二茂铑:修订间差异
无编辑摘要 |
无编辑摘要 |
||
| 第45行: | 第45行: | ||
}} |
}} |
||
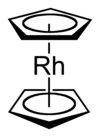
'''二茂铑'''({{lang-en|Rhodocene}}),也称'''双(η<sup>5</sup>-环戊二烯基)合铑(II)''',分子式为'''[Rh(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub>]'''。分子由两个η<sup>5</sup>的环戊二烯配体(哈普托数为5,表示环戊二烯基有五个原子参与配位)和一个铑原子构成,铑原子处在平行的两个环戊二烯基中间形成“[[三明治]]”夹心结构。二茂铑的自由基存在于150°C以上的条件下,可利用[[液氮]](−196°C)急速冷却的方法捕获到它。室温下,两个二茂铑自由基的环戊二烯基键合形成黄色的[[二聚体]]。<ref name="El_Murr_1979"/><ref name="Fischer_1966" |
'''二茂铑'''({{lang-en|Rhodocene}}),也称'''双(η<sup>5</sup>-环戊二烯基)合铑(II)''',分子式为'''[Rh(C<sub>5</sub>H<sub>5</sub>)<sub>2</sub>]'''。分子由两个η<sup>5</sup>的环戊二烯配体(哈普托数为5,表示环戊二烯基有五个原子参与配位)和一个铑原子构成,铑原子处在平行的两个环戊二烯基中间形成“[[三明治]]”夹心结构。二茂铑的自由基存在于150°C以上的条件下,可利用[[液氮]](−196°C)急速冷却的方法捕获到它。室温下,两个二茂铑自由基的环戊二烯基键合形成黄色的[[二聚体]]。<ref name="El_Murr_1979"/><ref name="Fischer_1966"/><ref name="Keller_1967"/> |
||
有机金属化学的历史可以追溯到在19世纪发现的[[蔡司盐]]和[[路德维希·蒙德]]发现的[[四羰基镍]]。由于当时化学家所理解的化学键模型不适用于解释这些化合物,因此这些化合物的出现对化学家提出了挑战。进一步的挑战出现在像[[二茂铁]]、二茂铑这类[[茂金属]]化合物被发现之后。当时出乎意料的是,诸如二茂铁和与其有相似化学结构并带有一个正电荷的二茂铑、二茂钴、二茂铱阳离子都有比较稳定的化学性质。对包括这些化合物在内的有机金属化学的研究,催生出新的化学键模型,用它可以解释这些有机金属化合物的形成和稳定性。鉴于[[杰弗里·威尔金森]]和[[恩斯特·奥托·菲舍尔]]在包括二茂铑在内的夹心化合物方面的研究工作中做出的杰出贡献,两人在1977年被授予诺贝尔化学奖。 |
有机金属化学的历史可以追溯到在19世纪发现的[[蔡司盐]]<ref name="Zeise review"/><ref name="Zeise discovery"/>和[[路德维希·蒙德]]发现的[[四羰基镍]]<ref name="Crabtree"/>。由于当时化学家所理解的化学键模型不适用于解释这些化合物,因此这些化合物的出现对化学家提出了挑战。进一步的挑战出现在像[[二茂铁]]、二茂铑这类[[茂金属]]化合物被发现之后。当时出乎意料的是,诸如二茂铁和与其有相似化学结构并带有一个正电荷的二茂铑、二茂钴、二茂铱阳离子都有比较稳定的化学性质。对包括这些化合物在内的有机金属化学的研究,催生出新的化学键模型,用它可以解释这些有机金属化合物的形成和稳定性。鉴于[[杰弗里·威尔金森]]和[[恩斯特·奥托·菲舍尔]]在包括二茂铑在内的夹心化合物方面的研究工作中做出的杰出贡献,两人在1977年被授予诺贝尔化学奖。 |
||
== 参考资料 == |
|||
{{Reflist|2|refs= |
|||
<ref name="astruc-2007">{{cite book |last= Astruc |first=D. |authorlink= Didier Astruc |year= 2007 |title= Organometallic Chemistry and Catalysis |location= Berlin |publisher= Springer |pages= 41–43 |isbn= 978-3-540-46128-9 |url= http://books.google.com.au/books?id=aVyfo52-nRsC&pg=PA41}}</ref> |
|||
<ref name="baghurst-1989">{{Cite journal |last1= Baghurst |first1= D. R. |last2= Mingos |first2= D. M. P. |last3= Watson |first3= M. J. |year= 1989 |title= Application of Microwave Dielectric Loss Heating Effects for the Rapid and Convenient Synthesis of Organometallic Compounds |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 368 |issue= 3 |pages= C43–C45 |doi= 10.1016/0022-328X(89)85418-X}}</ref> |
|||
<ref name="baghurst-1990">{{Cite journal |last1= Baghurst |first1= D. R. |last2= Mingos |first2= D. M. P. |year= 1990 |title= Design and Application of a Reflux Modification for the Synthesis of Organometallic Compounds Using Microwave Dielectric Loss Heating Effects |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 384 |issue= 3 |pages= C57–C60 |doi= 10.1016/0022-328X(90)87135-Z}}</ref> |
|||
<ref name="Buchholz_1994">{{Cite journal |last1= Buchholz |first1= D. |last2= Astruc |first2= D. |year= 1994 |title= The First Decaisopropylmetallocene – One-Pot Synthesis of [Rh(C<sub>5</sub>''i''Pr<sub>5</sub>)<sub>2</sub>]PF<sub>6</sub> from [Rh(C<sub>5</sub>Me<sub>5</sub>)<sub>2</sub>]PF<sub>6</sub> by Formation of 20 Carbon–Carbon Bonds |journal= [[Angewandte Chemie|Angew. Chem. Int. Ed.]] |volume= 33 |issue= 15–16 |pages= 1637–1639 |doi =10.1002/anie.199416371 }}</ref> |
|||
<ref name="Chromatographia">{{cite journal |last1= Andre |first1=M. |last2= Schottenberger |first2=H. |last3= Tessadri |first3=R. |last4= Ingram |first4=G. |last5= Jaitner |first5=P. |last6= Schwarzhans |first6=K. E. |year=1990 |title= Synthesis and Preparative HPLC-Separation of Heteronuclear Oligometallocenes. Isolation of Cations of Rhodocenylferrocene, 1,1'-Dirhodocenylferrocene, and 1-Cobaltocenyl-1'-rhodocenylferrocene |journal= [[Chromatographia]] |volume= 30 |issue= 9–10 |pages= 543–545 |doi= 10.1007/BF02269802}}</ref> |
|||
<ref name="clarke-1999">{{cite book |author1= Clarke, M. J. |author2= Sadler, P. J. |year= 1999 |title= Metallopharmaceuticals: Diagnosis and therapy |location= Berlin |publisher= Springer |isbn= 3-540-65308-2}}</ref> |
