元素氧化态列表:修订间差异
删除的内容 添加的内容
Hacter Chang(留言 | 贡献) 无编辑摘要 标签:HTML註解 |
Hacter Chang(留言 | 贡献) 无编辑摘要 标签:HTML註解 |
||
| 第899行: | 第899行: | ||
| |
| |
||
| style="text-align:right;" | [[1族元素|1]] |
| style="text-align:right;" | [[1族元素|1]] |
||
| |
| <!-- |
||
--><ref name="G&E Lithides"/> |
|||
|- |
|- |
||
| 38 |
| 38 |
||
| 第920行: | 第921行: | ||
| |
| |
||
| style="text-align:right;" | [[2族元素|2]] |
| style="text-align:right;" | [[2族元素|2]] |
||
| |
| <!-- |
||
--><ref>Sr(I) has been observed in [[strontium monofluoride]] (SrF); see {{cite journal|url=http://bernath.uwaterloo.ca/media/149.pdf |title=High-Resolution Infrared Emission Spectrum of Strontium Monofluoride |journal=Journal of Molecular Spectroscopy |volume=175 |issue=1 |pages=158–171 |year=1996 |author=P. Colarusso |bibcode=1996JMoSp.175..158C |doi=10.1006/jmsp.1996.0019 |last2=Guo |first2=B. |last3=Zhang |first3=K.-Q. |last4=Bernath |first4=P.F. |display-authors=etal |url-status=dead |archive-url=https://web.archive.org/web/20120308063843/http://bernath.uwaterloo.ca/media/149.pdf |archive-date=2012-03-08 }}</ref><ref name="Ca0"/> |
|||
|- |
|- |
||
| 39 |
| 39 |
||
| 第941行: | 第943行: | ||
| |
| |
||
| style="text-align:right;" | [[3族元素|3]] |
| style="text-align:right;" | [[3族元素|3]] |
||
| |
| <!-- |
||
--><ref name="Cloke1993"/><!-- |
|||
--><ref>Y(I) has been observed in [[yttrium(I) bromide]] (YBr); see {{cite web|url=http://www.openmopac.net/data_normal/yttrium(i)%20bromide_jmol.html |title=Yttrium: yttrium(I) bromide compound data |access-date=2007-12-10 |publisher=OpenMOPAC.net |url-status=dead |archive-url=https://web.archive.org/web/20110723233118/http://www.openmopac.net/data_normal/yttrium%28i%29%20bromide_jmol.html |archive-date=2011-07-23 }}</ref><!-- |
|||
--><ref>Y(II) has been observed in <!-- [[yttrium(II) hydride]] (YH<sub>2</sub>); see {{cite web|url=http://www.webelements.com/webelements/compounds/text/Y/H2Y1-13598351.html|title=Yttrium: yttrium(II) hydride compound data|access-date=2007-12-10|publisher=WebElements.com}} Isn't YH<sub>2</sub> electride-like?-->[(18-crown-6)K][(C<sub>5</sub>H<sub>4</sub>SiMe<sub>3</sub>)<sub>3</sub>Y]; see {{cite journal|last1=MacDonald|first1=M. R.|last2=Ziller|first2=J. W.|last3=Evans|first3=W. J.|year=2011|title=Synthesis of a Crystalline Molecular Complex of Y<sup>2+</sup>, [(18-crown-6)K][(C<sub>5</sub>H<sub>4</sub>SiMe<sub>3</sub>)<sub>3</sub>Y]|journal=J. Am. Chem. Soc.|volume=133|issue=40|pages=15914–17|doi=10.1021/ja207151y|pmid=21919538}}</ref> |
|||
|- |
|- |
||
| 40 |
| 40 |
||
| 第962行: | 第967行: | ||
| |
| |
||
| style="text-align:right;" | [[4族元素|4]] |
| style="text-align:right;" | [[4族元素|4]] |
||
| <!----><ref name="Carbonyls"/><!-- |
|||
| |
|||
--><ref>Zr(−1) has been reported in [Zr([[2,2'-Bipyridine|bipy]])<sub>3</sub>]<sup>−</sup> (see {{Greenwood&Earnshaw|page=960}} and {{cite book|publisher=Walter de Gruyter|year=1995|edition=101|pages=1413|isbn=978-3-11-012641-9|title=Lehrbuch der Anorganischen Chemie|first=Arnold F.|last=Holleman |author2=Wiberg, Egon |author3=Wiberg, Nils|language=de|chapter=Zirconium}}), but was later shown to be Zr(+4); see {{cite journal|doi=10.1021/ic302799s|pmid=23387926|title=Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study|year=2013|last1=Bowman|first1=A. C.|last2=England|first2=J.|last3=Sprouls|first3=S.|last4=Weihemüller|first4=T.|last5=Wieghardt|first5=K.|journal=Inorganic Chemistry|volume=52|issue=4|pages=2242–56}}</ref><!-- |
|||
--><ref name="Zr0">Zr(0) and Hf(0) occur in (η<sup>6</sup>-(1,3,5-<sup>''t''</sup>Bu)<sub>3</sub>C<sub>6</sub>H<sub>3</sub>)<sub>2</sub>M (M=Zr, Hf) and [(η<sup>5</sup>-C<sub>5</sub>R<sub>5</sub>M(CO)<sub>4</sub>]<sup>−</sup>, see {{cite book|publisher=Elsevier Ltd.|year=2007|volume=4|pages=697–739|doi=10.1016/B0-08-045047-4/00062-5|title=Comprehensive Organometallic Chemistry III. From Fundamentals to Applications|first=P. J.|last=Chirik |author2=Bradley, C. A. |chapter=4.06 - Complexes of Zirconium and Hafnium in Oxidation States 0 to ii|isbn=9780080450476}}</ref> |
|||
|- |
|- |
||
| 41 |
| 41 |
||
| 第983行: | 第990行: | ||
| |
| |
||
| style="text-align:right;" | [[5族元素|5]] |
| style="text-align:right;" | [[5族元素|5]] |
||
| |
| <!-- |
||
--><ref name="Carbonyls"/><!-- |
|||
--><ref name="NbTa0">Complexes of Nb(0) and Ta(0) have been observed, see {{cite book|publisher=Newnes|year=2003|edition=2|volume=4|pages=297–299|editor1=J. A. McCleverty|editor2=T.J. Meyer|isbn=978-0-08-091316-2|title=Comprehensive Coordination Chemistry II: From Biology to Nanotechnology|first=Arnold F.|last=Holleman |author2=Wiberg, Egon |author3=Wiberg, Nils|chapter=4.5.7. Niobium(0) and Tantalum(0)}}</ref><!-- |
|||
--><ref name="NbTa">Nb(I) and Ta(I) occur in [[cyclopentadienyl|Cp]]Nb(CO)<sub>4</sub> and [[cyclopentadienyl|Cp]]Ta(CO)<sub>4</sub>, see {{cite book|publisher=Walter de Gruyter|year=1995|edition=101| pages=1430|isbn=978-3-11-012641-9|title=Lehrbuch der Anorganischen Chemie|first=Arnold F.|last=Holleman |author2=Wiberg, Egon |author3=Wiberg, Nils|language=de|chapter=Tantal}} and {{cite book|publisher=Academic Press|year=1969|pages=11|isbn=978-0-32-315996-8|title=Transition-Metal Organometallic Chemistry: An Introduction|first=R. Bruce|last=King}}</ref> |
|||
|- |
|- |
||
| 42 |
| 42 |
||
| 第1,004行: | 第1,014行: | ||
| |
| |
||
| style="text-align:right;" | [[6族元素|6]] |
| style="text-align:right;" | [[6族元素|6]] |
||
| |
| <!-- |
||
--><ref name="Carbonyls"/> |
|||
|- |
|- |
||
| 43 |
| 43 |
||
| 第1,046行: | 第1,057行: | ||
| |
| |
||
| style="text-align:right;" | [[8族元素|8]] |
| style="text-align:right;" | [[8族元素|8]] |
||
| |
| <!-- |
||
--><ref name="Carbonyls"/><!-- |
|||
--><ref name="MetalAnions"/> |
|||
|- |
|- |
||
| 45 |
| 45 |
||
| 第1,067行: | 第1,080行: | ||
| |
| |
||
| style="text-align:right;" | [[9族元素|9]] |
| style="text-align:right;" | [[9族元素|9]] |
||
| |
| <!-- |
||
--><ref name="Carbonyls"/><!-- |
|||
--><ref>{{cite journal |journal= Chemical Physics Letters |volume= 108 |issue= 6 |year= 1984 |pages= 627–630 |title= Electron paramagnetic resonance spectroscopic studies on the zero-valent rhodium complex [Rh(P(OPr<sup>''i''</sup>)<sub>3</sub>)<sub>4</sub>] at X-and Q-band frequencies |first1= G.N. |last1= George |first2= S.I. |last2= Klein |first3= J.F. |last3= Nixon |doi= 10.1016/0009-2614(84)85069-1|bibcode= 1984CPL...108..627G }}</ref><ref>Rh(VII) is known in the RhO<sub>3</sub><sup>+</sup> cation, see {{cite journal |title=The Highest Oxidation State of Rhodium: Rhodium(VII) in [RhO3]+ |journal=Angew. Chem. Int. Ed. |date=2022 |doi=10.1002/anie.202207688}}</ref> |
|||
|- |
|- |
||
| 46 |
| 46 |
||
| 第1,088行: | 第1,103行: | ||
| |
| |
||
| style="text-align:right;" | [[10族元素|10]] |
| style="text-align:right;" | [[10族元素|10]] |
||
