3-甲基苯基)哌啶。[8]
它和氯磺酸在三氯甲烷中反应,可以得到4-溴-2-甲苯磺酰氯。[10]它可以被氧气、过氧化叔丁醇等氧化剂催化氧化为3-溴苯甲酸。[11][12][13]
参考文献
[编辑]- ^ Hussey, Allen S.; Dyer, John R. The Monomagnesium Derivatives of Dibromotoluenes. Journal of the American Chemical Society. 2002, 73 (2): 603–604. ISSN 0002-7863. doi:10.1021/ja01146a031.
- ^ 2.0 2.1 "PhysProp" data were obtained from Syracuse Research Corporation of Syracuse, New York (US). Retrieved from SciFinder. [2021-07-11].
- ^ Wang, Shuguo; Zhou, Peng; Jiang, Liang; Zhang, Zehui; Deng, Kejian; Zhang, Yuhua; Zhao, Yanxi; Li, Jinlin; Bottle, Steven; Zhu, Huaiyong. Selective deoxygenation of carbonyl groups at room temperature and atmospheric hydrogen pressure over nitrogen-doped carbon supported Pd catalyst. Journal of Catalysis. 2018, 368: 207–216. ISSN 0021-9517. doi:10.1016/j.jcat.2018.10.017.
- ^ Beletskaya, Irina; Sigeev, Alexander; Peregudov, Alexander; Petrovskii, Pavel. Catalytic Sandmeyer Bromination. Synthesis. 2007, 2007 (16): 2534–2538. ISSN 0039-7881. doi:10.1055/s-2007-983784.
- ^ Phuc Tran, Dat; Nomoto, Akihiro; Mita, Soichiro; Dong, Chun-ping; Kodama, Shintaro; Mizuno, Takumi; Ogawa, Akiya. Metal- and base-free synthesis of aryl bromides from arylhydrazines. Tetrahedron Letters. 2020, 61 (23): 151959. ISSN 0040-4039. doi:10.1016/j.tetlet.2020.151959.
- ^ Wang, Ai-E; Zhong, Jun; Xie, Jian-Hua; Li, Kai; Zhou, Qi-Lin. Highly Efficient Suzuki Cross-Coupling Catalyzed by Palladium/Phosphine-Imidazolium Carbene System. Advanced Synthesis & Catalysis. 2004, 346 (6): 595–598. ISSN 1615-4150. doi:10.1002/adsc.200404015.
- ^ Pal, Anwesha; Ghosh, Raju; Adarsh, N.N.; Sarkar, Amitabha. Pyrazole-tethered phosphine ligands for Pd(0): useful catalysts for Stille, Kumada and Hiyama cross-coupling reactions. Tetrahedron. 2010, 66 (29): 5451–5458. ISSN 0040-4020. doi:10.1016/j.tet.2010.05.026.
- ^ Mudithanapelli, Chandrashekar; Dhorma, Lama Prema; Kim, Mi-hyun. PIFA-Promoted, Solvent-Controlled Selective Functionalization of C(sp2)–H or C(sp3)–H: Nitration via C–N Bond Cleavage of CH3NO2, Cyanation, or Oxygenation in Water. Organic Letters. 2019, 21 (9): 3098–3102. ISSN 1523-7060. doi:10.1021/acs.orglett.9b00751.
- ^ Chen, Jingbo; Zhang, Yushun; Yang, Liquan; Zhang, Xiang; Liu, Jianping; Li, Liang; Zhang, Hongbin. A practical palladium catalyzed dehalogenation of aryl halides and α-haloketones. Tetrahedron. 2007, 63 (20): 4266–4270. ISSN 0040-4020. doi:10.1016/j.tet.2007.03.061.
- ^ Chen, Qiao-Hong; Rao, P.N. Praveen; Knaus, Edward E. Design, synthesis, and biological evaluation of N-acetyl-2-carboxybenzenesulfonamides: a novel class of cyclooxygenase-2 (COX-2) inhibitors. Bioorganic & Medicinal Chemistry. 2005, 13 (7): 2459–2468. ISSN 0968-0896. doi:10.1016/j.bmc.2005.01.039.
- ^ Zheng, Kun; Yan, Xiaoyu; Zhang, Guofu; Yan, Xinhuan; Li, Xiaoqing; Xu, Xiangsheng. Photoinduced Carbon Tetrabromide Initiated Aerobic Oxidation of Substituted Toluenes to Carboxylic Acids. Synlett. 2019, 31 (03): 272–274. ISSN 0936-5214. doi:10.1055/s-0039-1691534.
- ^ Ozen, Recep. Pyridinium Chlorochromate Catalyzed Oxidation of Toluenes to Aromatic Carboxylic Acids with Molecular Oxygen in Sub-critical Water. Asian Journal of Chemistry. 2014, 26 (3): 941–942. ISSN 0970-7077. doi:10.14233/ajchem.2014.16651.
- ^ Mohammadpour, Pegah; Safaei, Elham. Catalytic C–H aerobic and oxidant-induced oxidation of alkylbenzenes (including toluene derivatives) over VO2+ immobilized on core–shell Fe3O4@SiO2 at room temperature in water. RSC Advances. 2020, 10 (40): 23543–23553. ISSN 2046-2069. doi:10.1039/D0RA03483E.