吗啡全合成:修订间差异
外观
删除的内容 添加的内容
Mountainninja(留言 | 贡献) 增加或调整化学家内部链接 |
无编辑摘要 |
||
| 第1行: | 第1行: | ||
[[Image:Morphin - Morphine.svg|200px|right|thumbnail|吗啡]] |
[[Image:Morphin - Morphine.svg|200px|right|thumbnail|吗啡]] |
||
'''吗啡全合成'''是指化学中吗啡样[[生物碱]]的合成描述了天然[[吗啡喃]]类[[生物碱]]的[[全合成]],其包括[[可待因]],[[吗啡]],[[奥利平]],和[[蒂巴因]]及其密切相关的[[半合成]]类似物[[丁丙诺啡]],[[氢可酮]],[[异可待因]],[[纳曲酮]],[[纳洛酮]],[[纳布啡]]和{{le|羟考酮|Oxycodone}}<ref name="pmid21630507">{{cite journal | author = Chida N | title = Recent advances in the synthesis of morphine and related alkaloids | journal = Top Curr Chem | volume = 299 | issue = | pages = 1–28 | year = 2011 | pmid = 21630507 | doi = 10.1007/128_2010_73 }}</ref><ref name="pmid21547687">{{cite journal |vauthors=Rinner U, Hudlicky T | title = Synthesis of morphine alkaloids and derivatives | journal = Top Curr Chem | volume = 309 | issue = | pages = 33–66 | year = 2012 | pmid = 21547687 | doi = 10.1007/128_2011_133 | quote = Morphine's synthesis remains a serious challenge to this day. }}</ref>。 |
|||
| ⚫ | |||
吗啡的结构并不是特别复杂,然而,相邻结合原子的静电极化在整个结构中不均匀交替。这种“不一致的连接性”使得键的形成更加困难,并且因此使应用于该分子家族的任何合成策略明显的复杂化<ref name="pmid21547687"/>。 |
|||
| ⚫ | 首次全合成由{{link-en|马歇尔·D·盖茨|Marshall D. Gates, Jr.}}于1952年完成,并被视为这个领域中的经典之作。<ref name="Doijaa">{{cite journal|doi=10.1021/ja01588a033|year=1956|last1=Gates|first1=Marshall|last2=Tschudi|first2=Gilg|journal=Journal of the American Chemical Society|volume=78|issue=7|pages=1380}}</ref><ref name="Doijaa" /> |
||
此后,许多化学家提出了新的合成路线,其中值得注意的有以下研究者率领的团队所提出的路线:赖斯、<ref>{{cite journal|doi=10.1021/jo01303a045|title=Synthetic opium alkaloids and derivatives. A short total synthesis of (.+-.)-dihydrothebainone, (.+-.)-dihydrocodeinone, and (.+-.)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners|year=1980|last1=Rice|first1=Kenner C.|journal=The Journal of Organic Chemistry|volume=45|issue=15|pages=3135}}</ref>埃文斯、<ref>{{cite journal|doi=10.1016/S0040-4039(00)86810-0|title=Studies directed towards the total synthesis of morphine alkaloids|year=1982|last1=Evans|first1=D.A.|last2=Mitch|first2=C.H.|journal=Tetrahedron Letters|volume=23|issue=3|pages=285}}</ref>富克斯、<ref>{{cite journal|doi=10.1021/jo00255a008|title=Studies culminating in the total synthesis of (dl)-morphine|year=1988|last1=Toth|first1=J. E.|last2=Hamann|first2=P. R.|last3=Fuchs|first3=P. L.|journal=The Journal of Organic Chemistry|volume=53|issue=20|pages=4694}}</ref>帕克、<ref>{{cite journal|doi=10.1021/ja00050a075|title=Convergent synthesis of (.+-.)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (.+-.)