| |
| <!-- |
||
--><ref>Pd(I) has been observed; see {{cite journal|last1= Crabtree|first1= R. H.|title= CHEMISTRY: A New Oxidation State for Pd?|journal= [[Science (journal)|Science]]|volume= 295|pages= 288–289|year= 2002|doi= 10.1126/science.1067921|pmid= 11786632|issue= 5553|s2cid= 94579227}}</ref><!-- |
|||
--><ref>Pd(III) has been observed; see {{cite book|last1=Powers |first1=D. C. |last2=Ritter |first2=T. |title=Palladium(III) in Synthesis and Catalysis |journal=Top. Organomet. Chem. |volume=35 |pages=129–156 |date=2011 |doi=10.1007/978-3-642-17429-2_6 |pmid=21461129 |url=http://www.chem.harvard.edu/groups/ritter/pdf/2011-129t.pdf |series=Topics in Organometallic Chemistry |isbn=978-3-642-17428-5 |url-status=dead |archive-url=https://web.archive.org/web/20130612065217/http://www.chem.harvard.edu/groups/ritter/pdf/2011-129t.pdf |archive-date=June 12, 2013 |pmc=3066514|bibcode=2011hoso.book..129P }}</ref><ref>Palladium(V) has been identified in complexes with organosilicon compounds containing pentacoordinate palladium; see {{cite journal |first1=Shigeru |last1=Shimada |first2=Yong-Hua |last2=Li |first3=Yoong-Kee |last3=Choe |first4=Masato |last4=Tanaka |first5=Ming |last5=Bao |first6=Tadafumi |last6=Uchimaru |title=Multinuclear palladium compounds containing palladium centers ligated by five silicon atoms |doi=10.1073/pnas.0700450104 |journal=Proceedings of the National Academy of Sciences |volume=104 |year=2007 |issue=19 |pages=7758–7763}}</ref><ref>Palladium(VI) has been claimed to exist in {{cite journal |title=NEW PALLADIUM OXIDATION STATE? |journal=Chem. Eng. News |date=2002 |volume=80 |issue=2 |page=8 |doi=10.1021/cen-v080n002.p008}}, but this has been refuted showing it is a Palladium(II).</ref> |
|||
|- |
|- |
||
| 47 |
| 47 |
||
| 第1,109行: | 第1,126行: | ||
| |
| |
||
| style="text-align:right;" | [[11族元素|11]] |
| style="text-align:right;" | [[11族元素|11]] |
||
| |
| <!-- |
||
--><ref name="MetalAnions"/><!-- |
|||
--><ref>The Ag<sup>−</sup> ion has been observed in metal ammonia solutions: see {{cite journal|doi=10.1021/ic000333x|title=Metal Ammonia Solutions: Solutions Containing Argentide Ions|year=2001|last1=Tran|first1=N. E.|last2=Lagowski|first2=J. J.|journal=Inorganic Chemistry|volume=40|issue=5|pages=1067–68}}</ref><!-- |
|||
--><ref>Ag(0) has been observed in carbonyl complexes in low-temperature matrices: see {{cite journal|doi=10.1021/ja00427a018|title=Synthesis using metal vapors. Silver carbonyls. Matrix infrared, ultraviolet-visible, and electron spin resonance spectra, structures, and bonding of silver tricarbonyl, silver dicarbonyl, silver monocarbonyl, and disilver hexacarbonyl|year=1976|last1=McIntosh|first1=D.|last2=Ozin|first2=G. A.|journal=J. Am. Chem. Soc.|volume=98|issue=11|pages=3167-75}}</ref> |
|||
|- |
|- |
||
| 48 |
| 48 |
||
| 第1,130行: | 第1,150行: | ||
| |
| |
||
| style="text-align:right;" | [[12族元素|12]] |
| style="text-align:right;" | [[12族元素|12]] |
||
| |
| <!-- |
||
--><ref name="MetalAnions"/><!-- |
|||
--><ref>Cd(I) has been observed in [[cadmium(I) tetrachloroaluminate]] (Cd<sub>2</sub>(AlCl<sub>4</sub>)<sub>2</sub>); see {{cite book|publisher= Walter de Gruyter|year= 1985|edition= 91–100|pages= 1056–1057|isbn= 978-3-11-007511-3|title= Lehrbuch der Anorganischen Chemie|last1= Holleman|first1= Arnold F.|last2= Wiberg|first2=Egon |last3=Wiberg |first3=Nils |language= de|chapter= Cadmium}}</ref> |
|||
|- |
|- |
||
| 49 |
| 49 |
||
| 第1,151行: | 第1,173行: | ||
| |
| |
||
| style="text-align:right;" | [[13族元素|13]] |
| style="text-align:right;" | [[13族元素|13]] |
||
| |
| <!-- |
||
--><ref name="Zintl"/><!-- |
|||
--><ref>In(–5) has been observed in La<sub>3</sub>InGe, see {{cite journal|doi=10.1021/ic951378e|title=Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties|year=1996|last1=Guloy|first1=A. M.|last2=Corbett|first2=J. D.|journal=Inorganic Chemistry|volume=35|issue=9|pages=2616–22|pmid=11666477}}</ref><!-- |
|||
--><ref>In(−2) has been observed in Na<sub>2</sub>In, see [https://books.google.com/books?id=v-04Kn758yIC&pg=PA69&lpg=PA69&dq=zintl+anions+Na2In&source=bl&ots=aXLYIpkfYq&sig=Mqh8WdnvGOt2J2OPVLNqn79YVyk&hl=ru&sa=X&ei=XNDkVNeSJeXOyQOb8oBA&ved=0CBsQ6AEwADgK#v=onepage&q&f=false], p. 69.</ref><!-- |
|||
--><ref>Unstable In(0) carbonyls and clusters have been detected, see [https://www.researchgate.net/profile/Anthony-Downs-2/publication/6589844_Development_of_the_Chemistry_of_Indium_in_Formal_Oxidation_States_Lower_than_3/links/5a82db2a0f7e9bda869fb52c/Development-of-the-Chemistry-of-Indium-in-Formal-Oxidation-States-Lower-than-3.pdf?origin=publication_detail], p. 6.</ref> |
|||
|- |
|- |
||
| 50 |
| 50 |
||
| 第1,172行: | 第1,198行: | ||
| |
| |
||
| style="text-align:right;" | [[14族元素|14]] |
| style="text-align:right;" | [[14族元素|14]] |
||
| |
| <!-- |
||
--><ref name="Zintl"/><!-- |
|||
--><ref>Sn(−3) has been observed in [Sn<sub>2</sub>]<sup>6−</sup>, e.g. in (Ba<sub>2</sub>)<sup>4+</sup>(Mg<sub>4</sub>)<sup>8+</sup>Sn<sup>4−</sup>(Sn<sub>2</sub>)<sup>6−</sup>Sn<sup>2−</sup> (with square (Sn<sup>2−</sup>)<sub>n</sub> sheets), see {{cite journal |last1=Papoian |first1=Garegin A. |last2=Hoffmann |first2=Roald |year=2000 |title=Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks |journal=Angew. Chem. Int. Ed. |volume=2000 |issue= 39|pages=2408–2448 |url=https://www.researchgate.net/publication/12379848 |access-date=2015-02-23 |doi=10.1002/1521-3773(20000717)39:14<2408::aid-anie2408>3.0.co;2-u}}</ref><!-- |
|||
--><ref>Sn(I) and Sn(III) have been observed in [[organotin compounds]]</ref><!-- |
|||
--><ref name=ZeroValentTin/> |
|||
|- |
|- |
||
| 51 |
| 51 |
||
| 第1,193行: | 第1,223行: | ||
| |
| |
||
| style="text-align:right;" | [[15族元素|15]] |
| style="text-align:right;" | [[15族元素|15]] |
||
| |
| <!-- |
||
--><ref name="Zintl"/><!-- |
|||
--><ref>Sb(−2) has been observed in [Sb<sub>2</sub>]<sup>4−</sup>, e.g. in RbBa<sub>4</sub>[Sb<sub>2</sub>][Sb][O], see {{cite journal |last1=Boss |first1=Michael |last2=Petri |first2=Denis |last3=Pickhard |first3=Frank |last4=Zönnchen |first4=Peter |last5=Röhr |first5=Caroline |year=2005 |title=Neue Barium-Antimonid-Oxide mit den Zintl-Ionen [Sb]<sup>3−</sup>, [Sb<sub>2</sub>]<sup>4−</sup> und <sup>1</sup><sub>∞</sub>[Sb<sub>n</sub>]<sup>n−</sup> / New Barium Antimonide Oxides containing Zintl Ions [Sb]<sup>3−</sup>, [Sb<sub>2</sub>]<sup>4−</sup> and <sup>1</sup><sub>∞</sub>[Sb<sub>n</sub>]<sup>n−</sup> |journal=Zeitschrift für Anorganische und Allgemeine Chemie |volume=631 |issue= 6–7|pages=1181–1190 |language=de |doi= 10.1002/zaac.200400546}}</ref><!-- |
|||
--><ref>Sb(0) has been observed, see {{cite news|url=https://pdfs.semanticscholar.org/8817/39f9dfc007d7f77dd7baa63fe12e6079f8ef.pdf|author=Anastas Sidiropoulos|title=Studies of N-heterocyclic Carbene (NHC) Complexes of the Main Group Elements|page=39|doi=10.4225/03/5B0F4BDF98F60|s2cid=132399530}} |