|||
<ref name="clarke-2002">{{Cite journal |last1= Clarke |first1= M. J. |year= 2002 |title= Ruthenium Metallopharmaceuticals |journal= Coord. Chem. Rev. |volume= 232 |issue= 1–2 |pages= 69–93 |doi= 10.1016/S0010-8545(02)00025-5}}</ref> |
|||
<ref name="cobaltocenium">{{cite journal |last1= Connelly |first1=N. G. |author2= Geiger |first2=W. E. |title= Chemical Redox Agents for Organometallic Chemistry |journal= [[Chemical Reviews|Chem. Rev.]] |year= 1996 |volume= 96 |issue= 2 |pages= 877–910 |doi= 10.1021/cr940053x |pmid= 11848774}}</ref> |
|||
<ref name="Collins_1995">{{Cite journal |last1= Collins |first1= J. E. |last2= Castellani |first2= M. P. |last3= Rheingold |first3= A. L. |last4= Miller |first4= E. J. |last5= Geiger |first5= W. E. |last6= Rieger |first6= A. L. |last7= Rieger |first7= P. H. |year= 1995 |title= Synthesis, Characterization, and Molecular-Structure of Bis(tetraphenylcyclopentadienyl)rhodium(II) |journal= Organometallics |volume= 14 |issue= 3 |pages= 1232–1238 |doi= 10.1021/om00003a025}}</ref> |
|||
<ref name="Crabtree">{{cite book |last= Crabtree |first= R. H.|year= 2009 |title= The Organometallic Chemistry of the Transition Metals |edition= 5th |location= Hoboken, NJ |publisher= John Wiley and Sons |isbn= 978-0-470-25762-3 |page= 2 |url= http://books.google.com.au/books?id=WLb962AKlSEC&pg=PA2 |quote= An industrial application of transition metal organometallic chemistry appeared as early as the 1880s, when Ludwig Mond showed that nickel can be purified by using CO to pick up nickel in the form of gaseous Ni(CO)<sub>4</sub> that can easily be separated from solid impurities and later be thermally decomposed to give pure nickel.<p>... Recent work has shown the existence of a growing class of metalloenzymes having organometallic ligand environments – considered as the chemistry of metal ions having C-donor ligands such as CO or the methyl group}}</ref> |
|||
<ref name="decamethylferrocenium">{{cite journal |last1= Noviandri |first1=I. |last2= Brown |first2=K. N. |last3= Fleming |first3=D. S. |last4= Gulyas |first4=P. T. |last5= Lay |first5=P. A. |last6= Masters |first6=A. F. |last7= Phillips |first7=L. |year= 1999 |title= The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer |journal= [[Journal of Physical Chemistry B|J. Phys. Chem. B]] |volume= 103 |issue= 32 |pages= 6713–6722 |doi= 10.1021/jp991381}}</ref> |
|||
<ref name="decamethylrhodocenium">{{cite journal |last1= Gusev |first1=O. V. |last2= Denisovich |first2=L. I. |last3= Peterleitner |first3=M. G. |last4= Rubezhov|first4=A. Z. |first5=Nikolai A. |last5= Ustynyuk |last6= Maitlis |first6=P. M. |authorlink6= Peter Maitlis |year= 1993 |title= Electrochemical Generation of 19- and 20-electron Rhodocenium Complexes and Their Properties |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 452 |issue= 1–2 |pages= 219–222 |doi= 10.1016/0022-328X(93)83193-Y}}</ref> |
|||
<ref name="decamethyliridocenium">{{cite journal |last1= Gusev |first1=O. V. |last2= Peterleitner |first2=M. G. |last3= Ievlev |first3=M. A. |last4= Kal'sin|first4=A. M. |last5= Petrovskii|first5=P. V. |last6= Denisovich |first6=L. I. |first7=Nikolai A. |last7= Ustynyuk |year= 1997 |title= Reduction of Iridocenium Salts [Ir(η<sup>5</sup>-C<sub>5</sub>Me<sub>5</sub>)(η<sup>5</sup>-L)]+ (L= C<sub>5</sub>H<sub>5</sub>, C<sub>5</sub>Me<sub>5</sub>, C<sub>9</sub>H<sub>7</sub>); Ligand-to-Ligand Dimerisation Induced by Electron Transfer |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 531 |issue= 1–2 |pages= 95–100 |doi= 10.1016/S0022-328X(96)06675-2}}</ref> |
|||
<ref name="Dewar-Chatt-Duncanson">{{cite journal |last= Mingos |first=D. M. P. |year= 2001 |title= A Historical Perspective on Dewar's Landmark Contribution to Organometallic Chemistry |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 635 |issue= 1–2 |pages= 1–8 |doi= 10.1016/S0022-328X(01)01155-X}}</ref> |