-morphine|year=1992|last1=Parker|first1=Kathlyn A.|last2=Fokas|first2=Demosthenes|journal=Journal of the American Chemical Society|volume=114|issue=24|pages=9688}}</ref>奥尔曼、<ref>{{cite journal|doi=10.1021/ja00076a086|title=Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (-)- and (+)-dihydrocodeinone and (-)- and (+)-morphine|year=1993|last1=Hong|first1=Chang Y.|last2=Kado|first2=Noriyuki|last3=Overman|first3=Larry E.|journal=Journal of the American Chemical Society|volume=115|issue=23|pages=11028}}</ref>木泽尔·特劳纳、<ref>{{cite journal|doi=10.1002/anie.199628301|title=Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition|year=1996|last1=Mulzer|first1=Johann|last2=Dürner|first2=Gerd|last3=Trauner|first3=Dirk|journal=Angewandte Chemie International Edition in English|volume=35|issue=2324|pages=2830}}</ref>怀特、<ref>{{cite journal|doi=10.1021/jo990905z|title=Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion|year=1999|last1=White|first1=James D.|last2=Hrnciar|first2=Peter|last3=Stappenbeck|first3=Frank|journal=The Journal of Organic Chemistry|volume=64|issue=21|pages=7871}}</ref>泰伯、<ref>{{cite journal|doi=10.1021/ja027882h|title=Synthesis of (−)-Morphine|year=2002|last1=Taber|first1=Douglass F.|last2=Neubert|first2=Timothy D.|last3=Rheingold|first3=Arnold L.|journal=Journal of the American Chemical Society|volume=124|issue=42|pages=12416–7|pmid=12381175}}</ref>[[巴里·特罗斯特|特罗斯特]]、<ref>{{cite journal|doi=10.1021/ja0283394|title=Enantioselective Synthesis of (−)-Codeine and (−)-Morphine|year=2002|last1=Trost|first1=Barry M.|last2=Tang|first2=Weiping|journal=Journal of the American Chemical Society|volume=124|issue=49|pages=14542–3|pmid=12465957}}</ref>[[福山透|福山]]、<ref>{{cite journal|doi=10.1021/ol062112m|title=Total Synthesis of (±)-Morphine|year=2006|last1=Uchida|first1=Kenji|last2=Yokoshima|first2=Satoshi|last3=Kan|first3=Toshiyuki|last4=Fukuyama|first4=Tohru|journal=Organic Letters|volume=8|issue=23|pages=5311–3|pmid=17078705}}</ref>吉尤<ref>{{cite journal|doi=10.1002/chem.200800744|title=Diastereoselective Total Synthesis of (±)-Codeine|year=2008|last1=Varin|first1=Marie|last2=Barré|first2=Elvina|last3=Iorga|first3=Bogdan|last4=Guillou|first4=Catherine|journal=Chemistry - A European Journal|volume=14|issue=22|pages=6606}}</ref>和[[吉尔伯特·斯托克|斯托克]]。<ref>{{cite journal|doi=10.1021/ja9038505|title=Regiospecific and Stereoselective Syntheses of (±) Morphine, Codeine, and Thebaine via a Highly Stereocontrolled Intramolecular 4 + 2 Cycloaddition Leading to a Phenanthrofuran System|year=2009|last1=Stork|first1=Gilbert|last2=Yamashita|first2=Ayako|last3=Adams|first3=Julian|last4=Schulte|first4=Gary R.|last5=Chesworth|first5=Richard|last6=Miyazaki|first6=Yoji|last7=Farmer|first7=Jay J.|journal=Journal of the American Chemical Society|volume=131|issue=32|pages=11402–6|pmid=19624126}}</ref> |