|||
</ref><!-- |
|||
--><ref>Sb(I) and Sb(II) have been observed in [[organoantimony compounds]]; for Sb(I), see {{cite journal |last1=Šimon |first1=Petr |last2=de Proft |first2=Frank |last3=Jambor |first3=Roman |last4=Růžička |first4=Aleš |last5=Dostál |first5=Libor |year=2010 |title=Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures |journal=Angewandte Chemie International Edition |volume=49 |issue=32 |pages=5468–5471 |doi= 10.1002/anie.201002209|pmid=20602393}}</ref><!-- |
|||
--><ref>Sb(IV) has been observed in {{chem2|[SbCl6](2-)}}, see {{cite journal|doi=10.1246/bcsj.73.1599|title=Production of Sb(IV) Chloro Complex by Flash Photolysis of the Corresponding Sb(III) and Sb(V) Complexes in CH3CN and CHCl3|year=2000|author1=Nobuyoshi Shinohara|author2=Masaaki Ohsima|journal=Bulletin of the Chemical Society of Japan|volume=73|issue=7|pages=1599–1604}}</ref> |
|||
|- |
|- |
||
| 52 |
| 52 |
||
| 第1,214行: | 第1,250行: | ||
| |
| |
||
| style="text-align:right;" | [[16族元素|16]] |
| style="text-align:right;" | [[16族元素|16]] |
||
| |
| <!-- |
||
--><ref name="Zintl"/><!-- |
|||
--><ref>Te(0) has been observed in tellurolates.</ref><!-- |
|||
--><ref>Te(I) has been observed in [[tellurium iodide]] (TeI), see {{cite web|url=http://www.webelements.com/compounds/tellurium/tellurium_iodide.html|title=Tellurium: tellurium iodide|access-date=2015-02-23|publisher=WebElements.com}}</ref><!-- |
|||
--><ref>Te(III) has been observed in [Te(N(Si[[methyl|Me]]<sub>3</sub>)<sub>2</sub>)<sub>2</sub>]<sup>+</sup>, see {{cite journal |last1=Heinze |first1=Thorsten |last2=Roesky |first2=Herbert W. |last3=Pauer |first3=Frank |last4=Stalke |first4=Dietmar |last5=Sheldrick |first5=George M. |year=1991 |title=Synthesis and Structure of the First Tellurium(III) Radical Cation |journal=Angewandte Chemie International Edition |volume=30 |issue=12 |pages=1678 |doi= 10.1002/anie.199116771 |url=https://www.researchgate.net/publication/237225046 |access-date=2015-02-23}}</ref><!-- |
|||
--><ref>Te(V) is mentioned by Greenwood and Earnshaw, but they do not give any example of a Te(V) compound. What was long thought to be [[ditellurium decafluoride]] (Te<sub>2</sub>F<sub>10</sub>) is actually bis(pentafluorotelluryl) oxide, F<sub>5</sub>TeOTeF<sub>5</sub>: see {{cite journal |author= Watkins, P. M. |title= Ditellurium decafluoride - A Continuing Myth |journal= Journal of Chemical Education |year= 1974 |volume= 51 |issue= 9 |pages= 520–521 |doi= 10.1021/ed051p520|bibcode= 1974JChEd..51..520W }} However, Te(V) has been observed in {{chem2|HTeO-}}, {{chem2|TeO-}}, {{chem2|HTeO2-}}, and {{chem2|TeO3-}}; see {{cite journal|doi=10.1021/jp010577i|title=Tellurium(V). A Pulse Radiolysis Study|year=2001|last1=Kläning|first1=Ulrik K.|last2=Sehested|first2=K.|journal=The Journal of Physical Chemistry A|volume=105|issue=27|pages=6637–45|bibcode=2001JPCA..105.6637K|url=http://orbit.dtu.dk/en/publications/tellurium-5-a-pulse-radiolysis-study(58c2417f-34c0-436d-8a46-211f3d752423).html}}</ref> |
|||
|- |
|- |
||
| 53 |
| 53 |
||
| 第1,235行: | 第1,276行: | ||
| |
| |
||
| style="text-align:right;" | [[17族元素|17]] |
| style="text-align:right;" | [[17族元素|17]] |
||
| |
| <!-- |
||
--><ref>I(II) is known to exist in monoxide (IO); see {{cite journal|last1=Nikitin|first1=I V|title=Halogen monoxides|journal=Russian Chemical Reviews|date=31 August 2008|volume=77|issue=8|pages=739–749|doi=10.1070/RC2008v077n08ABEH003788|bibcode=2008RuCRv..77..739N}}</ref><!-- |
|||
--><ref>I(IV) has been observed in [[Iodine oxide|iodine dioxide]] (IO<sub>2</sub>); see {{cite book|publisher= Dover Publications, Inc.|year= 1988|edition= 3rd|page= 259|isbn= 978-0-486-65622-9|title= General Chemistry|first= Linus|last= Pauling|chapter= Oxygen Compounds of Nonmetallic Elements}}</ref><!-- |
|||
--><ref>I(VI) has been observed in IO<sub>3</sub>, IO<sub>4</sub><sup>2−</sup>, H<sub>5</sub>IO<sub>6</sub><sup>−</sup>, H<sub>2</sub>IO<sub>5</sub><sup>2−</sup>, H<sub>4</sub>IO<sub>6</sub><sup>2−</sup>, and HIO<sub>5</sub><sup>3−</sup>; see {{cite journal|doi=10.1039/F19817701707|title=Laser flash photolysis and pulse radiolysis of iodate and periodate in aqueous solution. Properties of iodine(VI)|year=1981|last1=Kläning|first1=Ulrik K.|last2=Sehested|first2=Knud|last3=Wolff|first3=Thomas|journal=J. Chem. Soc., Faraday Trans. 1|volume=77|issue=7|pages=1707–18}}</ref> |
|||
|- |
|- |
||
|- style="background:#B2FFFF" |
|- style="background:#B2FFFF" |
||
| 第1,257行: | 第1,301行: | ||
| |
| |
||
| style="text-align:right;" | [[18族元素|18]] |
| style="text-align:right;" | [[18族元素|18]] |
||
| |
| <!-- |
||
--><ref>Xe compounds: see [[Xenon#Compounds|Xenon]]</ref><!-- |
|||
--><ref>Xe(0) has been observed in [[tetraxenonogold(II)]] (AuXe<sub>4</sub><sup>2+</sup>).</ref><!-- |
|||
--><ref>Xe(I) has been reported in [[xenon hexafluoroplatinate]] and [[xenon hexafluororhodate]] (see {{cite book|publisher= Dover Publications, Inc.|year= 1988|edition= 3rd|page= 250|isbn= 978-0-486-65622-9|title= General Chemistry|first= Linus|last= Pauling}}), however these compounds were later found to contain Xe(II).</ref> |
|||
<!--- 第六週期 ---> |
<!--- 第六週期 ---> |
||
|- |
|- |
||
2023年5月28日 (日) 07:35的版本
元素氧化态列表列出目前已知的118種化学元素之所有已知的整数氧化态,其中最常见的氧化态以粗体标记。所有元素以单质存在時(無論單原子或多原子,包括同素異形體)氧化态均为0,因此下表中氧化態0的欄位只列出已發現在化合物中存在氧化態0的元素。
该列表主要参考《元素化学》(Chemistry of the Elements)[1],显示出元素周期律在元素价态上的一些趋势。
+1 粗體表示其為該元素之主要氧化態
| 化學元素氧化態列表 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 元素 | 負氧化態 | 正氧化態 | 族 | 註解 | |||||||||||||||
| −5 | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | |||||
| Z | |||||||||||||||||||
| 1 | 氫 | H | −1 | +1 | 1 | ||||||||||||||
| 2 | 氦 | He | 0 | 18 | [2] | ||||||||||||||
| 3 | 鋰 | Li | 0 | +1 | 1 | [3][4] | |||||||||||||
| 4 | 鈹 | Be | 0 | +1 | +2 | 2 | [5][6] | ||||||||||||
| 5 | 硼 | B | −5 | −1 | 0 | +1 | +2 | +3 | 13 | [7][8][9] | |||||||||
| 6 | 碳 | C | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | |||||||
| 7 | 氮 | N | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | [10] | ||||||
| 8 | 氧 | O | −2 | −1 | 0 | +1 | +2 | 16 | |||||||||||
| 9 | 氟 | F | −1 | 0 | 17 | [11][12] | |||||||||||||
| 10 | 氖 | Ne | 0 | 18 | [13] | ||||||||||||||
| 11 | 鈉 | Na | −1 | 0 | +1 | 1 | [3][14] | ||||||||||||
| 12 | 鎂 | Mg | 0 | +1 | +2 | 2 | [15][16] | ||||||||||||
| 13 | 鋁 | Al | −2 | −1 | 0 | +1 | +2 | +3 | 13 | [17][18][19][20] | |||||||||
| 14 | 矽 | Si | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [21] | ||||||
| 15 | 磷 | P | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | [22] | ||||||
| 16 | 硫 | S | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | |||||||
