Anthocyanone A
外观
| Anthocyanone A | |
|---|---|
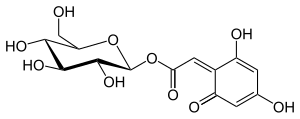
| |
| IUPAC名 [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] 2-(2,6-dihydroxy-4-oxocyclohexa-2,5-dien-1-ylidene)acetate | |
| 别名 | 8-β-d-glucopyranosyl-2,4-dihydroxy-6-oxo-cyclohexa-2,4-dienyl acetic acid |
| 识别 | |
| CAS号 | |
| PubChem | 139031050 |
| ChemSpider | 29784776 |
| SMILES |
|
| InChI |
|
| InChIKey | VOWKJMFKRCLSJJ-BFYJNFCABS |
| 性质 | |
| 化学式 | C14H16O10 |
| 摩尔质量 | 344.27 g·mol−1 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
Anthocyanone A是一种有机化合物,分子式C14H16O10,是锦葵素-3-O-葡萄糖苷在酸性条件下经拜耳-维利格反应被H2O2氧化降解的产物[1][2],存在于葡萄酒中[3],形成于葡萄酒陈酿过程,引起葡萄酒色度下降。[1]
参考文献
[编辑]- ^ 1.0 1.1 张欣珂,赵旭,刘沛通,等. 红葡萄酒的花色苷:来源、呈色与反应. 食品科学. 2023, 44 (23): 342-352.
- ^ Lopes, P; Richard, T; Saucier, C; Teissedre, PL; Monti, JP; Glories, Y. Anthocyanone A: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation. Journal of Agricultural and Food Chemistry. 2007, 55 (7): 2698–704. PMID 17338545. doi:10.1021/jf062875o.
- ^ Saucier, Cédric. How do wine polyphenols evolve during wine ageing?. Cerevisia. 2010, 35: 11–15. doi:10.1016/j.cervis.2010.05.002.