此后,许多化学家提出了新的合成路线,其中值得注意的有以下研究者率领的团队所提出的路线:赖斯、<ref>{{cite journal|doi=10.1021/jo01303a045|title=Synthetic opium alkaloids and derivatives. A short total synthesis of (.+-.)-dihydrothebainone, (.+-.)-dihydrocodeinone, and (.+-.)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners|year=1980|last1=Rice|first1=Kenner C.|journal=The Journal of Organic Chemistry|volume=45|issue=15|pages=3135}}</ref>埃文斯、<ref>{{cite journal|doi=10.1016/S0040-4039(00)86810-0|title=Studies directed towards the total synthesis of morphine alkaloids|year=1982|last1=Evans|first1=D.A.|last2=Mitch|first2=C.H.|journal=Tetrahedron Letters|volume=23|issue=3|pages=285}}</ref>富克斯、<ref>{{cite journal|doi=10.1021/jo00255a008|title=Studies culminating in the total synthesis of (dl)-morphine|year=1988|last1=Toth|first1=J. E.|last2=Hamann|first2=P. R.|last3=Fuchs|first3=P. L.|journal=The Journal of Organic Chemistry|volume=53|issue=20|pages=4694}}</ref>帕克、<ref>{{cite journal|doi=10.1021/ja00050a075|title=Convergent synthesis of (.+-.)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (.+-.)-morphine|year=1992|last1=Parker|first1=Kathlyn A.|last2=Fokas|first2=Demosthenes|journal=Journal of the American Chemical Society|volume=114|issue=24|pages=9688}}</ref>奥尔曼、<ref>{{cite journal|doi=10.1021/ja00076a086|title=Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (-)- and (+)-dihydrocodeinone and (-)- and (+)-morphine|year=1993|last1=Hong|first1=Chang Y.|last2=Kado|first2=Noriyuki|last3=Overman|first3=Larry E.|journal=Journal of the American Chemical Society|volume=115|issue=23|pages=11028}}</ref>木泽尔·特劳纳、<ref>{{cite journal|doi=10.1002/anie.199628301|title=Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition|year=1996|last1=Mulzer|first1=Johann|last2=Dürner|first2=Gerd|last3=Trauner|first3=Dirk|journal=Angewandte Chemie International Edition in English|volume=35|issue=2324|pages=2830}}</ref>怀特、<ref>{{cite journal|doi=10.1021/jo990905z|title=Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion|year=1999|last1=White|first1=James D.|last2=Hrnciar|first2=Peter|last3=Stappenbeck|first3=Frank|journal=The Journal of Organic Chemistry|volume=64|issue=21|pages=7871}}</ref>泰伯、<ref>{{cite journal|doi=10.1021/ja027882h|title=Synthesis of (−)-Morphine|year=2002|last1=Taber|first1=Douglass F.