腺苷脫氨酶
外观
(重定向自腺苷脱氨酶)
| Adenosine/AMP deaminase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
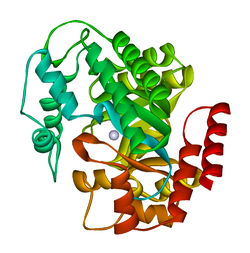
 crystal structure of plasmodium yoelii adenosine deaminase (py02076) | |||||||||
| 鑑定 | |||||||||
| 標誌 | A_deaminase | ||||||||
| Pfam | PF00962(旧版) | ||||||||
| Pfam宗系 | CL0034(旧版) | ||||||||
| InterPro | IPR001365 | ||||||||
| PROSITE | PDOC00419 | ||||||||
| SCOP | 1add / SUPFAM | ||||||||
| |||||||||
| Adenosine deaminase (editase) domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 鑑定 | |||||||||
| 標誌 | A_deamin | ||||||||
| Pfam | PF02137(旧版) | ||||||||
| InterPro | IPR002466 | ||||||||
| PROSITE | PDOC00419 | ||||||||
| SCOP | 1add / SUPFAM | ||||||||
| |||||||||
| Adenosine/AMP deaminase N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 鑑定 | |||||||||
| 標誌 | A_deaminase_N | ||||||||
| Pfam | PF08451(旧版) | ||||||||
| InterPro | IPR013659 | ||||||||
| |||||||||
腺苷脫氨酶(英語:Adenosine deaminase)的是一種參與嘌呤代謝作用的酶。它是用作拆解食物組織中的核酸中的腺苷。在人體中,它主要參與了免疫細胞的製造。若該酶突變,會造成T細胞、B細胞及自然殺手細胞皆無法表現的嚴重複合型免疫缺乏症(SCID)。
反應
[编辑]腺苷脫氨酶對腺苷進行不可逆轉的脫氨作用,轉化腺苷為相關的核苷肌苷,移除氨基酸組。
病理學
[编辑]腺苷脫氨酶的突變基因使之不容易被表達,這是一些嚴重複合型免疫缺乏症(SCID)的原因。基因突變令ADA被過度產生是溶血性貧血原因之一。有一些證據表明,一個不同的等位基因(ADA2)可能會導致自閉症。
ADA活性異常與多種疾病相關,如當人體發生結核桿菌侵襲時,人體主要啓動細胞免疫,T淋巴細胞和單核巨噬細胞被分枝桿菌激活可引起ADA的活性增強,且間皮細胞吞噬分枝桿菌後產生的特異性細胞因子亦誘導ADA的活性增強。
参考文献
[编辑]深入阅读
[编辑]- da Cunha JG. [Adenosine deaminase. A pluridisciplinary enzyme]. Acta médica portuguesa. 1992, 4 (6): 315–23. PMID 1807098.
- Franco R, Casadó V, Ciruela F; et al. Cell surface adenosine deaminase: much more than an ectoenzyme. Prog. Neurobiol. 1997, 52 (4): 283–94. PMID 9247966. doi:10.1016/S0301-0082(97)00013-0.
- Valenzuela A, Blanco J, Callebaut C; et al. HIV-1 envelope gp120 and viral particles block adenosine deaminase binding to human CD26. Adv. Exp. Med. Biol. 1997, 421: 185–92. PMID 9330696.
- Moriwaki Y, Yamamoto T, Higashino K. Enzymes involved in purine metabolism--a review of histochemical localization and functional implications. Histol. Histopathol. 1999, 14 (4): 1321–40. PMID 10506947.
- Hirschhorn R. Identification of two new missense mutations (R156C and S291L) in two ADA- SCID patients unusual for response to therapy with partial exchange transfusions. Hum. Mutat. 1993, 1 (2): 166–8. PMID 1284479. doi:10.1002/humu.1380010214.
- Berkvens TM, van Ormondt H, Gerritsen EJ; et al. Identical 3250-bp deletion between two AluI repeats in the ADA genes of unrelated ADA-SCID patients. Genomics. 1990, 7 (4): 486–90. PMID 1696926. doi:10.1016/0888-7543(90)90190-6.
- Aran JM, Colomer D, Matutes E; et al. Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy. J. Histochem. Cytochem. 1991, 39 (8): 1001–8. PMID 1856451.
- Bielat K, Tritsch GL. Ecto-enzyme activity of human erythrocyte adenosine deaminase. Mol. Cell. Biochem. 1989, 86 (2): 135–42. PMID 2770711. doi:10.1007/BF00222613.
- Hirschhorn R, Tzall S, Ellenbogen A, Orkin SH. Identification of a point mutation resulting in a heat-labile adenosine deaminase (ADA) in two unrelated children with partial ADA deficiency. J. Clin. Invest. 1989, 83 (2): 497–501. PMC 303706
 . PMID 2783588. doi:10.1172/JCI113909.
. PMID 2783588. doi:10.1172/JCI113909. - Murray JL, Perez-Soler R, Bywaters D, Hersh EM. Decreased adenosine deaminase (ADA) and 5'nucleotidase (5NT) activity in peripheral blood T cells in Hodgkin disease. Am. J. Hematol. 1986, 21 (1): 57–66. PMID 3010705. doi:10.1002/ajh.2830210108.
- Wiginton DA, Kaplan DJ, States JC; et al. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1987, 25 (25): 8234–44. PMID 3028473. doi:10.1021/bi00373a017.
- Akeson AL, Wiginton DA, Dusing MR; et al. Mutant human adenosine deaminase alleles and their expression by transfection into fibroblasts. J. Biol. Chem. 1988, 263 (31): 16291–6. PMID 3182793.
- Glader BE, Backer K. Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases. Br. J. Haematol. 1988, 68 (2): 165–8. PMID 3348976. doi:10.1111/j.1365-2141.1988.tb06184.x.
- Petersen MB, Tranebjaerg L, Tommerup N; et al. New assignment of the adenosine deaminase gene locus to chromosome 20q13 X 11 by study of a patient with interstitial deletion 20q. J. Med. Genet. 1987, 24 (2): 93–6. PMC 1049896
 . PMID 3560174. doi:10.1136/jmg.24.2.93.
. PMID 3560174. doi:10.1136/jmg.24.2.93. - Orkin SH, Goff SC, Kelley WN, Daddona PE. Transient expression of human adenosine deaminase cDNAs: identification of a nonfunctional clone resulting from a single amino acid substitution. Mol. Cell. Biol. 1985, 5 (4): 762–7. PMC 366780
 . PMID 3838797.
. PMID 3838797. - Valerio D, Duyvesteyn MG, Dekker BM; et al. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985, 4 (2): 437–43. PMC 554205
 . PMID 3839456.
. PMID 3839456. - Bonthron DT, Markham AF, Ginsburg D, Orkin SH. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J. Clin. Invest. 1985, 76 (2): 894–7. PMC 423929
 . PMID 3839802. doi:10.1172/JCI112050.
. PMID 3839802. doi:10.1172/JCI112050. - Daddona PE, Shewach DS, Kelley WN; et al. Human adenosine deaminase. cDNA and complete primary amino acid sequence. J. Biol. Chem. 1984, 259 (19): 12101–6. PMID 6090454.
- Valerio D, Duyvesteyn MG, Meera Khan P; et al. Isolation of cDNA clones for human adenosine deaminase. Gene. 1984, 25 (2-3): 231–40. PMID 6198240. doi:10.1016/0378-1119(83)90227-5.