討論:分子內親核取代反應
|
本條目頁依照頁面評級標準評為小作品級。 本條目頁屬於下列維基專題範疇: |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||
未翻譯內容
[編輯]未翻譯內容如下--Flame 歡迎泡茶 2011年5月1日 (日) 00:28 (UTC)
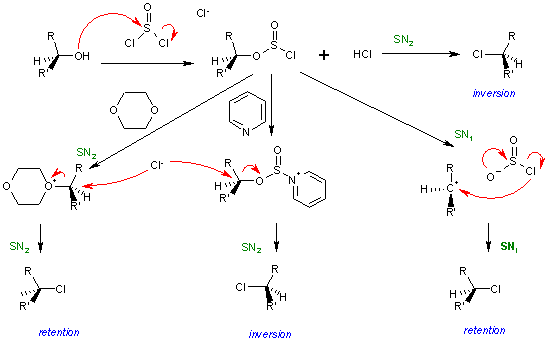
A typical representative organic reaction displaying this mechanism is the chlorination of alcohols with thionyl chloride, or the decomposition of alkyl chloroformates, the main feature is retention of stereochemical configuration. Some examples for this reaction were reported by Edward S. Lewis and Charles E. Boozer in 1952.[1] Mechanistic and kinetic studies were reported few years later by various researchers.[2] [3]
Thionyl chloride first reacts with the alcohol to form an alkyl chloro sulfite, actually forming an intimate ion pair. The second step is the concerted loss of a sulfur dioxide molecule and its replacement by the chloride, which was attached to the sulfite group. The difference between SN1 and SNi is actually that the ion pair is not completely dissociated, and therefore no real carbocation is formed, which else would lead to a racemisation.
This reaction type is linked to many forms of neighbouring group participation, for instance the reaction of the sulfur or nitrogen lone pair in sulfur mustard or nitrogen mustard to form the cationic intermediate.
This reaction mechanism is supported by the observation that addition of pyridine to the reaction leads to inversion. The reasoning behind this finding is that pyridine reacts with the intermediate sulfite replacing chlorine. The dislodged chloride has to resort to nucleophilic attack from the rear as in a regular nucleophilic substitution.[2]
In the complete picture for this reaction the sulfite reacts with a chlorine ion in a standard SN2 reaction with inversion of configuration. When the solvent is also a nucleophile such as dioxane two successive SN2 reactions take place and the stereochemistry is again retention. With standard SN1 reaction conditions the reaction outcome is retention via a competing SNi mechanism and not racemization and with pyridine added the result is again inversion. [4] [2]
- ^ Lewis, E.S.; Boozer, C.E. J. Am. Chem. Soc. 1952, 74, 308.
- ^ 2.0 2.1 2.2 Cram, D. J., J. Am. Chem. Soc. 1953, 75, 332-338.
- ^ Lee, C. C. ; Clayton, J. W.; Lee, D. G.; Finlayson, A. J. Tetrahedron, 1962, 18 1395-1402
- ^ Smith, M. B; March, J., March’s Advanced Organic Chemistry, 6th ed.; John Wiley and Sons: 2007, pp. 468 – 469