|last2=Neubert|first2=Timothy D.|last3=Rheingold|first3=Arnold L.|journal=Journal of the American Chemical Society|volume=124|issue=42|pages=12416–7|pmid=12381175}}</ref>[[巴里·特罗斯特|特罗斯特]]、<ref>{{cite journal|doi=10.1021/ja0283394|title=Enantioselective Synthesis of (−)-Codeine and (−)-Morphine|year=2002|last1=Trost|first1=Barry M.|last2=Tang|first2=Weiping|journal=Journal of the American Chemical Society|volume=124|issue=49|pages=14542–3|pmid=12465957}}</ref>[[福山透|福山]]、<ref>{{cite journal|doi=10.1021/ol062112m|title=Total Synthesis of (±)-Morphine|year=2006|last1=Uchida|first1=Kenji|last2=Yokoshima|first2=Satoshi|last3=Kan|first3=Toshiyuki|last4=Fukuyama|first4=Tohru|journal=Organic Letters|volume=8|issue=23|pages=5311–3|pmid=17078705}}</ref>吉尤<ref>{{cite journal|doi=10.1002/chem.200800744|title=Diastereoselective Total Synthesis of (±)-Codeine|year=2008|last1=Varin|first1=Marie|last2=Barré|first2=Elvina|last3=Iorga|first3=Bogdan|last4=Guillou|first4=Catherine|journal=Chemistry - A European Journal|volume=14|issue=22|pages=6606}}</ref>和[[吉尔伯特·斯托克|斯托克]]。<ref>{{cite journal|doi=10.1021/ja9038505|title=Regiospecific and Stereoselective Syntheses of (±) Morphine, Codeine, and Thebaine via a Highly Stereocontrolled Intramolecular 4 + 2 Cycloaddition Leading to a Phenanthrofuran System|year=2009|last1=Stork|first1=Gilbert|last2=Yamashita|first2=Ayako|last3=Adams|first3=Julian|last4=Schulte|first4=Gary R.|last5=Chesworth|first5=Richard|last6=Miyazaki|first6=Yoji|last7=Farmer|first7=Jay J.|journal=Journal of the American Chemical Society|volume=131|issue=32|pages=11402–6|pmid=19624126}}</ref> |
||
2016年11月10日 (四) 07:21的版本

吗啡全合成是指化学中吗啡样生物碱的合成描述了天然吗啡喃类生物碱的全合成,其包括可待因,吗啡,奥利平,和蒂巴因及其密切相关的半合成类似物丁丙诺啡,氢可酮,异可待因,纳曲酮,纳洛酮,纳布啡和羟考酮[1][2]。
吗啡的结构并不是特别复杂,然而,相邻结合原子的静电极化在整个结构中不均匀交替。这种“不一致的连接性”使得键的形成更加困难,并且因此使应用于该分子家族的任何合成策略明显的复杂化[2]。
首次全合成由马歇尔·D·盖茨于1952年完成,并被视为这个领域中的经典之作。[3][3]
此后,许多化学家提出了新的合成路线,其中值得注意的有以下研究者率领的团队所提出的路线:赖斯、[4]埃文斯、[5]富克斯、[6]帕克、[7]奥尔曼、[8]木泽尔·特劳纳、[9]怀特、[10]泰伯、[11]特罗斯特、[12]福山、[13]吉尤[14]和斯托克。[15]
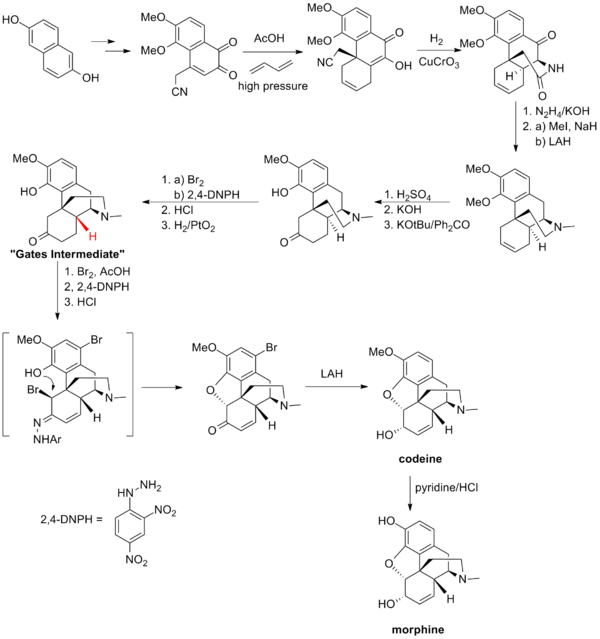
盖茨的合成路线
盖茨的吗啡全合成路线是在全合成中运用Diels-Alder反应的首个例子。
参考文献
- ^ Chida N. Recent advances in the synthesis of morphine and related alkaloids. Top Curr Chem. 2011, 299: 1–28. PMID 21630507. doi:10.1007/128_2010_73.
- ^ 2.0 2.1 Rinner U, Hudlicky T. Synthesis of morphine alkaloids and derivatives. Top Curr Chem. 2012, 309: 33–66. PMID 21547687. doi:10.1007/128_2011_133.
Morphine's synthesis remains a serious challenge to this day.