|||
<ref name="eiland-1952">{{Cite journal |first1= P. F. |last1= Eiland |first2= R. |last2= Pepinsky |year= 1952 |title= X-ray Examination of Iron Biscyclopentadienyl |journal= [[美国化学会志|J. Am. Chem. Soc.]] |volume= 74 |issue= 19 |page= 4971 |doi= 10.1021/ja01139a527}}</ref> |
|||
<ref name="El_Murr_1979">{{Cite journal |last1= El Murr |first1= N. |last2= Sheats |first2= J. E. |last3= Geiger |first3= W. E. |last4= Holloway |first4= J. D. L. |year= 1979 |title= Electrochemical Reduction Pathways of the Rhodocenium Ion. Dimerization and Reduction of Rhodocene |journal= Inorg. Chem. |volume= 18 |issue= 6 |pages= 1443–1446 |doi= 10.1021/ic50196a007}}</ref> |
|||
<ref name="EOFischer">{{Cite journal |last1= Fischer |first1= E. O. |authorlink1= 恩斯特·奥托·菲舍尔 |last2= Pfab |first2= W. |title= Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels |journal= Z. Anorg. Allg. Chem. |language= German |year= 1952 |volume= 7 |issue= 6 |pages= 377–379 |doi= 10.1002/zaac.19532740603}}</ref> |
|||
<ref name="FerroceneHistory">{{Cite journal |first1= A. |last2= Pelegrino |first2= A. C. |last3= Darin |first3= V. A. |year= 2004 |title= Ferrocene: 50 Years of Transition Metal Organometallic Chemistry — From Organic and Inorganic to Supramolecular Chemistry |last1= Federman Neto |journal= ChemInform |volume= 35 |issue= 43 |doi= 10.1002/chin.200443242}} (Abstract; original published in ''Trends Organomet. Chem.'', '''4''':147–169, 2002)</ref> |
|||
<ref name="ferrocene bonding">{{cite book |author1= Mehrotra, R. C. |author2= Singh, A. |year= 2007 |title= Organometallic Chemistry: A Unified Approach |edition= 2nd |location= New Delhi |publisher= New Age International |pages= 261–267 |isbn= 978-81-224-1258-1 |url= http://books.google.com.au/books?id=NSQy3mFKRM8C&pg=PA262}}</ref> |
|||
<ref name="ferrocenium redox couple">{{cite journal |last1= Pavlishchuk |first1=V. V. |last2= Addison |first2=A. W. |year= 2000 |title= Conversion Constants for Redox Potentials Measured Versus Different Reference Electrodes in Acetonitrile Solutions at 25 °C |journal= Inorg. Chim. Acta |volume= 298 |issue= 1 |pages= 97–102 |doi= 10.1016/S0020-1693(99)00407-7}}</ref> |
|||
<ref name="Fischer_1966">{{Cite journal |last1= Fischer |first1= E. O. |last2= Wawersik |first2= H. |authorlink1= 恩斯特·奥托·菲舍尔 |year= 1966 |title= Über Aromatenkomplexe von Metallen. LXXXVIII. Über Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Über Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 5 |issue= 6 |pages= 559–567 |language= German |doi= 10.1016/S0022-328X(00)85160-8}}</ref> |
|||
<ref name="fouda-2007">{{Cite journal |last1= Fouda |first1= M. F. R. |last2= Abd-Elzaher |first2= M. M. |last3= Abdelsamaia |first3= R. A. |last4= Labib |first4= A. A. |year= 2007 |title= On the Medicinal Chemistry of Ferrocene |journal= [[Applied Organometallic Chemistry|Appl. Organomet. Chem.]] |volume= 21 |issue= 8 |pages= 613–625 |doi= 10.1002/aoc.1202}}</ref> |
|||
<ref name="gagne-1980">{{cite journal |author1= Gagne, R. R. |author2= Koval, C. A. |author3= Lisensky, G. C. |year= 1980 |title= Ferrocene as an Internal Standard for Electrochemical Measurements |journal= [[Inorganic Chemistry (journal)|Inorg. Chem.]] |volume= 19 |issue= 9 |pages= 2854–2855 |doi= 10.1021/ic50211a080}}</ref> |
|||
<ref name="goldschmidt-1988">{{cite journal |last1= Goldschmidt |first1= Z. |last2= Crammer |first2= B. |year= 1988 |title= Vinylcyclopropane Rearrangements |journal= [[Chemical Society Reviews|Chem. Soc. Rev.]] |volume= 17 |issue= |pages= 229–267 |doi= 10.1039/CS9881700229}}</ref> |
|||
<ref name="green-1959">{{cite journal |last1= Green|first1=M. L. H. |last2= Pratt|first2=L. |last3= Wilkinson|first3=G. |authorlink3= Geoffrey Wilkinson |year= 1959 |title= 760. A New Type of Transition Metal–Cyclopentadiene Compound |journal= [[Journal of the Chemical Society|J. Chem. Soc.]] |pages= 3753–3767 |doi= 10.1039/JR9590003753}}</ref> |
