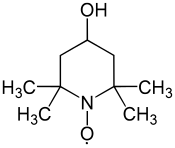
4-羟基-2,2,6,6-四甲基-1-氧化哌啶
外观
| 4-羟基-2,2,6,6-四甲基-1-氧化哌啶 | |
|---|---|
| |

| |
| IUPAC名 (4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)oxyl | |
| 别名 | tempol; tanol; TMPN; 4-Oxypiperidol; nitroxyl 2; HyTEMPO |
| 识别 | |
| CAS号 | 2226-96-2 |
| PubChem | 137994 |
| ChemSpider | 121639 |
| SMILES |
|
| InChI |
|
| InChIKey | UZFMOKQJFYMBGY-UHFFFAOYSA-N |
| 性质 | |
| 化学式 | C9H18NO2 |
| 摩尔质量 | 172.24 g·mol−1 |
| 外观 | 橙色晶体 |
| 熔点 | 71—73 °C(344—346 K)[1] |
| 溶解性(水) | 629.3 g/L (20 °C) |
| 危险性 | |
GHS危险性符号 [2] [2]
| |
| GHS提示词 | Warning[2] |
| H-术语 | H302, H315, H319, H335[2] |
| P-术语 | P261, P305+351+338[2] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
4-羟基-2,2,6,6-四甲基-1-氧化哌啶(4-羟基-TEMPO,TEMPOL)是一种有机化合物,化学式为C9H18NO2。在工业上,它可由下图所示方法合成:[3]
它和乙酰氯反应,可以得到4-乙酰氧基-TEMPO;[4]类似地,它和甲磺酰氯反应,可以得到4-甲磺酰氧基-TEMPO。[5]
参考文献
[编辑]- ^ Zakrzewski, Jerzy; Krawczyk, Maria. Reactions of Nitroxides. Part XII [1]. – 2,2,6,6-Tetramethyl-1-oxyl- 4-piperidyl Chloroformate – A New Reactive Nitroxyl Radical. A One-pot Synthesis of 2,2,6,6-Tetramethyl-1-oxyl-4-piperidyl N,N-Dialkyl-carbamates. Zeitschrift für Naturforschung B. 1 January 2011, 66 (5). doi:10.1515/znb-2011-0509.
- ^ 2.0 2.1 2.2 2.3 来源:Sigma-Aldrich Co., 4-Hydroxy-TEMPO (2015-08-24查阅).
- ^ Ciriminna, Rosaria; Pagliaro, Mario. Industrial Oxidations with Organocatalyst TEMPO and Its Derivatives. Organic Process Research & Development. 15 January 2010, 14 (1): 245–251. doi:10.1021/op900059x.
- ^ Nayereh Mohebbati, Adrian Prudlik, Anton Scherkus, Aija Gudkova, Robert Francke. TEMPO‐Modified Polymethacrylates as Mediators in Electrosynthesis – Redox Behavior and Electrocatalytic Activity toward Alcohol Substrates. ChemElectroChem. 2021-10-13, 8 (20): 3837–3843 [2021-11-10]. ISSN 2196-0216. doi:10.1002/celc.202100768 (英语).
- ^ N. G. Bushmakina, A. Yu. Misharin. A Simple Synthesis of 4-Amino-2,2,6,6-tetramethyl-1-piperidinyloxy Radical. Synthesis. 1986, 1986 (11): 966–966 [2021-11-10]. ISSN 0039-7881. doi:10.1055/s-1986-31841. (原始内容存档于2018-06-03) (英语).