| 17 | 氯 | Cl | −1 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 17 | [23] | |||||||
| 18 | 氬 | Ar | 0 | 18 | [24] | ||||||||||||||
| 19 | 鉀 | K | −1 | +1 | 1 | [3] | |||||||||||||
| 20 | 鈣 | Ca | +1 | +2 | 2 | [25][26] | |||||||||||||
| 21 | 鈧 | Sc | 0 | +1 | +2 | +3 | 3 | [27][28][29] | |||||||||||
| 22 | 鈦 | Ti | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 4 | [30][31][32][33] | ||||||||
| 23 | 釩 | V | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 5 | [31] | |||||||
| 24 | 鉻 | Cr | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [31] | |||||
| 25 | 錳 | Mn | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | |||||
| 26 | 鐵 | Fe | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 8 | [34][35][36] | ||||
| 27 | 鈷 | Co | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 9 | [31] | |||||||
| 28 | 鎳 | Ni | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 10 | [37] | ||||||||
| 29 | 銅 | Cu | −2 | 0 | +1 | +2 | +3 | +4 | 11 | [36][38] | |||||||||
| 30 | 鋅 | Zn | −2 | 0 | +1 | +2 | 12 | [36][39][40][41] | |||||||||||
| 31 | 鎵 | Ga | −5 | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | 13 | [18][42][43][44] | ||||||
| 32 | 鍺 | Ge | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [45][21] | ||||||
| 33 | 砷 | As | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | [18][46][47][48] | ||||||
| 34 | 硒 | Se | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [49][50][51][52][53] | ||||||
| 35 | 溴 | Br | −1 | +1 | +2 | +3 | +4 | +5 | +7 | 17 | [54] | ||||||||
| 36 | 氪 | Kr | 0 | +1 | +2 | 18 | |||||||||||||
| 37 | 銣 | Rb | −1 | +1 | 1 | [3] | |||||||||||||
| 38 | 鍶 | Sr | +1 | +2 | 2 | [55][26] | |||||||||||||
| 39 | 釔 | Y | 0 | +1 | +2 | +3 | 3 | [56][57][58] | |||||||||||
| 40 | 鋯 | Zr | −2 | 0 | +1 | +2 | +3 | +4 | 4 | [31][59][60] | |||||||||
| 41 | 鈮 | Nb | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 5 | [31][61][62] | |||||||
| 42 | 鉬 | Mo | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | [31] | |||||
| 43 | 鎝 | Tc | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 44 | 釕 | Ru | −4 | −2 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | [31][36] | ||||
| 45 | 銠 | Rh | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 9 | [31][63][64] | |||||
| 46 | 鈀 | Pd | 0 | +1 | +2 | +3 | +4 | +5 | 10 | [65][66][67][68] | |||||||||
| 47 | 銀 | Ag | −2 | −1 | 0 | +1 | +2 | +3 | 11 | [36][69][70] | |||||||||
| 48 | 鎘 | Cd | −2 | +1 | +2 | 12 | [36][71] | ||||||||||||
| 49 | 銦 | In | −5 | −2 | −1 | 0 | +1 | +2 | +3 | 13 | [18][72][73][74] | ||||||||
| 50 | 錫 | Sn | −4 | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | [18][75][76][21] | ||||||
| 51 | 銻 | Sb | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | [18][77][78][79][80] | ||||||
| 52 | 碲 | Te | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 16 | [18][81][82][83][84] | ||||||
| 53 | 碘 | I | −1 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 17 | [85][86][87] | |||||||
| 54 | 氙 | Xe | 0 | +2 | +4 | +6 | +8 | 18 | [88][89][90] | ||||||||||
| 55 | 銫 | Cs | −1 | +1 | 1 | ||||||||||||||
| 56 | 鋇 | Ba | +1 | +2 | 2 | ||||||||||||||
| 57 | 鑭 | La | 0 | +1 | +2 | +3 | f區元素 | ||||||||||||
| 58 | 鈰 | Ce | +2 | +3 | +4 | f區元素 | |||||||||||||
| 59 | 鐠 | Pr | 0 | +1 | +2 | +3 | +4 | +5 | f區元素 | ||||||||||
| 60 | 釹 | Nd | 0 | +2 | +3 | +4 | f區元素 | ||||||||||||
| 61 | 鉕 | Pm | +2 | +3 | f區元素 | ||||||||||||||
| 62 | 釤 | Sm | 0 | +1 | +2 | +3 | f區元素 | ||||||||||||
| 63 | 銪 | Eu | 0 | +2 | +3 | f區元素 | |||||||||||||
| 64 | 釓 | Gd | 0 | +1 | +2 | +3 | f區元素 | ||||||||||||
| 65 | 鋱 | Tb | 0 | +1 | +2 | +3 | +4 | f區元素 | |||||||||||
| 66 | 鏑 | Dy | 0 | +2 | +3 | +4 | f區元素 | ||||||||||||
| 67 | 鈥 | Ho | 0 | +2 | +3 | f區元素 | |||||||||||||
| 68 | 鉺 | Er | 0 | +2 | +3 | f區元素 | |||||||||||||
| 69 | 銩 | Tm | 0 | +1 | +2 | +3 | f區元素 | ||||||||||||
| 70 | 鐿 | Yb | 0 | +1 | +2 | +3 | f區元素 | ||||||||||||
| 71 | 鎦 | Lu | 0 | +2 | +3 | 3 | |||||||||||||
| 72 | 鉿 | Hf | −2 | 0 | +1 | +2 | +3 | +4 | 4 | ||||||||||
| 73 | 鉭 | Ta | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 5 | ||||||||
| 74 | 鎢 | W | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 6 | ||||||
| 75 | 錸 | Re | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | 7 | ||||||
| 76 | 鋨 | Os | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | 8 | ||||
| 77 | 銥 | Ir | −3 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | 9 | ||||
| 78 | 鉑 | Pt | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | +6 | 10 | ||||||
| 79 | 金 | Au | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +5 | 11 | ||||||||
| 80 | 汞 | Hg | −2 | +1 | +2 | 12 | |||||||||||||
| 81 | 鉈 | Tl | −5 | −2 | −1 | +1 | +2 | +3 | 13 | ||||||||||
| 82 | 鉛 | Pb | −4 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | 14 | ||||||||
| 83 | 鉍 | Bi | −3 | −2 | −1 | 0 | +1 | +2 | +3 | +4 | +5 | 15 | |||||||
| 84 | 釙 | Po | −2 | +2 | +4 | +5 | +6 | 16 | |||||||||||
| 85 | 砈 | At | −1 | +1 | +3 | +5 | +7 | 17 | |||||||||||
| 86 | 氡 | Rn | +2 | +6 | 18 | ||||||||||||||
| 87 | 鍅 | Fr | +1 | 1 | |||||||||||||||
| 88 | 鐳 | Ra | +2 | 2 | |||||||||||||||
| 89 | 錒 | Ac | +3 | f區元素 | |||||||||||||||
| 90 | 釷 | Th | −1 | +1 | +2 | +3 | +4 | f區元素 | |||||||||||
| 91 | 鏷 | Pa | +2 | +3 | +4 | +5 | f區元素 | ||||||||||||
| 92 | 鈾 | U | −1 | +1 | +2 | +3 | +4 | +5 | +6 | f區元素 | |||||||||
| 93 | 錼 | Np | +2 | +3 | +4 | +5 | +6 | +7 | f區元素 | ||||||||||
| 94 | 鈽 | Pu | +2 | +3 | +4 | +5 | +6 | +7 | +8 | f區元素 | |||||||||
| 95 | 鋂 | Am | +2 | +3 | +4 | +5 | +6 | +7 | f區元素 | ||||||||||
| 96 | 鋦 | Cm | +3 | +4 | +5 | +6 | f區元素 | ||||||||||||
| 97 | 鉳 | Bk | +2 | +3 | +4 | +5 | f區元素 | ||||||||||||
| 98 | 鉲 | Cf | +2 | +3 | +4 | +5 | f區元素 | ||||||||||||
| 99 | 鑀 | Es | +2 | +3 | +4 | f區元素 | |||||||||||||
| 100 | 鐨 | Fm | +2 | +3 | f區元素 | ||||||||||||||
| 101 | 鍆 | Md | +2 | +3 | f區元素 | ||||||||||||||
| 102 | 鍩 | No | +2 | +3 | f區元素 | ||||||||||||||
| 103 | 鐒 | Lr | +3 | 3 | |||||||||||||||
| 104 | 鑪 | Rf | +4 | 4 | |||||||||||||||
| 105 | 𨧀 | Db | +5 | 5 | |||||||||||||||
| 106 | 𨭎 | Sg | 0 | +6 | 6 | ||||||||||||||
| 107 | 𨨏 | Bh | +7 | 7 | |||||||||||||||
| 108 | 𨭆 | Hs | +8 | 8 | |||||||||||||||
| 109 | 䥑 | Mt | 9 | ||||||||||||||||
| 110 | 鐽 | Ds | 10 | ||||||||||||||||
| 111 | 錀 | Rg | 11 | ||||||||||||||||
| 112 | 鎶 | Cn | +2 | 12 | |||||||||||||||
| 113 | 鉨 | Nh | 13 | ||||||||||||||||
| 114 | 鈇 | Fl | 14 | ||||||||||||||||
| 115 | 鏌 | Mc | 15 | ||||||||||||||||
| 116 | 鉝 | Lv | 16 | ||||||||||||||||
| 117 | 鿬 | Ts | 17 | ||||||||||||||||
| 118 | 鿫 | Og | 18 | ||||||||||||||||
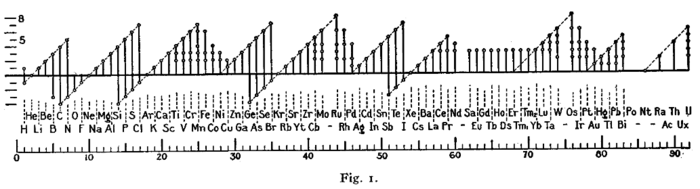
下图是欧文·朗缪尔1919年在研究八隅体规则时所画:[91]
参考资料
- ^ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements, 2nd Edition, Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4, p. 28.