- ^ 3.0 3.1 Gates, Marshall; Tschudi, Gilg. Journal of the American Chemical Society. 1956, 78 (7): 1380. doi:10.1021/ja01588a033. 缺少或
|title=为空 (帮助) - ^ Rice, Kenner C. Synthetic opium alkaloids and derivatives. A short total synthesis of (.+-.)-dihydrothebainone, (.+-.)-dihydrocodeinone, and (.+-.)-nordihydrocodeinone as an approach to a practical synthesis of morphine, codeine, and congeners. The Journal of Organic Chemistry. 1980, 45 (15): 3135. doi:10.1021/jo01303a045.
- ^ Evans, D.A.; Mitch, C.H. Studies directed towards the total synthesis of morphine alkaloids. Tetrahedron Letters. 1982, 23 (3): 285. doi:10.1016/S0040-4039(00)86810-0.
- ^ Toth, J. E.; Hamann, P. R.; Fuchs, P. L. Studies culminating in the total synthesis of (dl)-morphine. The Journal of Organic Chemistry. 1988, 53 (20): 4694. doi:10.1021/jo00255a008.
- ^ Parker, Kathlyn A.; Fokas, Demosthenes. Convergent synthesis of (.+-.)-dihydroisocodeine in 11 steps by the tandem radical cyclization strategy. A formal total synthesis of (.+-.)-morphine. Journal of the American Chemical Society. 1992, 114 (24): 9688. doi:10.1021/ja00050a075.
- ^ Hong, Chang Y.; Kado, Noriyuki; Overman, Larry E. Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (-)- and (+)-dihydrocodeinone and (-)- and (+)-morphine. Journal of the American Chemical Society. 1993, 115 (23): 11028. doi:10.1021/ja00076a086.
- ^ Mulzer, Johann; Dürner, Gerd; Trauner, Dirk. Formal Total Synthesis of(—)-Morphine by Cuprate Conjugate Addition. Angewandte Chemie International Edition in English. 1996, 35 (2324): 2830. doi:10.1002/anie.199628301.
- ^ White, James D.; Hrnciar, Peter; Stappenbeck, Frank. Asymmetric Total Synthesis of (+)-Codeine via Intramolecular Carbenoid Insertion. The Journal of Organic Chemistry. 1999, 64 (21): 7871. doi:10.1021/jo990905z.
- ^ Taber, Douglass F.; Neubert, Timothy D.; Rheingold, Arnold L. Synthesis of (−)-Morphine. Journal of the American Chemical Society. 2002, 124 (42): 12416–7. PMID 12381175. doi:10.1021/ja027882h.
- ^ Trost, Barry M.; Tang, Weiping. Enantioselective Synthesis of (−)-Codeine and (−)-Morphine. Journal of the American Chemical Society. 2002, 124 (49): 14542–3. PMID 12465957. doi:10.1021/ja0283394.
- ^ Uchida, Kenji; Yokoshima, Satoshi; Kan, Toshiyuki; Fukuyama, Tohru. Total Synthesis of (±)-Morphine. Organic Letters. 2006, 8 (23): 5311–3. PMID 17078705. doi:10.1021/ol062112m.
- ^ Varin, Marie; Barré, Elvina; Iorga, Bogdan; Guillou, Catherine. Diastereoselective Total Synthesis of (±)-Codeine. Chemistry - A European Journal. 2008, 14 (22): 6606. doi:10.1002/chem.200800744.
- ^ Stork, Gilbert; Yamashita, Ayako; Adams, Julian; Schulte, Gary R.; Chesworth, Richard; Miyazaki, Yoji; Farmer, Jay J. Regiospecific and Stereoselective Syntheses of (±) Morphine, Codeine, and Thebaine via a Highly Stereocontrolled Intramolecular 4 + 2 Cycloaddition Leading to a Phenanthrofuran System. Journal of the American Chemical Society. 2009, 131 (32): 11402–6. PMID 19624126. doi:10.1021/ja9038505.
