硼化钽
外观

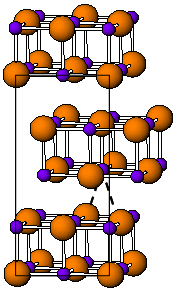
硼化钽(化学式:TaB,TaB2)是钽和硼组成的化合物,它的硬度极高。
性质
[编辑]TaB和TaB2薄片和晶体的维氏硬度为30GPa。[1][2][3] 在700℃以下时,这些物质很耐氧化和酸腐蚀,性质是稳定的。[1][3]
TaB2与大多数二硼化物(AlB2, MgB2等)具有相同的六方形结构。[4] 这些硼化物具有以下分类空间:TaB(正交晶系,碘化亚铊型,CMCM),Ta5B6 (Cmmm), Ta3B4 (Immm), TaB2 (六方晶系, 二硼化铝型,P6/mmm)。[3]
制取
[编辑]采用区域熔炼法可制得TaB,Ta5B6,Ta3B4或TaB2单晶(直径约1cm,长度约6cm)。[2][3]
使用TaCl5-BCl3-H2-Ar混合气体在540~800°C范围内时沉积硼化钽薄片。在源气流量比(BCl3/TaCl5)为6,温度在600°C以上时沉积TaB2(单晶)。在BCl3/TaCl5 = 2/4 ,温度在600-700°C时沉积TaB(单晶)。[1]
以NaBH4和Ta2O5为原料,在700~900°C下,摩尔质量比M:B为1:4时,在氩气中还原30分钟,就可以合成TaB2。[5]
- Ta2O5 + 6.5 NaBH4 → 2 TaB2 + 4 Na(g,l) + 2.5 NaBO2+ 13 H2(g)
参考文献
[编辑]- ^ 1.0 1.1 1.2 Motojima, Seiji; Kito, Kazuhito; Sugiyama, Kohzo. Low-temperature deposition of TaB and TaB2 by chemical vapor deposition. Journal of Nuclear Materials (Elsevier BV). 1982, 105 (2-3): 262–268. ISSN 0022-3115. doi:10.1016/0022-3115(82)90383-x.
- ^ 2.0 2.1 Otani, S; Korsukova, M.M; Mitsuhashi, T. Floating zone growth and high-temperature hardness of NbB2 and TaB2 single crystals. Journal of Crystal Growth (Elsevier BV). 1998, 194 (3-4): 430–433. ISSN 0022-0248. doi:10.1016/s0022-0248(98)00691-5.
- ^ 3.0 3.1 3.2 3.3 Okada, Shigeru; Kudou, Kunio; Higashi, Iwarni; Lundström, Torsten. Single crystals of TaB, Ta5B6, Ta3B4 and TAB2, as obtained from high-temperature metal solutions, and their properties. Journal of Crystal Growth (Elsevier BV). 1993, 128 (1-4): 1120–1124. ISSN 0022-0248. doi:10.1016/s0022-0248(07)80109-6.
- ^ Chen, Xing-Qiu; Fu, C. L.; Krčmar, M.; Painter, G. S. Electronic and Structural Origin of Ultraincompressibility of5dTransition-Metal DiboridesMB2(M=W, Re, Os). Physical Review Letters (American Physical Society (APS)). 2008-05-16, 100 (19): 196403. ISSN 0031-9007. doi:10.1103/physrevlett.100.196403.
- ^ Zoli, Luca; Galizia, Pietro; Silvestroni, Laura; Sciti, Diletta. Synthesis of group IV and V metal diboride nanocrystals via borothermal reduction with sodium borohydride (PDF). Journal of the American Ceramic Society. 2018-01-23, 101 (6): 2627–2637 [2020-07-22]. doi:10.1111/jace.15401. (原始内容存档 (PDF)于2019-05-04).