- ^ Disodium helide, (Na+)2He(e-)2, has been synthesized at high pressure, see Dong, Xiao; Oganov, Artem R.; Goncharov, Alexander F.; Stavrou, Elissaios; Lobanov, Sergey; Saleh, Gabriele; Qian, Guang-Rui; Zhu, Qiang; Gatti, Carlo; Deringer, Volker L.; Dronskowski, Richard; Zhou, Xiang-Feng; Prakapenka, Vitali B.; Konôpková, Zuzana; Popov, Ivan A.; Boldyrev, Alexander I.; Wang, Hui-Tian. A stable compound of helium and sodium at high pressure. Nature Chemistry. 6 February 2017, 9 (5): 440–445. Bibcode:2017NatCh...9..440D. PMID 28430195. S2CID 20459726. arXiv:1309.3827
 . doi:10.1038/nchem.2716.
. doi:10.1038/nchem.2716.
- ^ 3.0 3.1 3.2 3.3 Na(−1), K(−1), Rb(−1), and Cs(−1) are known in alkalides; the table by Greenwood and Earnshaw shows −1 only for Na and also erroneously for Li; no lithides are described.
- ^ Li(0) atoms have been observed in various small lithium-chloride clusters; see Milovanović, Milan; Veličković, Suzana; Veljkovićb, Filip; Jerosimić, Stanka. Structure and stability of small lithium-chloride LinClm(0,1+) (n ≥ m, n = 1–6, m = 1–3) clusters. Physical Chemistry Chemical Physics. October 30, 2017, (45). doi:10.1039/C7CP04181K.
- ^ Be(0) has been observed; see Beryllium(0) Complex Found. Chemistry Europe. 13 June 2016.
- ^ Be(I) has been observed in beryllium monohydride (BeH); see Shayesteh, A.; Tereszchuk, K.; Bernath, P. F.; Colin, R. Infrared Emission Spectra of BeH and BeD (PDF). J. Chem. Phys. 2003, 118 (3): 1158 [2007-12-10]. Bibcode:2003JChPh.118.1158S. doi:10.1063/1.1528606. (原始内容 (PDF)存档于2007-12-02). and in [(CAAC)2Be]+• [CAAC = cyclic (alkyl)(amino)carbene], see Wang, Guocang; Walley, Jacob E.; Dickie, Diane E.; Pan, Sudip; Frenking, Gernot; Gilliard Jr., Robert G. A Stable, Crystalline Beryllium Radical Cation. J. Am. Chem. Soc. 2020, 142 (10): 4560–4 [2020-11-17]. PMID 32088963. S2CID 211262005. doi:10.1021/jacs.9b13777.
- ^ B(−5) has been observed in Al3BC, see Schroeder, Melanie. Eigenschaften von borreichen Boriden und Scandium-Aluminium-Oxid-Carbiden. : 139 (德语).
- ^ B(−1) has been observed in magnesium diboride (MgB2), see Keeler, James; Wothers, Peter. Chemical Structure and Reactivity: An Integrated Approach. Oxford University Press. 2014. ISBN 9780199604135.
- ^ B(0) has been observed in diborynes, see Braunschweig, H.; Dewhurst, R. D.; Hammond, K.; Mies, J.; Radacki, K.; Vargas, A. Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond. Science. 2012, 336 (6087): 1420–2. Bibcode:2012Sci...336.1420B. PMID 22700924. S2CID 206540959. doi:10.1126/science.1221138.
- ^ Tetrazoles contain a pair of double-bonded nitrogen atoms with oxidation state 0 in the ring. A Synthesis of the parent 1H-tetrazole, CH2N4 (two atoms N(0)) is given in Ronald A. Henry and William G. Finnegan, "An Improved Procedure for the Deamination of 5-Aminotetrazole", _J. Am. Chem. Soc._ (1954), 76, 1, 290–291, https://doi.org/10.1021/ja01630a086.
- ^ Gold heptafluoride is calculated to be the pentafluoride with a molecular F2 ligand. Himmel, Daniel; Riedel, Sebastian. After 20 Years, Theoretical Evidence That 'AuF7' Is Actually AuF5•F2. Inorganic Chemistry. 2007, 46 (13): 5338–5342. PMID 17511450. doi:10.1021/ic700431s.
- ^ A cluster of elusive SF6+ with helium atoms is known to have fluorine(0) atom as a ligand; see Albertini, Simon; Bergmeister, Stefan; Laimer, Felix; Martini, Paul; Gruber, Elisabeth; Zappa, Fabio; Ončák, Milan; Scheier, Paul; Echt, Olof. SF 6 + : Stabilizing Transient Ions in Helium Nanodroplets. The Journal of Physical Chemistry Letters. 2021-04-22, 12 (17): 4112–4117. ISSN 1948-7185. PMC 8154854
 . PMID 33886323. doi:10.1021/acs.jpclett.1c01024
. PMID 33886323. doi:10.1021/acs.jpclett.1c01024  (英语).
(英语).
- ^ Ne(0) has been observed in Cr(CO)5Ne. Perutz, Robin N.; Turner, James J. Photochemistry of the Group 6 hexacarbonyls in low-temperature matrices. III. Interaction of the pentacarbonyls with noble gases and other matrices. Journal of the American Chemical Society. August 1975, 97 (17): 4791–4800. doi:10.1021/ja00850a001.
- ^ The compound NaCl has been shown in experiments to exists in several unusual stoichiometries under high pressure, including Na3Cl in which contains a layer of sodium(0) atoms; see Zhang, W.; Oganov, A. R.; Goncharov, A. F.; Zhu, Q.; Boulfelfel, S. E.; Lyakhov, A. O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V. B.; Konôpková, Z. Unexpected Stable Stoichiometries of Sodium Chlorides. Science. 2013, 342 (6165): 1502–1505. Bibcode:2013Sci...342.1502Z. PMID 24357316. S2CID 15298372. arXiv:1310.7674
 . doi:10.1126/science.1244989.
. doi:10.1126/science.1244989.
- ^ Low valent magnesium compounds with Mg(I) have been obtained using bulky ligands; see Green, S. P.; Jones C.; Stasch A. Stable Magnesium(I) Compounds with Mg-Mg Bonds. Science. December 2007, 318 (5857): 1754–1757. Bibcode:2007Sci...318.1754G. PMID 17991827. S2CID 40657565. doi:10.1126/science.1150856.
- ^ Mg(0) has been synthesized in a compound containing a Na2Mg22+ cluster coordinated to a bulky organic ligand; see Rösch, B.; Gentner, T. X.; Eyselein, J.; Langer, J.; Elsen, H.; Li, W.; Harder, S. Strongly reducing magnesium(0) complexes. Nature. 2021, 592 (7856): 717–721. Bibcode:2021Natur.592..717R. PMID 33911274. S2CID 233447380. doi:10.1038/s41586-021-03401-w
- ^ Al(II) has been observed in aluminium(II) oxide (AlO); see Tyte, D.C. Red (B2Π–A2σ) Band System of Aluminium Monoxide. Nature. 1964, 202 (4930): 383–384. Bibcode:1964Natur.202..383T. S2CID 4163250. doi:10.1038/202383a0, and in dialanes (R2Al—AlR2); see Uhl, Werner. Organoelement Compounds Possessing Al—Al, Ga—Ga, In—In, and Tl—Tl Single Bonds. Advances in Organometallic Chemistry. 2004, 51: 53–108. doi:10.1016/S0065-3055(03)51002-4.
- ^ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Negative oxidation states of p-block metals (Al, Ga, In, Sn, Tl, Pb, Bi, Po) and metalloids (Si, Ge, As, Sb, Te, At) may occur in Zintl phases, see: Riedel, Erwin (编). Moderne Anorganische Chemie. 2007: 259 (德语), and Vorlesung Intermetallische Phasen § 6.2 Binäre Zintl-Phasen (德语).
- ^ Unstable carbonyl of Al(0) has been detected in reaction of Al2(CH3)6 with carbon monoxide; see Sanchez, Ramiro; Arrington, Caleb; Arrington Jr., C. A. Reaction of trimethylaluminum with carbon monoxide in low-temperature matrixes. American Chemical Society. December 1, 1989, 111 (25): 9110-9111. doi:10.1021/ja00207a023.