|||
<ref name="He_PhD">{{Cite book |last= He |first= H. T. |title= Synthesis and Characterisation of Metallocenes Containing Bulky Cyclopentadienyl Ligands |type= PhD thesis |year= 1999 |location= [[University of Sydney]] |oclc=222646266}}</ref> |
|||
<ref name="hill-2002">{{cite book |last= Hill |first= A. F. |authorlink= Anthony F. Hill |year= 2002 |title= Organotransition Metal Chemistry |location= Cambridge, UK |publisher= Royal Society of Chemistry |pages= 4–7 |isbn= 0-85404-622-4 |url= http://books.google.com.au/books?id=A_DD2-boJiUC&pg=RA1-PA4}}</ref> |
|||
<ref name="Hoffman">{{Cite journal |last1= Laszlo |first1= P. |last2= Hoffmann |first2= R. |authorlink2= Roald Hoffman |year= 2000 |title= Ferrocene: Ironclad History or Rashomon Tale? |journal= [[Angewandte Chemie|Angew. Chem. Int. Ed.]] |volume= 39 |issue=1 |pages= 123–124 |doi= 10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z |pmid=10649350}}</ref> |
|||
<ref name="hughes-1999">{{cite journal |author1= Hughes, R. P. |author2= Trujillo, H. A. |author3= Egan, J. W. |author4= Rheingold, A. L. |title= Skeletal Rearrangement during Rhodium-Promoted Ring Opening of 1,2-Diphenyl-3-vinyl-1-cyclopropene. Preparation and Characterization of 1,2- and 2,3-Diphenyl-3,4-pentadienediyl Rhodium Complexes and Their Ring Closure to a 1,2-Diphenylcyclopentadienyl Complex |year= 1999 |journal= [[Organometallics]] |volume= 18 |issue= 15 |pages= 2766–2772 |doi= 10.1021/om990159o}}</ref> |
|||
<ref name="JACS_1953">{{Cite journal|last1= Cotton |first1= F. A. |authorlink1= F. Albert Cotton |last2= Whipple |first2= R. O. |last3= Wilkinson |first3= G. |authorlink3= Geoffrey Wilkinson |year= 1953 |title= Bis-Cyclopentadienyl Compounds of Rhodium(III) and Iridium(III) |journal= [[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |volume= 75 |issue= 14 |pages= 3586–3587 |doi= 10.1021/ja01110a504}}</ref> |
|||
<ref name="JACS_1982">{{Cite journal |last1= Jacobson |first1= D. B. |last2= Byrd |first2= G. D. |last3= Freiser |first3= B. S. |year= 1982 |title= Generation of Titanocene and Rhodocene Cations in the Gas Phase by a Novel Metal-Switching Reaction |journal= [[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |volume= 104 |issue= 8 |pages= 2320–2321 |doi= 10.1021/ja00372a041}}</ref> |
|||
<ref name="jones-2007">{{cite book |last1= Jones |first1=C. J. |last2= Thornback |first2=J. |year= 2007 |title= Medicinal Applications of Coordination Chemistry |location= Cambridge, UK |publisher= RSC Publishing |isbn= 978-0-85404-596-9}}</ref> |
|||
<ref name="Keller_1967">{{Cite journal |last1= Keller |first1= H. J. |last2= Wawersik |first2= H. |year= 1967 |title= Spektroskopische Untersuchungen an Komplexverbindungen. VI. EPR-spektren von (C<sub>5</sub>H<sub>5</sub>)<sub>2</sub>Rh und (C<sub>5</sub>H<sub>5</sub>)<sub>2</sub>Ir |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 8 |issue= 1 |pages= 185–188 |language= German |doi= 10.1016/S0022-328X(00)84718-X}}</ref> |
|||
<ref name="koelle-1991">{{Cite journal|last1= Kölle |first1= U. |last2= Kläui |first2= W. Z.l |year= 1991 |title= Darstellung und Redoxverhalten einer Serie von Cp*/aqua/tripod-Komplexen des Co, Rh und Ru |language= German |journal= Z. Naturforsch. B. |volume= 46 |issue= 1 |pages= 75–83}}</ref> |
|||
<ref name="Kotz et al.">{{cite book |last1= Kotz |first1=J. C. |last2= Treichel |first2=P. M. |last3= Townsend |first3=J. R. |authorlink2= Paul M. Treichel |year= 2009 |title= Chemistry and Chemical Reactivity, Volume 2 |edition= 7th |location= Belmont, CA |publisher= Cengage Learning |pages= 1050–1053 |isbn= 978-0-495-38703-9 |url= http://books.google.com.au/books?id=s4NPzJ3H90IC&pg=PA1050}}</ref> |
|||
