白花丹素
外观
| 白花丹素 | |
|---|---|
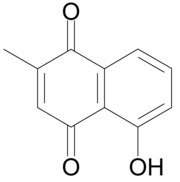
| |

| |
| IUPAC名 5-hydroxy-2-methyl-naphthalene-1,4-dione | |
| 识别 | |
| CAS号 | 481-42-5 |
| PubChem | 10205 |
| ChemSpider | 9790 |
| SMILES |
|
| InChI |
|
| InChIKey | VCMMXZQDRFWYSE-UHFFFAOYAB |
| ChEBI | 8273 |
| KEGG | C10387 |
| 性质 | |
| 化学式 | C11H8O3 |
| 摩尔质量 | 188.17942 g/mol g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
白花丹素(Plumbagin,5-hydroxy-2-methyl-1,4-naphthoquinone)为萘醌衍生物,为黄色染料。[1]其得名于植物白花丹(Plumbago)。[2]白花丹素常见于茅膏菜和猪笼草体内。[3]
药理性质
[编辑]各种细胞和动物模型试验中得出了以下药理学特性:
参考文献
[编辑]- ^ Black Walnut (页面存档备份,存于互联网档案馆). Drugs.com.
- ^ Van der Vijver, L.M. 1972. Distribution of plumbagin in the Plumbaginaceae. Phytochemistry 11: 3247–3248.
- ^ Wang, W., X. Luo, & H. Li 2010. Terahertz and infrared spectra of plumbagin, juglone, and menadione. Carnivorous Plant Newsletter 39(3): 82–88.
- ^ Didry, N., L. Dubrevil & M. Pinkas 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Die Pharmazie 49(9): 681–683.
- ^ Paiva, S.R.d., M.R. Figueiredo, T. V. Aragão, M.A.C. Kaplan 2003. Antimicrobial Activity in Vitro of Plumbagin Isolated from Plumbago Species.PDF (511 KiB) Mem Inst Oswaldo Cruz 98(7): 959–961.
- ^ Likhitwitayawuid, K., R. Kaewamatawong, N. Ruangrungsi & J. Krungkrai 1998. Antimalarial naphthoquinones from Nepenthes thorelii. Planta Medica 64(3): 237–241.
- ^ Checker R., Sharma D., Sandur S.K., Subrahmanyam G., Krishnan S., Poduval T.B., Sainis K.B. "Plumbagin inhibits proliferative and inflammatory responses of T cells independent of ROS generation but by modulating intracellular thiols" Journal of Cellular Biochemistry 2010 110:5 (1082-1093)
- ^ Parimala, R. & P. Sachdanandam 1993. Effect of plumbagin on some glucose metabolizing enzymes studied in rats in experimental hepatoma. Molecular and Cellular Biochemistry 12(1): 59–63.
- ^ Hsu, Y.-L., C.-Y. Cho, P.-L. Kuo, Y.-T. Huang & C.-C. Lin 2006. Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) Induces Apoptosis and Cell Cycle Arrest in A549 Cells through p53 Accumulation via c-Jun NH2-Terminal Kinase-Mediated Phosphorylation at Serine 15 in Vitro and in Vivo. Journal of Pharmacology and Experimental Therapeutics 318(2): 484–494. doi:10.1124/jpet.105.098863 PMID 16632641
- ^ Itoigawa, M., K. Takeya & H. Furukawa 1991. Cardiotonic action of plumbagin on guinea-pig papillary muscle. Planta Medica 57(4): 317–319.
- ^ McKallip, Robert J. Lombard, Catherine. Sun, Jingping. Ramakrishnan, Rupal. "Plumbagin-induced apoptosis in lymphocytes is mediated through increased reactive oxygen species production, upregulation of Fas, and activation of the caspase cascade."Toxicology & Applied Pharmacology. 247(1):41-52, 2010 Aug 15.
- ^ Bhargava, S.K. 1984. Effects of plumbagin on reproductive function of male dog. Indian Journal of Experimental Biology 22(3): 153–156.
- ^ Son TG. Camandola S. Arumugam TV. Cutler RG. Telljohann RS. Mughal MR. Moore TA. Luo W. Yu QS. Johnson DA. Johnson JA. Greig NH. Mattson MP. Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia.
- ^ Ding, Y., Z.-J. Chen, S. Liu, D. Che, M. Vetter, C.-H. Chang 2005. Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. Journal of Pharmacy and Pharmacology 57(1): 111.
