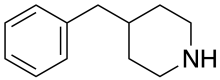
4-苄基哌啶
 | |
 | |
| 法律規範狀態 | |
|---|---|
| 法律規範 |
|
| 識別資訊 | |
| |
| CAS號 | 31252-42-3 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.045.926 |
| 化學資訊 | |
| 化學式 | C12H17N |
| 摩爾質量 | 175.28 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
4-苄基哌啶(化學式:C12H17N)是一種單胺釋放劑,釋放多巴胺的選擇性是血清素的20到48倍。它可由哌啶和苯甲醛反應,再經催化氫化得到。[1]它和三氟乙酸酐在二氯甲烷中反應,可以得到N-三氟甲酰基-4-苄基哌啶。[2]
參考文獻[編輯]
- ^ Zeng Hong, Xin Ge, Shaodong Zhou. Underlying Mechanisms of Reductive Amination on Pd-Catalysts: The Unique Role of Hydroxyl Group in Generating Sterically Hindered Amine. International Journal of Molecular Sciences. 2022-07-10, 23 (14): 7621 [2023-03-07]. ISSN 1422-0067. doi:10.3390/ijms23147621. (原始內容存檔於2022-10-01) (英語).
- ^ Karine Alarcon, Adeline Martz, Laetitia Mony, Jacques Neyton, Pierre Paoletti, Maurice Goeldner, Bernard Foucaud. Reactive derivatives for affinity labeling in the ifenprodil site of NMDA receptors. Bioorganic & Medicinal Chemistry Letters. 2008-05, 18 (9): 2765–2770 [2023-03-07]. doi:10.1016/j.bmcl.2008.04.019. (原始內容存檔於2022-08-02) (英語).