<ref name="leigh-2002">{{cite book |editor1-last= Leigh |editor1-first= G. J. |editor2-last= Winterton |editor2-first= N. |year= 2002 |chapter= Section D: Transition Metal Complexes of Olefins, Acetylenes, Arenes and Related Isolobal COmpounds |title= Modern Coordination Chemistry: The Legacy of Joseph Chatt |location= Cambridge, UK |publisher= RSC Publishing |pages= 101–110 |isbn= 0-85404-469-8 |url= http://books.google.com.au/books?id=VoBxtPb5zCcC&pg=PA101}}</ref> |
|||
<ref name="linked metallocenes">{{cite journal |last1= Barlow |first1=S. |last2= O'Hare |first2=D. |year= 1997 |title= Metal–Metal Interactions in Linked Metallocenes |journal= [[Chemical Reviews|Chem. Rev.]] |volume= 97 |issue= 3 |pages= 637–670 |doi= 10.1021/cr960083v}}</ref> |
|||
<ref name="Mass spec">{{cite journal |last1= Zagorevskii |first1=D. V. |last2= Holmes |first2=J. L. |year= 1992 |title= Observation of Rhodocenium and Substituted-Rhodocenium Ions and their Neutral Counterparts by Mass Spectrometry |journal= [[Organometallics]] |volume= 11 |issue= 10 |pages= 3224–3227 |doi= 10.1021/om00046a018}}</ref> |
|||
<ref name="Nobel Prize">{{Cite web |url= http://nobelprize.org/nobel_prizes/chemistry/laureates/1973/ |title= The Nobel Prize in Chemistry 1973 |publisher= [[Nobel Foundation]] |accessdate= 12 September 2010}}</ref> |
|||
<ref name="okuda-1992">{{Cite book |last= Okuda |first= J. |year= 1992 |chapter= Transition-Metal Complexes of Sterically Demanding Cyclopentadienyl Ligands |editor-first=Herrmann |editor-last=W. A. |title=Transition Metal Coordination Chemistry |series= Topics in Current Chemistry |volume=160 |pages= 97–145 |location=Berlin |publisher=Springer-Verlag |doi= 10.1007/3-540-54324-4_3 |isbn=3-540-54324-4}}</ref> |
|||
<ref name="organ distrib">{{Cite journal |last1= Wenzel |first1= M. |last2= Wu |first2= Y. F. |year= 1987 |title= Abtrennung von [<sup>103''m''</sup>Rh]Rhodocen-Derivaten von den Analogen [<sup>103</sup>Ru]Ruthenocen-Derivaten und deren Organ-Verteilung |language= German |journal= Int. J. Rad. Appl. Instrum. A. |volume= 38 |issue= 1 |pages= 67–69 |pmid= 3030970 |doi= 10.1016/0883-2889(87)90240-1}}</ref> |
|||
<ref name="Pauson_Kealy">{{Cite journal |last1= Kealy |first1= T. J. |last2= Pauson |first2= P. L. |year= 1951 |title= A New Type of Organo-Iron Compound |journal= [[Nature (journal)|Nature]] |volume= 168 |issue= 4285 |pages= 1039–1040 |doi= 10.1038/1681039b0|bibcode = 1951Natur.168.1039K }}</ref> |
|||
<ref name="precious metals">{{cite book |first= Cotton |last=S. A. |year= 1997 |title= Chemistry of Precious Metals |chapter= Rhodium and Iridium |quote= Both metals exhibit an extensive chemistry, principally in the +3 oxidation state, with +1 also being important, and a significant chemistry of +4 iridium existing. Few compounds are known in the +2 state, in contrast to the situation for cobalt, their lighter homologue (factors responsible include the increased stability of the +3 state consequent upon the greater stabilization of the low spin d<sup>6</sup> as 10 Dq increases)." (p. 78) |location= London |publisher= Blackie Academic and Professional |pages= 78–172 |isbn= 0-7514-0413-6 |url= http://books.google.com/books?id=6VKAs6iLmwcC&pg=PA78}}</ref> |
|||
<ref name="Progress in IC">{{cite journal |last1= De Bruin |first1= B. |last2= Hetterscheid |first2=D. G. H. |last3= Koekkoek |first3=A. J. J. |last4= Grützmacher |first4=H. |year= 2007 |title= The Organometallic Chemistry of Rh–, Ir–, Pd–, and Pt–Based Radicals: Higher Valent Species |journal= Prog. Inorg. Chem. |volume=55 |pages= 247–354 |isbn= 978-0-471-68242-4 |url= http://books.google.com.au/books?id=N7yOMUn9HWIC&pg=PA281 |doi=10.1002/9780470144428.ch5}}</ref> |
|||
<ref name="Rh in medicine">{{cite book |last= Pruchnik |first=F. P. |year= 2005 |chapter= <sub>45</sub>Rh — Rhodium in Medicine |editor1-last= Gielen |editor1-first= M. |editor2-last= Tiekink |editor2-first= E. R. T |title= Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine |location= Hoboken, NJ |publisher= Wiley |pages= 379–398 |isbn= 0-470-86403-6 |url= http://books.google.com.au/books?id=vJBLE6G0aIAC&pg=PA379 |doi=10.1002/0470864052.ch20}}</ref> |