- ^ Al(−2) has been observed in Sr14[Al4]2[Ge]3, see Wemdorff, Marco; Röhr, Caroline. Sr14[Al4]2[Ge]3: Eine Zintl-Phase mit isolierten [Ge]4–- und [Al4]8–-Anionen / Sr14[Al4]2[Ge]3: A Zintl Phase with Isolated [Ge]4–- and [Al4]8– Anions. Zeitschrift für Naturforschung B. 2007, 62 (10): 1227. S2CID 94972243. doi:10.1515/znb-2007-1001 (德语).
- ^ 21.0 21.1 21.2 New Type of Zero-Valent Tin Compound. Chemistry Europe. 27 August 2016.
- ^ P(0) has been observed, see Wang, Yuzhong; Xie, Yaoming; Wei, Pingrong; King, R. Bruce; Schaefer, Iii; Schleyer, Paul v. R.; Robinson, Gregory H. Carbene-Stabilized Diphosphorus. Journal of the American Chemical Society. 2008, 130 (45): 14970–1. PMID 18937460. doi:10.1021/ja807828t.
- ^ The equilibrium Cl2O6⇌2ClO3 is mentioned by Greenwood and Earnshaw, but it has been refuted, see Lopez, Maria; Juan E. Sicre. Physicochemical properties of chlorine oxides. 1. Composition, ultraviolet spectrum, and kinetics of the thermolysis of gaseous dichlorine hexoxide. J. Phys. Chem. 1990, 94 (9): 3860–3863. doi:10.1021/j100372a094., and Cl2O6 is actually chlorine(V,VII) oxide. However, ClO3 has been observed, see Grothe, Hinrich; Willner, Helge. Chlorine Trioxide: Spectroscopic Properties, Molecular Structure, and Photochemical Behavior. Angew. Chem. Int. Ed. 1994, 33 (14): 1482–1484. doi:10.1002/anie.199414821.
- ^ Ar(0) has been observed in argon fluorohydride (HArF) and ArCF22+, see Lockyear, J.F.; Douglas, K.; Price, S.D.; Karwowska, M.; et al. Generation of the ArCF22+ Dication. Journal of Physical Chemistry Letters. 2010, 1: 358. doi:10.1021/jz900274p.
- ^ Ca(I) has been observed; see Krieck, Sven; Görls, Helmar; Westerhausen, Matthias. Mechanistic Elucidation of the Formation of the Inverse Ca(I) Sandwich Complex [(thf)3Ca(μ-C6H3-1,3,5-Ph3)Ca(thf)3] and Stability of Aryl-Substituted Phenylcalcium Complexes. Journal of the American Chemical Society. 2010, 132 (35): 12492–501. PMID 20718434. doi:10.1021/ja105534w.
- ^ 26.0 26.1 Octacarbonyl complexes isolated of Ca, Sr, Ba have been observed in a neon matrix, but it remains unclear whether these are metal(0) complexes because calculations disagree whether the metal is covalently or ionically bonded to the ligands; see Wu, X.; Zhao, L.; Jin, J.; Pan, S.; Li, W.; Jin, X.; Wang, G.; Zhou, M.; Frenking, G. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science. 2018, 361 (6405): 912–916. Bibcode:2018Sci...361..912W. PMID 30166489. S2CID 52131470. doi:10.1126/science.aau0839
- ^ Sc(0) has been observed; see F. Geoffrey N. Cloke; Karl Khan & Robin N. Perutz. η-Arene complexes of scandium(0) and scandium(II). J. Chem. Soc., Chem. Commun. 1991, (19): 1372–1373. doi:10.1039/C39910001372.
- ^ Sc(I) has been observed; see Polly L. Arnold; F. Geoffrey; N. Cloke; Peter B. Hitchcock & John F. Nixon. The First Example of a Formal Scandium(I) Complex: Synthesis and Molecular Structure of a 22-Electron Scandium Triple Decker Incorporating the Novel 1,3,5-Triphosphabenzene Ring. J. Am. Chem. Soc. 1996, 118 (32): 7630–7631. doi:10.1021/ja961253o.
- ^ Sc(II) has been observed; see Woen, David H.; Chen, Guo P.; Ziller, Joseph W.; Boyle, Timothy J.; Furche, Filipp; Evans, William J. Solution Synthesis, Structure, and CO Reduction Reactivity of a Scandium(II) Complex. Angewandte Chemie International Edition. January 2017, 56 (8): 2050–2053. PMID 28097771. doi:10.1002/anie.201611758.
- ^ Ti(I) has been observed in [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2); see Calderazzo, Fausto; Ferri, Isabella; Pampaloni, Guido; Englert, Ulli; Green, Malcolm L. H. Synthesis of [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2), the First Titanium(I) Derivatives. Organometallics. 1997, 16 (14): 3100–3101. doi:10.1021/om970155o.
- ^ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 31.7 31.8 Ti(−2), V(−3), Cr(−4), Co(−3), Zr(−2), Nb(−3), Mo(−4), Ru(−2), Rh(−3), Hf(−2), Ta(−3), and W(−4) occur in anionic binary metal carbonyls; see [1], p. 4 (in German); [2], pp. 97–100; [3], p. 239
- ^ Ti(−1) has been reported in [Ti(bipy)3]−, but was later shown to be Ti(+3); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study. Inorganic Chemistry. 2013, 52 (4): 2242–56. PMID 23387926. doi:10.1021/ic302799s. However, Ti(−1) occurs in [Ti(η-C6H6]− and [Ti(η-C6H5CH3)]−, see Bandy, J. A.; Berry, A.; Green, M. L. H.; Perutz, R. N.; Prout, K.; Verpeautz, J.-N. Synthesis of anionic sandwich compounds: [Ti(η-C6H5R)2]– and the crystal structure of [K(18-crown-6)(µ-H)Mo(η-C5H5)2]. Inorganic Chemistry. 1984, 52 (4): 729–731. doi:10.1039/C39840000729.
- ^ Jilek, Robert E.; Tripepi, Giovanna; Urnezius, Eugenijus; Brennessel, William W.; Young, Victor G. Jr.; Ellis, John E. Zerovalent titanium–sulfur complexes. Novel dithiocarbamato derivatives of Ti(CO)6: [Ti(CO)4(S2CNR2)]−. Chem. Commun. 2007, (25): 2639–2641. PMID 17579764. doi:10.1039/B700808B.
- ^ Fe(VII) has been observed in [FeO4]−; see Lu, Jun-Bo; Jian, Jiwen; Huang, Wei; Lin, Hailu; Zhou, Mingfei. Experimental and theoretical identification of the Fe(VII) oxidation state in FeO4−. Physical Chemistry Chemical Physics. 2016, 18 (45): 31125–31131. Bibcode:2016PCCP...1831125L. PMID 27812577. doi:10.1039/C6CP06753K.
- ^ Fe(VIII) has been reported; see Yurii D. Perfiliev; Virender K. Sharma. Higher Oxidation States of Iron in Solid State: Synthesis and Their Mössbauer Characterization – Ferrates – ACS Symposium Series (ACS Publications). Platinum Metals Review. 2008, 48 (4): 157–158. doi:10.1595/147106704X10801. However, its existence has been disputed.
- ^ 36.0 36.1 36.2 36.3 36.4 36.5 Fe(−4), Ru(−4), and Os(−4) have been observed in metal-rich compounds containing octahedral complexes [MIn6−xSnx]; Pt(−3) (as a dimeric anion [Pt–Pt]6−), Cu(−2), Zn(−2), Ag(−2), Cd(−2), Au(−2), and Hg(−2) have been observed (as dimeric and monomeric anions; dimeric ions were initially reported to be [T–T]2− for Zn, Cd, Hg, but later shown to be [T–T]4− for all these elements) in La2Pt2In, La2Cu2In, Ca5Au3, Ca5Ag3, Ca5Hg3, Sr5Cd3, Ca5Zn3(structure (AE2+)5(T–T)4−T2−⋅4e−), Yb3Ag2, Ca5Au4, and Ca3Hg2; Au(–3) has been observed in ScAuSn and in other 18-electron half-Heusler compounds. See Changhoon Lee; Myung-Hwan Whangbo. Late transition metal anions acting as p-metal elements. Solid State Sciences. 2008, 10 (4): 444–449. Bibcode:2008SSSci..10..444K. doi:10.1016/j.solidstatesciences.2007.12.001. and Changhoon Lee; Myung-Hwan Whangbo; Jürgen Köhler. Analysis of Electronic Structures and Chemical Bonding of Metal-rich Compounds. 2. Presence of Dimer (T–T)4– and Isolated T2– Anions in the Polar Intermetallic Cr5B3-Type Compounds AE5T3 (AE = Ca, Sr; T = Au, Ag, Hg, Cd, Zn). Zeitschrift für Anorganische und Allgemeine Chemie. 2010, 636 (1): 36–40. doi:10.1002/zaac.200900421.
- ^ Ni(−2) has been observed in Li2[Ni(1,5-COD)2], see Jonas, Klaus. Dilithium-Nickel-Olefin Complexes. Novel Bimetal Complexes Containing a Transition Metal and a Main Group Metal. Angew. Chem. Int. Ed. 1975, 14 (11): 752–753. doi:10.1002/anie.197507521. and Ellis, John E. Adventures with Substances Containing Metals in Negative Oxidation States. Inorganic Chemistry. 2006, 45 (8): 3167–86. PMID 16602773. doi:10.1021/ic052110i.
