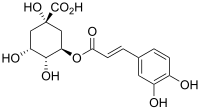
綠原酸
| 綠原酸 | |
|---|---|
| |
| IUPAC名 (1S,3R,4R,5R)-3[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy1,4,5-trihydroxycyclohexanecarboxylic acid | |
| 英文名 | Chlorogenic acid |
| 识别 | |
| CAS号 | 327-97-9 |
| PubChem | 1794427 |
| ChemSpider | 1405788 |
| SMILES |
|
| InChI |
|
| InChIKey | CWVRJTMFETXNAD-JUHZACGLBD |
| ChEBI | 16112 |
| RTECS | GU8480000 |
| 性质 | |
| 化学式 | C16H18O9 |
| 摩尔质量 | 354.31 g·mol−1 |
| 密度 | 1.28 g/cm3 |
| 熔点 | 207—209 °C(405—408 °F;480—482 K) |
| 危险性 | |
| 警示术语 | R:- |
| 安全术语 | S:S24 S25 S28 S37 S45 |
| NFPA 704 | |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
綠原酸(英語:Chlorogenic acid,縮寫CGA),又名氯吉酸、氯原酸、咖啡单宁酸、杜仲绿原酸、咖啡酰奎尼酸等[1][來源可靠?],是一天然的化合物,由咖啡酸及(−)-奎尼酸酯化而成。绿原酸(chlorogenic)一名来自希腊语χλωρός(“绿色”)和后缀-γένος(“产生...的”),因为绿原酸氧化后会变绿而得名。
綠原酸是一種重要的生物合成中間體[2]。綠原酸是木質素(lignin)的生物合成的重要中間生成物。綠原素作為一種抗氧化劑,可令餐後葡萄醣釋出進入血液的過程減慢[3]。
綠原酸這個名詞也泛指羥基肉桂酸(即:咖啡酸、阿魏酸及对香豆酸)與奎尼酸的酯化物[4]。
结构与性质
[编辑]綠原酸是咖啡酸與L-奎尼酸和3號位羥基縮合形成的酯。[5]綠原酸的異構體包括奎尼酸其它位置羥基酯化的產物,如4-O-咖啡酰奎尼酸(cryptochlorogenic acid或簡稱4-CQA,隐綠原酸)和5-O-咖啡酰奎尼酸(neochlorogenic acid或稱5-CQA,新綠原酸)。一位羥基的異構體尚未在自然界中發現。[4]
兩個羥基被酯化則稱異綠原酸(isochlorogenic acid),存在於咖啡中。[6]例如3,4-二咖啡酰奎尼酸和3,5-二咖啡酰奎尼酸。[7] 和1,5-二咖啡酰奎尼酸。

自然来源
[编辑]据报道,绿原酸在葵花籽壳和葵花籽仁中均存在,葵花籽中绿原酸含量为1.5%~3.3%,葵花籽仁中绿原酸含量为2.1%~3.5%。[8]由于葵花籽中含有较多的绿原酸,因此可以做为提取绿原酸的原料,除了葵花籽外,提取绿原酸的原料还常用生咖啡豆、金银花、杜仲等。[9]此外,在榨取葵花籽油或制取葵花籽蛋白时,因为其中的绿原酸被氧化后呈绿色,会影响葵花籽油、葵花籽蛋白的品质,需要将其中的绿原酸给除去。[10]綠
原酸是金銀花的主要抗菌、抗病毒有效藥理成分之一。[11]朝鲜蓟中也含有较多的绿原酸。[12]
此外,綠原酸还存在于毛竹(Phyllostachys edulis)[13]以及其它植物中[14],也是桃中常见的酚类化合物[15]、健康的帚石楠(Calluna vulgaris)嫩芽中[16]。
參考文獻
[编辑]- ^ 绿原酸 CAS: 327-97-9. 2016-08-05 [2016-08-07]. (原始内容存档于2016-08-14) (中文(简体)).
- ^ Boerjan, Wout; Ralph, John; Baucher, Marie. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54: 519–46. PMID 14503002. doi:10.1146/annurev.arplant.54.031902.134938.
- ^ Johnston, K. L.; Clifford, M. N.; Morgan, L. M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutrit.. 2003-10, 78 (4): 728–733 [2016-08-07]. PMID 14522730. (原始内容存档于2010-04-21) (英语).
- ^ 4.0 4.1 Clifford, M. N.; Johnston, K. L.; Knigh, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. Journal of Agricultural and Food Chemistry. 2003, 51 (10): 2900–2911. PMID 12720369. doi:10.1021/jf026187q.
- ^ Clifford, M. N. Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J. Sci. Food Agr. 1999, 79 (3): 362–372. doi:10.1002/(SICI)1097-0010(19990301)79:3<362::AID-JSFA256>3.0.CO;2-D.
- ^ Isochlorogenic Acid. Isolation from Coffee and Structure Studies. H. M. Barnes, J. R. Feldman and W. V. White, J. Am. Chem. Soc., 1950, volume 72, issue 9, pages 4178–4182, doi:10.1021/ja01165a095
- ^ Corse, J.; Lundin, R. E.; Waiss, A. C. Identification of several components of isochlorogenic acid. Phytochem. May 1965, 4 (3): 527–529. doi:10.1016/S0031-9422(00)86209-3.
- ^ 梁少华. 植物油料资源综合利用 第2版. 南京: 东南大学出版社. 2009.10: 221–222. ISBN 978-7-5641-1881-5.
- ^ 李新兰,朱建如,李汉帆 (编). 保健食品开发及应用. 武汉: 华中理工大学出版社. 1999.10: 104–105.
- ^ 郑竟成; 曹博睿; 何东平; 田华. 葵花籽油加工技术. 北京: 中国轻工业出版社. 2021.01: 151. ISBN 978-7-5184-3135-9.
- ^ Ding, Yue; Cai, Zeyu. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Scientific Reports. 2017, 7: 45723. PMC 5385491
 . PMID 28393840. doi:10.1038/srep45723.
. PMID 28393840. doi:10.1038/srep45723.
- ^ 宋曙辉; 张宝海, 王文琪, 何洪巨, 唐晓伟. 朝鲜蓟叶中绿原酸提取技术的研究. 中国农学通报. 2010, (20): 98–101.
- ^ Kweon, Mee-Hyang; Hwang, Han-Joon; Sung, Ha-Chin. Identification and Antioxidant Activity of Novel Chlorogenic Acid Derivatives from Bamboo (Phyllostachys edulis). Journal of Agricultural and Food Chemistry. 2001, 49 (20): 4646–46552. doi:10.1021/jf010514x.
- ^ Clifford, M. N. 14. The analysis and characterization of chlorogenic acids and other cinnamates. C. Santos-Buelga & G. Williamson (Eds.) (编). Methods in Polyphenol Analysis. Cambridge: Royal Society of Chemistry. 2003: 314–337. ISBN 0-85404-580-5.
- ^ Cheng, G. W.; Crisosto, C. H. Browning Potential, Phenolic Composition, and Polyphenoloxidase Activity of Buffer Extracts of Peach and Nectarine Skin Tissue (PDF). J. Amer. Soc. Hort. Sci. September 1995, 120 (5): 835–838 [2016-08-07]. (原始内容 (PDF)存档于2014-05-14).
- ^ Jalal, Mahbubul A.F.; Read, David J.; Haslam, E. Phenolic composition and its seasonal variation in Calluna vulgaris. Phytochem. 1982, 21 (6): 1397–1401. doi:10.1016/0031-9422(82)80150-7.