|||
<ref name="sazek-1991">{{cite journal |last1= Szajek |first1=L. P. |last2= Shapley |first2=J. R. |year= 1991 |title= Unexpected Synthesis of CpIr(η<sup>4</sup>-C<sub>5</sub>H<sub>6</sub>) and a Proton and Carbon-13 NMR Comparison with its Cobalt and Rhodium Congeners |journal= [[Organometallics]] |volume= 10 |issue= 7 |pages= 2512–2515 |doi= 10.1021/om00053a066}}</ref> |
|||
<ref name="stojanovic-1993">{{cite journal |first1= R. S. |last1= Stojanovic |first2= A. M. |last2= Bond |year= 1993 |title= Examination of Conditions under which the Reduction of the Cobaltocenium Cation can be used as a Standard Voltammetric Reference Process in Organic and Aqueous Solvents |journal= [[Analytical Chemistry (journal)|Anal. Chem.]] |volume= 65 |issue= 1 |pages= 56–64 |doi= 10.1021/ac00049a012}}</ref> |
|||
<ref name="termetallocenes">{{cite journal |last1= Jaitner|first1=P. |last2= Schottenberger|first2=H. |last3= Gamper|first3=S. |last4= Obendorf|first4=D. |year= 1994 |title= Termetallocenes |journal= [[Journal of Organometallic Chemistry|J. Organomet. Chem.]] |volume= 475 |issue= 1–2 |pages= 113–120 |doi= 10.1016/0022-328X(94)84013-X}}</ref> |
|||
<ref name="tris(acetone)">{{cite journal |title= Synthesis of η<sup>5</sup>-1,2,3,4,5-Pentamethylcyclopentadienyl-Platinum Complexes |author1= Gusev, O. V. |author2= Morozovaa, L. N. |author3= Peganovaa, T. A. |author4= Petrovskiia, P. V. |author5= Ustynyuka N. A. |author6= Maitlis, P. M. |authorlink6= Peter Maitlis |journal= [[J. Organomet. Chem.]] |volume= 472 |issue= 1–2 |year= 1994 |pages= 359–363 |doi= 10.1016/0022-328X(94)80223-8}}</ref> |
|||
<ref name="vinylcyclopropene_1997">{{cite journal |last1= Donovan-Merkert |first1=B. T. |last2= Tjiong |first2=H. I. |last3= Rhinehart |first3=L. M. |last4= Russell |first4=R. A. |last5= Malik |first5= J. |year= 1997 |title= Facile, Redox-Promoted Formation of Rhodocenium Complexes Bearing the 1,2,3-Tri-tert-butylcyclopentadienyl Ligan |journal= [[Organometallics]] |volume= 16 |issue= 5 |pages= 819–821 |doi= 10.1021/om9608871}}</ref> |
|||
<ref name="vinylcyclopropene_1998">{{cite journal |last1= Donovan-Merkert |first1=B. T. |last2= Clontz |first2=C. R. |last3= Rhinehart |first3=L. M. |last4= Tjiong |first4=H. I. |last5= Carlin |first5=C. M. |last6= Cundari |year= 1998 |first6= Thomas R. |last7= Rheingold |first7= Arnold L. |last8= Guzei |first8= Ilia |title= Rhodocenium Complexes Bearing the 1,2,3-Tri-''tert''-butylcyclopentadienyl Ligand: Redox-Promoted Synthesis and Mechanistic, Structural and Computational Investigations |journal= [[Organometallics]] |volume= 17 |issue= 9 |pages= 1716–1724 |doi= 10.1021/om9707735}}</ref> |
|||
<ref name="Wagner_2006">{{cite journal |last= Wagner |first=M. |year= 2006 |title= A New Dimension in Multinuclear Metallocene Complexes |journal= [[Angewandte Chemie|Angew. Chem. Int. Ed.]] |volume= 45 |issue= 36 |pages= 5916–5918 |doi= 10.1002/anie.200601787}}</ref> |
|||
<ref name="Wenzel">{{Cite journal |last1= Wenzel |first1= M. |last2= Wu |first2= Y. |year= 1988 |title= Ferrocen-, Ruthenocen-bzw. Rhodocen-analoga von Haloperidol Synthese und Organverteilung nach Markierung mit <sup>103</sup>Ru-bzw. <sup>103''m''</sup>Rh |language= German |journal= Int. J. Rad. Appl. Instrum. A. |volume= 39 |issue= 12 |pages= 1237–1241 |pmid= 2851003 |doi= 10.1016/0883-2889(88)90106-2}}</ref> |
|||
<ref name="werner-2008">{{cite book |last= Werner |first=H. |year= 2008 |location= New York |title= Landmarks in Organo-Transition Metal Chemistry: A Personal View |publisher= Springer Science |pages= 161–163 |isbn= 978-0-387-09847-0 |url= http://books.google.com.au/books?id=dP4LTfaPzAMC&pg=PA161}}</ref> |
|||