- ^ Cu(0) has been observed in Cu(tris[2-(diisopropylphosphino)- phenyl]borane), see Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. A Polar Copper–Boron One-Electron σ-Bond. J. Am. Chem. Soc. 2013, 135 (10): 3792–3795. PMID 23418750. doi:10.1021/ja4006578.
- ^ Zn(0) has been observed; see Singh, Amit Pratap; Samuel, Prinson P.; Roesky, Herbert W.; Schwarzer, Martin C.; Frenking, Gernot; Sidhu, Navdeep S.; Dittrich, Birger. A Singlet Biradicaloid Zinc Compound and Its Nonradical Counterpart. J. Am. Chem. Soc. 2013, 135 (19): 7324–9. PMID 23600486. doi:10.1021/ja402351x. and Soleilhavoup, Michèle; Bertrand, Guy. Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48 (2): 256–266. PMID 25515548. doi:10.1021/ar5003494.
- ^ Zn(I) has been observed in decamethyldizincocene (Zn2(η5–C5Me5)2); see Resa, I.; Carmona, E.; Gutierrez-Puebla, E.; Monge, A. Decamethyldizincocene, a Stable Compound of Zn(I) with a Zn-Zn Bond. Science. 2004, 305 (5687): 1136–8. Bibcode:2004Sci...305.1136R. PMID 15326350. S2CID 38990338. doi:10.1126/science.1101356.
- ^ Zn(III) has been predicted to be stable in compounds with highly stabilized borane-based trianions, but no Zn(III) candidates are known experimentally; see Hong Fang; Huta Banjade; Deepika; Puru Jena. Realization of the Zn3+ oxidation state. Nanoscale. 2021, 13 (33): 14041–14048. PMID 34477685. S2CID 237400349. doi:10.1039/D1NR02816B (English).
- ^ Ga(−2), Ga(−4), and Ga(−5) have been observed in the magnesium gallides MgGa, Mg2Ga, and Mg5Ga2, respectively; see Patrick Hofmann. Colture. Ein Programm zur interaktiven Visualisierung von Festkörperstrukturen sowie Synthese, Struktur und Eigenschaften von binären und ternären Alkali- und Erdalkalimetallgalliden (PDF). : 72 (德语).
- ^ Ga(−3) has been observed in LaGa, see Dürr, Ines; Bauer, Britta; Röhr, Caroline. Lanthan-Triel/Tetrel-ide La(Al,Ga)x(Si,Ge)1-x. Experimentelle und theoretische Studien zur Stabilität intermetallischer 1:1-Phasen (PDF). Z. Naturforsch. 2011, 66b: 1107–1121. 已忽略未知参数
|lang=(建议使用|language=) (帮助) - ^ Ga(0) has been observed in Gallium monoiodide among other gallium's oxidation states
- ^ Ge(−1), Ge(−2), and Ge(−3) have been observed in germanides; see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils. Germanium. Lehrbuch der Anorganischen Chemie 101. Walter de Gruyter. 1995: 953–959. ISBN 978-3-11-012641-9 (德语).
- ^ As(0) has been observed; see Abraham, Mariham Y.; Wang, Yuzhong; Xie, Yaoming; Wei, Pingrong; Shaefer III, Henry F.; Schleyer, P. von R.; Robinson, Gregory H. Carbene Stabilization of Diarsenic: From Hypervalency to Allotropy. Chemistry: A European Journal. 2010, 16 (2): 432–5. PMID 19937872. doi:10.1002/chem.200902840.
- ^ As(I) has been observed in arsenic(I) iodide (AsI); see Ellis, Bobby D.; MacDonald, Charles L. B. Stabilized Arsenic(I) Iodide: A Ready Source of Arsenic Iodide Fragments and a Useful Reagent for the Generation of Clusters. Inorganic Chemistry. 2004, 43 (19): 5981–6. PMID 15360247. doi:10.1021/ic049281s.
- ^ As(IV) has been observed in arsenic(IV) hydroxide (As(OH)4) and HAsO−
; see Kläning, Ulrik K.; Bielski, Benon H. J.; Sehested, K. Arsenic(IV). A pulse-radiolysis study. Inorganic Chemistry. 1989, 28 (14): 2717–24. doi:10.1021/ic00313a007. - ^ Se(−1) has been observed in diselenides(2−) (Se22−).
- ^ A Se(0) atom has been identified using DFT in [ReOSe(2-pySe)3]; see Cargnelutti, Roberta; Lang, Ernesto S.; Piquini, Paulo; Abram, Ulrich. Synthesis and structure of [ReOSe(2-Se-py)3]: A rhenium(V) complex with selenium(0) as a ligand. Inorganic Chemistry Communications. 2014, 45: 48–50. ISSN 1387-7003. doi:10.1016/j.inoche.2014.04.003.
- ^ Se(I) has been observed in selenium(I) chloride (Se2Cl2); see Selenium: Selenium(I) chloride compound data. WebElements.com. [2007-12-10].
- ^ Se(III) has been observed in Se2NBr3; see Lau, Carsten; Neumüller, Bernhard; Vyboishchikov, Sergei F.; Frenking, Gernot; Dehnicke, Kurt; Hiller, Wolfgang; Herker, Martin. Se2NBr3, Se2NCl5, Se2NCl−6: New Nitride Halides of Selenium(III) and Selenium(IV). Chemistry: A European Journal. 1996, 2 (11): 1393–1396. doi:10.1002/chem.19960021108.
- ^ Se(V) has been observed in SeO−
3 and HSeO2−
4; see Kläning, Ulrik K.; Sehested, K. Selenium(V). A pulse radiolysis study. Inorganic Chemistry. 1986, 90 (21): 5460–4. doi:10.1021/j100412a112. - ^ Br(II) is known to occur in bromine monoxide radical; see [4]
- ^ Sr(I) has been observed in strontium monofluoride (SrF); see P. Colarusso; Guo, B.; Zhang, K.-Q.; Bernath, P.F.; et al. High-Resolution Infrared Emission Spectrum of Strontium Monofluoride (PDF). Journal of Molecular Spectroscopy. 1996, 175 (1): 158–171. Bibcode:1996JMoSp.175..158C. doi:10.1006/jmsp.1996.0019. (原始内容 (PDF)存档于2012-03-08).
- ^ 引用错误:没有为名为
Cloke1993的参考文献提供内容 - ^ Y(I) has been observed in yttrium(I) bromide (YBr); see Yttrium: yttrium(I) bromide compound data. OpenMOPAC.net. [2007-12-10]. (原始内容存档于2011-07-23).
- ^ Y(II) has been observed in [(18-crown-6)K][(C5H4SiMe3)3Y]; see MacDonald, M. R.; Ziller, J. W.; Evans, W. J. Synthesis of a Crystalline Molecular Complex of Y2+, [(18-crown-6)K][(C5H4SiMe3)3Y]. J. Am. Chem. Soc. 2011, 133 (40): 15914–17. PMID 21919538. doi:10.1021/ja207151y.
- ^ Zr(−1) has been reported in [Zr(bipy)3]− (see Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements 2nd. Oxford:Butterworth-Heinemann. 1997: 960. ISBN 0-7506-3365-4. and Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils. Zirconium. Lehrbuch der Anorganischen Chemie 101. Walter de Gruyter. 1995: 1413. ISBN 978-3-11-012641-9 (德语).), but was later shown to be Zr(+4); see Bowman, A. C.; England, J.; Sprouls, S.; Weihemüller, T.; Wieghardt, K. Electronic structures of homoleptic [tris(2,2'-bipyridine)M]n complexes of the early transition metals (M = Sc, Y, Ti, Zr, Hf, V, Nb, Ta; n = 1+, 0, 1-, 2-, 3-): an experimental and density functional theoretical study. Inorganic Chemistry. 2013, 52 (4): 2242–56. PMID 23387926. doi:10.1021/ic302799s.
- ^ Zr(0) and Hf(0) occur in (η6-(1,3,5-tBu)3C6H3)2M (M=Zr, Hf) and [(η5-C5R5M(CO)4]−, see Chirik, P. J.; Bradley, C. A. 4.06 - Complexes of Zirconium and Hafnium in Oxidation States 0 to ii. Comprehensive Organometallic Chemistry III. From Fundamentals to Applications 4. Elsevier Ltd. 2007: 697–739. ISBN 9780080450476. doi:10.1016/B0-08-045047-4/00062-5.
- ^ Complexes of Nb(0) and Ta(0) have been observed, see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils. 4.5.7. Niobium(0) and Tantalum(0). J. A. McCleverty; T.J. Meyer (编). Comprehensive Coordination Chemistry II: From Biology to Nanotechnology 4 2. Newnes. 2003: 297–299. ISBN 978-0-08-091316-2.
- ^ Nb(I) and Ta(I) occur in CpNb(CO)4 and CpTa(CO)4, see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils. Tantal. Lehrbuch der Anorganischen Chemie 101. Walter de Gruyter. 1995: 1430. ISBN 978-3-11-012641-9 (德语). and King, R. Bruce. Transition-Metal Organometallic Chemistry: An Introduction. Academic Press. 1969: 11. ISBN 978-0-32-315996-8.
