4-羥基苯基丙酮
外觀
此條目需要精通或熟悉化學的編者參與及協助編輯。 |
此條目需要擴充。 (2018年2月26日) |
| 4-羥基苯基丙酮 | |
|---|---|
| |
| IUPAC名 1-(4-Hydroxyphenyl)propan-2-one | |
| 別名 | p-Hydroxyphenylacetone; para-Hydroxyphenylacetone |
| 識別 | |
| CAS號 | 770-39-8 |
| PubChem | 7019274 |
| ChemSpider | 5382241 |
| SMILES |
|
| InChI |
|
| InChIKey | VWMVAQHMFFZQGD-UHFFFAOYSA-N |
| 性質 | |
| 化學式 | C9H10O2 |
| 摩爾質量 | 150.17 g·mol−1 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
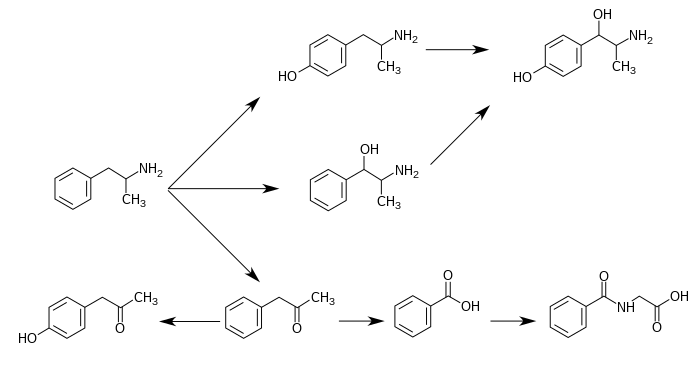
4-羥基苯基丙酮(4-Hydroxyphenylacetone)是苯基丙酮(苯丙胺在人體中的不活潑代謝產物)的羥基類似物。[1][2]當它作為苯丙胺的代謝產物出現時,它通常是由不活潑的代謝物苯基丙酮中直接產生。[1][3]
苯丙胺的代謝途徑[sources 1]
|
參注
[編輯]參考資料
[編輯]- ^ 1.0 1.1 Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G. Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection. J. Pharm. Biomed. Anal. September 2002, 30 (2): 247–55. PMID 12191709. doi:10.1016/S0731-7085(02)00330-8.
- ^ 4-Hydroxyphenylacetone. NCBI. PubChem Compound. [2013-10-25]. (原始內容存檔於2014-01-08).
- ^ 3.0 3.1 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 12–13. December 2013 [30 December 2013]. (原始內容存檔 (PDF)於2018-07-18).
- ^ Glennon RA. Phenylisopropylamine stimulants: amphetamine-related agents. Lemke TL, Williams DA, Roche VF, Zito W (編). Foye's principles of medicinal chemistry 7th. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013: 646–648 [2018-06-20]. ISBN 9781609133450. (原始內容存檔於2020-07-26).
- ^ Taylor KB. Dopamine-beta-hydroxylase. Stereochemical course of the reaction (PDF). J. Biol. Chem. January 1974, 249 (2): 454–458 [6 November 2014]. PMID 4809526. (原始內容存檔 (PDF)於2019-04-04).
- ^ Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. June 2005, 106 (3): 357–387. PMC 1828602
 . PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
. PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO (頁面存檔備份,存於網際網路檔案館) - ^ Cashman JR, Xiong YN, Xu L, Janowsky A. N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication. J. Pharmacol. Exp. Ther. March 1999, 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G. Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection. J. Pharm. Biomed. Anal. September 2002, 30 (2): 247–255. PMID 12191709. doi:10.1016/S0731-7085(02)00330-8.
- ^ Sjoerdsma A, von Studnitz W. Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate. Br. J. Pharmacol. Chemother. April 1963, 20: 278–284. PMC 1703637
 . PMID 13977820. doi:10.1111/j.1476-5381.1963.tb01467.x.
. PMID 13977820. doi:10.1111/j.1476-5381.1963.tb01467.x.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA. Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation. Expert Opin. Drug Metab. Toxicol. September 2013, 9 (9): 1139–1153. PMID 23650932. doi:10.1517/17425255.2013.796929.