<ref name="wilkinson-1952">{{Cite journal |last1= Wilkinson |first1= G. |authorlink1= Geoffrey Wilkinson |last2= Rosenblum |first2= M. |last3= Whiting |first3= M. C. |last4= Woodward |first4= R. B. |authorlink4= Robert Burns Woodward |year= 1952 |title= The Structure of Iron ''Bis''-Cyclopentadienyl |journal= [[Journal of the American Chemical Society|J. Am. Chem. Soc.]] |volume= 74 |issue= 8 |pages= 2125–2126 |doi= 10.1021/ja01128a527}}</ref> |
|||
<ref name="Zeise anion">{{cite journal |last1= Jarvis |first1=J. A. J. |last2= Kilbourn |first2=B. T. |last3= Owston |first3=P. G. |authorlink3= P. G. Owston |year= 1971 |title= A Re-determination of the Crystal and Molecular Structure of Zeise's salt, KPtCl<sub>3</sub>.C<sub>2</sub>H<sub>4</sub>.H<sub>2</sub>O |journal= [[Acta Crystallographica B|Acta Cryst. B]] |volume= 27 |issue= 2 |pages= 366–372 |doi= 10.1107/S0567740871002231}}</ref> |
|||
<ref name="Zeise discovery">{{cite journal |last1= Zeise |first1= W. C. |authorlink= William Christopher Zeise |year= 1831 |title= Von der Wirkung zwischen Platinchlorid und Alkohol, und von den dabei entstehenden neuen Substanzen |journal= [[Annalen der Physik|Ann. der Physik]] |language= German |volume= 97 |issue= 4 |pages= 497–541 |doi= 10.1002/andp.18310970402|bibcode = 1831AnP....97..497Z }}</ref> |
|||
<ref name="Zeise review">{{cite journal |last= Hunt |first=L. B. |year= 1984 |title= The First Organometallic Compounds: William Christopher Zeise and his Platinum Complexes |journal= [[Platinum Metals Review|Platinum Metals Rev.]] |volume= 28 |issue= 2 |pages= 76–83 |url= http://www.platinummetalsreview.com/pdf/pmr-v28-i2-076-083.pdf}}</ref> |
|||
}} |
|||
== 参考文献 == |
== 参考文献 == |
||
2012年9月9日 (日) 09:47的版本
| 二茂铑 | |
|---|---|
| |
| 别名 | 双环戊二烯铑 |
| 识别 | |
| CAS号 | 12318-21-7 |
| ChemSpider | 2339512 |
| SMILES |
|
| 性质 | |
| 化学式 | C10H10Rh |
| 摩尔质量 | 233.09 g·mol−1 |
| 外观 | 黄色固体(二聚物)[1] |
| 熔点 | 174℃分解(二聚物)[1] |
| 溶解性(水) | 稍溶于二氯甲烷(二聚物)[1] 溶于乙腈[1] |
| 相关物质 | |
| 相关化学品 | 二茂铁 二茂钴 二茂铱 二茂钌 二苯铬 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
二茂铑(英語:Rhodocene),也称双(η5-环戊二烯基)合铑(II),分子式为[Rh(C5H5)2]。分子由两个η5的环戊二烯配体(哈普托数为5,表示环戊二烯基有五个原子参与配位)和一个铑原子构成,铑原子处在平行的两个环戊二烯基中间形成“三明治”夹心结构。二茂铑的自由基存在于150°C以上的条件下,可利用液氮(−196°C)急速冷却的方法捕获到它。室温下,两个二茂铑自由基的环戊二烯基键合形成黄色的二聚体。[1][2][3]
有机金属化学的历史可以追溯到在19世纪发现的蔡司盐[4][5]和路德维希·蒙德发现的四羰基镍[6]。由于当时化学家所理解的化学键模型不适用于解释这些化合物,因此这些化合物的出现对化学家提出了挑战。进一步的挑战出现在像二茂铁、二茂铑这类茂金属化合物被发现之后。当时出乎意料的是,诸如二茂铁和与其有相似化学结构并带有一个正电荷的二茂铑、二茂钴、二茂铱阳离子都有比较稳定的化学性质。对包括这些化合物在内的有机金属化学的研究,催生出新的化学键模型,用它可以解释这些有机金属化合物的形成和稳定性。鉴于杰弗里·威尔金森和恩斯特·奥托·菲舍尔在包括二茂铑在内的夹心化合物方面的研究工作中做出的杰出贡献,两人在1977年被授予诺贝尔化学奖。
参考资料
- ^ 1.0 1.1 1.2 1.3 1.4 El Murr, N.; Sheats, J. E.; Geiger, W. E.; Holloway, J. D. L. Electrochemical Reduction Pathways of the Rhodocenium Ion. Dimerization and Reduction of Rhodocene. Inorg. Chem. 1979, 18 (6): 1443–1446. doi:10.1021/ic50196a007.(英文) 引用错误:带有name属性“El_Murr_1979”的
<ref>标签用不同内容定义了多次 - ^ Fischer, E. O.; Wawersik, H. Über Aromatenkomplexe von Metallen. LXXXVIII. Über Monomeres und Dimeres Dicyclopentadienylrhodium und Dicyclopentadienyliridium und Über Ein Neues Verfahren Zur Darstellung Ungeladener Metall-Aromaten-Komplexe. J. Organomet. Chem. 1966, 5 (6): 559–567. doi:10.1016/S0022-328X(00)85160-8 (German).
- ^ Keller, H. J.; Wawersik, H. Spektroskopische Untersuchungen an Komplexverbindungen. VI. EPR-spektren von (C5H5)2Rh und (C5H5)2Ir. J. Organomet. Chem. 1967, 8 (1): 185–188. doi:10.1016/S0022-328X(00)84718-X (German).
- ^ Hunt, L. B. The First Organometallic Compounds: William Christopher Zeise and his Platinum Complexes (PDF). Platinum Metals Rev. 1984, 28 (2): 76–83.
- ^ Zeise, W. C. Von der Wirkung zwischen Platinchlorid und Alkohol, und von den dabei entstehenden neuen Substanzen. Ann. der Physik. 1831, 97 (4): 497–541. Bibcode:1831AnP....97..497Z. doi:10.1002/andp.18310970402 (German).
- ^ Crabtree, R. H. The Organometallic Chemistry of the Transition Metals 5th. Hoboken, NJ: John Wiley and Sons. 2009: 2. ISBN 978-0-470-25762-3.