- ^ George, G.N.; Klein, S.I.; Nixon, J.F. Electron paramagnetic resonance spectroscopic studies on the zero-valent rhodium complex [Rh(P(OPri)3)4] at X-and Q-band frequencies. Chemical Physics Letters. 1984, 108 (6): 627–630. Bibcode:1984CPL...108..627G. doi:10.1016/0009-2614(84)85069-1.
- ^ Rh(VII) is known in the RhO3+ cation, see The Highest Oxidation State of Rhodium: Rhodium(VII) in [RhO3]+. Angew. Chem. Int. Ed. 2022. doi:10.1002/anie.202207688.
- ^ Pd(I) has been observed; see Crabtree, R. H. CHEMISTRY: A New Oxidation State for Pd?. Science. 2002, 295 (5553): 288–289. PMID 11786632. S2CID 94579227. doi:10.1126/science.1067921.
- ^ Pd(III) has been observed; see Powers, D. C.; Ritter, T. Palladium(III) in Synthesis and Catalysis (PDF). Topics in Organometallic Chemistry 35. 2011: 129–156. Bibcode:2011hoso.book..129P. ISBN 978-3-642-17428-5. PMC 3066514
 . PMID 21461129. doi:10.1007/978-3-642-17429-2_6. (原始内容 (PDF)存档于June 12, 2013).
. PMID 21461129. doi:10.1007/978-3-642-17429-2_6. (原始内容 (PDF)存档于June 12, 2013). |journal=被忽略 (帮助) - ^ Palladium(V) has been identified in complexes with organosilicon compounds containing pentacoordinate palladium; see Shimada, Shigeru; Li, Yong-Hua; Choe, Yoong-Kee; Tanaka, Masato; Bao, Ming; Uchimaru, Tadafumi. Multinuclear palladium compounds containing palladium centers ligated by five silicon atoms. Proceedings of the National Academy of Sciences. 2007, 104 (19): 7758–7763. doi:10.1073/pnas.0700450104.
- ^ Palladium(VI) has been claimed to exist in NEW PALLADIUM OXIDATION STATE?. Chem. Eng. News. 2002, 80 (2): 8. doi:10.1021/cen-v080n002.p008., but this has been refuted showing it is a Palladium(II).
- ^ The Ag− ion has been observed in metal ammonia solutions: see Tran, N. E.; Lagowski, J. J. Metal Ammonia Solutions: Solutions Containing Argentide Ions. Inorganic Chemistry. 2001, 40 (5): 1067–68. doi:10.1021/ic000333x.
- ^ Ag(0) has been observed in carbonyl complexes in low-temperature matrices: see McIntosh, D.; Ozin, G. A. Synthesis using metal vapors. Silver carbonyls. Matrix infrared, ultraviolet-visible, and electron spin resonance spectra, structures, and bonding of silver tricarbonyl, silver dicarbonyl, silver monocarbonyl, and disilver hexacarbonyl. J. Am. Chem. Soc. 1976, 98 (11): 3167–75. doi:10.1021/ja00427a018.
- ^ Cd(I) has been observed in cadmium(I) tetrachloroaluminate (Cd2(AlCl4)2); see Holleman, Arnold F.; Wiberg, Egon; Wiberg, Nils. Cadmium. Lehrbuch der Anorganischen Chemie 91–100. Walter de Gruyter. 1985: 1056–1057. ISBN 978-3-11-007511-3 (德语).
- ^ In(–5) has been observed in La3InGe, see Guloy, A. M.; Corbett, J. D. Synthesis, Structure, and Bonding of Two Lanthanum Indium Germanides with Novel Structures and Properties. Inorganic Chemistry. 1996, 35 (9): 2616–22. PMID 11666477. doi:10.1021/ic951378e.
- ^ In(−2) has been observed in Na2In, see [5], p. 69.
- ^ Unstable In(0) carbonyls and clusters have been detected, see [6], p. 6.
- ^ Sn(−3) has been observed in [Sn2]6−, e.g. in (Ba2)4+(Mg4)8+Sn4−(Sn2)6−Sn2− (with square (Sn2−)n sheets), see Papoian, Garegin A.; Hoffmann, Roald. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl–Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 2000 (39): 2408–2448 [2015-02-23]. doi:10.1002/1521-3773(20000717)39:14<2408::aid-anie2408>3.0.co;2-u.
- ^ Sn(I) and Sn(III) have been observed in organotin compounds
- ^ Sb(−2) has been observed in [Sb2]4−, e.g. in RbBa4[Sb2][Sb][O], see Boss, Michael; Petri, Denis; Pickhard, Frank; Zönnchen, Peter; Röhr, Caroline. Neue Barium-Antimonid-Oxide mit den Zintl-Ionen [Sb]3−, [Sb2]4− und 1∞[Sbn]n− / New Barium Antimonide Oxides containing Zintl Ions [Sb]3−, [Sb2]4− and 1∞[Sbn]n−. Zeitschrift für Anorganische und Allgemeine Chemie. 2005, 631 (6–7): 1181–1190. doi:10.1002/zaac.200400546 (德语).
- ^ Sb(0) has been observed, see Anastas Sidiropoulos. Studies of N-heterocyclic Carbene (NHC) Complexes of the Main Group Elements (PDF). : 39. S2CID 132399530. doi:10.4225/03/5B0F4BDF98F60.
- ^ Sb(I) and Sb(II) have been observed in organoantimony compounds; for Sb(I), see Šimon, Petr; de Proft, Frank; Jambor, Roman; Růžička, Aleš; Dostál, Libor. Monomeric Organoantimony(I) and Organobismuth(I) Compounds Stabilized by an NCN Chelating Ligand: Syntheses and Structures. Angewandte Chemie International Edition. 2010, 49 (32): 5468–5471. PMID 20602393. doi:10.1002/anie.201002209.
- ^ Sb(IV) has been observed in [SbCl
6]2−, see Nobuyoshi Shinohara; Masaaki Ohsima. Production of Sb(IV) Chloro Complex by Flash Photolysis of the Corresponding Sb(III) and Sb(V) Complexes in CH3CN and CHCl3. Bulletin of the Chemical Society of Japan. 2000, 73 (7): 1599–1604. doi:10.1246/bcsj.73.1599. - ^ Te(0) has been observed in tellurolates.
- ^ Te(I) has been observed in tellurium iodide (TeI), see Tellurium: tellurium iodide. WebElements.com. [2015-02-23].
- ^ Te(III) has been observed in [Te(N(SiMe3)2)2]+, see Heinze, Thorsten; Roesky, Herbert W.; Pauer, Frank; Stalke, Dietmar; Sheldrick, George M. Synthesis and Structure of the First Tellurium(III) Radical Cation. Angewandte Chemie International Edition. 1991, 30 (12): 1678 [2015-02-23]. doi:10.1002/anie.199116771.
- ^ Te(V) is mentioned by Greenwood and Earnshaw, but they do not give any example of a Te(V) compound. What was long thought to be ditellurium decafluoride (Te2F10) is actually bis(pentafluorotelluryl) oxide, F5TeOTeF5: see Watkins, P. M. Ditellurium decafluoride - A Continuing Myth. Journal of Chemical Education. 1974, 51 (9): 520–521. Bibcode:1974JChEd..51..520W. doi:10.1021/ed051p520. However, Te(V) has been observed in HTeO−
, TeO−
, HTeO−
2, and TeO−
3; see Kläning, Ulrik K.; Sehested, K. Tellurium(V). A Pulse Radiolysis Study. The Journal of Physical Chemistry A. 2001, 105 (27): 6637–45. Bibcode:2001JPCA..105.6637K. doi:10.1021/jp010577i. - ^ I(II) is known to exist in monoxide (IO); see Nikitin, I V. Halogen monoxides. Russian Chemical Reviews. 31 August 2008, 77 (8): 739–749. Bibcode:2008RuCRv..77..739N. doi:10.1070/RC2008v077n08ABEH003788.
- ^ I(IV) has been observed in iodine dioxide (IO2); see Pauling, Linus. Oxygen Compounds of Nonmetallic Elements. General Chemistry 3rd. Dover Publications, Inc. 1988: 259. ISBN 978-0-486-65622-9.
- ^ I(VI) has been observed in IO3, IO42−, H5IO6−, H2IO52−, H4IO62−, and HIO53−; see Kläning, Ulrik K.; Sehested, Knud; Wolff, Thomas. Laser flash photolysis and pulse radiolysis of iodate and periodate in aqueous solution. Properties of iodine(VI). J. Chem. Soc., Faraday Trans. 1. 1981, 77 (7): 1707–18. doi:10.1039/F19817701707.
- ^ Xe compounds: see Xenon
- ^ Xe(0) has been observed in tetraxenonogold(II) (AuXe42+).
- ^ Xe(I) has been reported in xenon hexafluoroplatinate and xenon hexafluororhodate (see Pauling, Linus. General Chemistry 3rd. Dover Publications, Inc. 1988: 250. ISBN 978-0-486-65622-9.), however these compounds were later found to contain Xe(II).
- ^ Langmuir, Irving. THE ARRANGEMENT OF ELECTRONS IN ATOMS AND MOLECULES.. Journal of the American Chemical Society. 1919-06, 41 (6): 868–934 [2022-03-25]. ISSN 0002-7863. doi:10.1021/ja02227a002. (原始内容存档于2020-05-09) (英语).