二乙酰基过氧化物
外观
| 二乙酰基过氧化物 | |
|---|---|
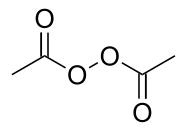
| |

| |
| IUPAC名 Acetic peroxyanhydride[1] | |
| 别名 | 过氧化乙酰 过氧化二乙酰 过氧乙酸酐 |
| 识别 | |
| CAS号 | 110-22-5 |
| PubChem | 8040 |
| ChemSpider | 7749 |
| SMILES |
|
| UN编号 | 2084 |
| EINECS | 203-748-8 |
| 性质 | |
| 化学式 | C4H6O4 |
| 摩尔质量 | 118.09 g·mol−1 |
| 外观 | 无色晶体[2] |
| 密度 | 1.163 g/cm3[2] |
| 熔点 | 30 °C[3] |
| 沸点 | 121.4 °C(760 mmHg) 63 °C(145 °F;336 K)(21 mmHg)[4][3] |
| 溶解性(水) | slight in cold water [2] |
| 危险性 | |
| 主要危害 | 氧化性;爆炸性 |
| NFPA 704 | |
| 闪点 | 32.2 °C(90.0 °F;305.3 K) (45 °C(113 °F;318 K)[5]) |
| 爆炸性 | |
| 撞击感度 | Very high / moderate when wet |
| 摩擦感度 | Very high / moderate when wet |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
二乙酰基过氧化物是一种有机过氧化物,化学式为(CH3CO2)2,它是白色固体或油状液体。[5]它最初在1858年由本杰明·柯林斯·布罗迪[6]通过乙酸和过氧化钡在无水乙醚中反应得到。[7]它可由过氧化氢和乙酸酐反应制得。[8]
参考文献
- ^ International Union of Pure and Applied Chemistry. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. 2014: 829. ISBN 978-0-85404-182-4. doi:10.1039/9781849733069.
- ^ 2.0 2.1 2.2 Lewis, R. J., Sr (编). Hawley's Condensed Chemical Dictionary 13th. New York, NY: John Wiley & Sons. 1997: 13.
- ^ 3.0 3.1 "Hazardous Substances Data Bank" data were obtained from the National Library of Medicine (US). Retrieved from SciFinder. [2024-02-21].
- ^ Lide, D. R. (编). CRC Handbook of Chemistry and Physics 79th. Boca Raton, Florida: CRC Press. 1998–1999: 3–250.
- ^ 5.0 5.1 Acetyl peroxide (PDF). NJ.gov.
- ^ Anniversary Meeting. Journal of the Chemical Society, Transactions. 30 March 1881, 39: 177–201 [17 February 2022]. doi:10.1039/CT8813900177.
- ^ Brodie, Benjamin Collins. I. Note on the formation of the peroxides of the radicals of the organic acids. Proceedings of the Royal Society of London. 1 January 1859, 9: 361–365. S2CID 97728118. doi:10.1098/rspl.1857.0087
 .
.
- ^ Chemical Safety: Synthesis Procedure. Chemical & Engineering News. 2011-01-10, 89 (2): 2.