An industrial application of transition metal organometallic chemistry appeared as early as the 1880s, when Ludwig Mond showed that nickel can be purified by using CO to pick up nickel in the form of gaseous Ni(CO)4 that can easily be separated from solid impurities and later be thermally decomposed to give pure nickel.
... Recent work has shown the existence of a growing class of metalloenzymes having organometallic ligand environments – considered as the chemistry of metal ions having C-donor ligands such as CO or the methyl group
引用错误:在<references>标签中name属性为“astruc-2007”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“baghurst-1989”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“baghurst-1990”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Buchholz_1994”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Chromatographia”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“clarke-1999”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“clarke-2002”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“cobaltocenium”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Collins_1995”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“decamethylferrocenium”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“decamethylrhodocenium”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“decamethyliridocenium”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Dewar-Chatt-Duncanson”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“eiland-1952”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“EOFischer”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“FerroceneHistory”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“ferrocene bonding”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“ferrocenium redox couple”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“fouda-2007”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“gagne-1980”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“goldschmidt-1988”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“green-1959”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“He_PhD”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“hill-2002”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Hoffman”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“hughes-1999”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“JACS_1953”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“JACS_1982”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“jones-2007”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“koelle-1991”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Kotz et al.”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“leigh-2002”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“linked metallocenes”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Mass spec”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Nobel Prize”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“okuda-1992”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“organ distrib”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Pauson_Kealy”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“precious metals”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Progress in IC”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Rh in medicine”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“sazek-1991”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“stojanovic-1993”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“termetallocenes”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“tris(acetone)”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“vinylcyclopropene_1997”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“vinylcyclopropene_1998”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Wagner_2006”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“Wenzel”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“werner-2008”的参考文献没有在文中使用
引用错误:在<references>标签中name属性为“wilkinson-1952”的参考文献没有在文中使用
<references>标签中name属性为“Zeise anion”的参考文献没有在文中使用