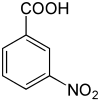
3-硝基苯甲酸
外观
| 3-硝基苯甲酸 | |||
|---|---|---|---|
| |||
| IUPAC名 3-Nitrobenzoic acid | |||
| 别名 | 间硝基苯甲酸 m-硝基苯甲酸 | ||
| 识别 | |||
| CAS号 | 121-92-6 | ||
| PubChem | 8497 | ||
| ChemSpider | 8183 | ||
| SMILES |
| ||
| InChI |
| ||
| InChIKey | AFPHTEQTJZKQAQ-UHFFFAOYAS | ||
| 性质 | |||
| 化学式 | C7H5NO4 | ||
| 摩尔质量 | 167.12 g·mol−1 | ||
| 外观 | 浅黄色透明晶体[1] | ||
| 密度 | 1.498 g·cm−3[1] | ||
| 熔点 | 140 °C(413 K)[2] | ||
| 溶解性(水) | 0.24 g/100 mL(15 °C) | ||
| pKa | 3.48±0.10(25 °C)[3] | ||
| 磁化率 | -80.22·10−6 cm3/mol | ||
| 相关物质 | |||
| 相关化学品 | 苯甲酸、硝基苯 2-硝基苯甲酸、4-硝基苯甲酸 3-硝基甲苯 | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
3-硝基苯甲酸是一种有机化合物,化学式为C7H5NO4,它是苯甲酸苯环间位上的氢被硝基取代的产物,是硝基苯甲酸的同分异构体之一。
制备
[编辑]3-硝基苯甲酸可由苯甲酸在较低温度下的硝化反应制备,其副产物为2-硝基苯甲酸(20%)和4-硝基苯甲酸(1.5%)。[4]苯甲酸甲酯硝化后水解也可得到3-硝基苯甲酸,但产率较低。[5]
其它制备方法还包括3-硝基苯甲醇[6]、3-硝基苯甲醛[7]或3-硝基甲苯[8]的氧化反应及3-硝基苯甲腈的水解反应[9]。
反应
[编辑]3-硝基苯甲酸具有酸的通性,如和氢氧化钾反应,得到3-硝基苯甲酸钾。[10]
3-硝基苯甲酸和氯化亚砜回流反应,得到3-硝基苯甲酰氯。[11]它和甲醇在酸催化下反应,得到3-硝基苯甲酸甲酯。[12]它被还原剂(如氢等)还原,可以得到3-氨基苯甲酸。[13]
参考文献
[编辑]- ^ 1.0 1.1 Dhaneshwar, N. N.; Kulkarni, A. G.; Tavale, S. S.; Pant, L. M. The crystal structure of a second modification of m -nitrobenzoic acid. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 1975-07-01, 31 (7): 1978–1980. ISSN 0567-7408. doi:10.1107/S0567740875006620.
- ^ Mayuranathan, P. S. Thermal decomposition of the mercuric and silver salts of the isomeric methyl hydrogen 3-nitrophthalates. Journal of the Chemical Society, 1957. 493-495. ISSN: 0368-1769.
- ^ Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02. Retrieved from SciFinder. [2020-07-13]
- ^ Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 2000-06-15. ISBN 978-3-527-30385-4. doi:10.1002/14356007.a03_555.
- ^ m-NITROBENZOIC ACID. Organic Syntheses. 1923, 3: 73. doi:10.15227/orgsyn.003.0073.
- ^ Banerjee, Amalendu; Dutt, Sachchidananda; Banerjee, Gopal Chandra; Hazra, Banasri; Datta, Hasi; Banerjee, Santanu. Studies on the oxidation of aromatic aldehydes and arylmethanols by vanadate and dichromate in dilute perchloric acid. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1990. 29B (3): 257-262. ISSN: 0376-4699.
- ^ Hunsen, Mo. Carboxylic Acids from Primary Alcohols and Aldehydes by a Pyridinium Chlorochromate Catalyzed Oxidation. Synthesis. 2005-07-20, 2005 (15): 2487–2490. ISSN 0039-7881. doi:10.1055/s-2005-872085.
- ^ Yamazaki, Shigekazu. Chromium(VI) Oxide-Catalyzed Benzylic Oxidation with Periodic Acid. Organic Letters, 1999. 1 (13): 2129-2132. ISSN: 1523-7060. DOI: 10.1021/ol991175k.
- ^ Meth-Cohn, Otto; Wang, Mei-Xiang. An in-depth study of the biotransformation of nitriles into amides and/or acids using Rhodococcus rhodochrous AJ270. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry, 1997. 8. 1099-1104. ISSN: 0300-922X.
- ^ Rouhi-Saadabad, Hamed; Akhlaghinia, Batool. Facile and direct synthesis of symmetrical acid anhydrides using a newly prepared powerful and efficient mixed reagent. Chemical Papers. 2015-01-01, 69 (3). ISSN 1336-9075. doi:10.1515/chempap-2015-0042.
- ^ Xu, Yiming; Liang, Pengyun; Rashid, Haroon ur; Wu, Lichuan; Xie, Peng; Wang, Haodong; Zhang, Shuyan; Wang, Lisheng; Jiang, Jun. Design, synthesis, and biological evaluation of matrine derivatives possessing piperazine moiety as antitumor agents. Medicinal Chemistry Research. 2019, 28 (10): 1618–1627. ISSN 1054-2523. doi:10.1007/s00044-019-02398-2.
- ^ Zaheer, Muhammad; Zia-Ur-Rehman, Muhammad; Rahman, Salma; Ahmed, Naveed; Chaudhary, Muhammad Nawaz. Microwave assisted synthesis of biologically active 4-hydroxy-N'-(phenylcarbonyl)-2H-1,2-benzothiazine-3-carbohydrazide 1,1-dioxide derivatives.. Journal of the Chilean Chemical Society. 2012, 57 (4): 1492–1496. ISSN 0717-9707. doi:10.4067/S0717-97072012000400031.
- ^ Rahman, MD Taifur; Wharry, Scott; Smyth, Megan; Manyar, Haresh; Moody, Thomas S. FAST Hydrogenations as a Continuous Platform for Green Aromatic Nitroreductions. Synlett. 2020, 31 (06): 581–586. ISSN 0936-5214. doi:10.1055/s-0037-1610751.