柚皮素
| 柚皮素 | |
|---|---|
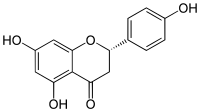
| |
| IUPAC名 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one | |
| 别名 | Naringetol; Salipurol; Salipurpol; 4',5,7-Trihydroxyflavanone |
| 识别 | |
| CAS号 | 480-41-1 |
| PubChem | 439246 |
| ChemSpider | 388383 |
| SMILES |
|
| ChEBI | 50202 |
| DrugBank | DB03467 |
| 性质 | |
| 化学式 | C15H12O5 |
| 摩尔质量 | 272.25 g·mol−1 |
| 熔点 | 251 °C(524 K) |
| 溶解性(水) | 475 mg/L[1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
柚皮素是一种无味,颜色由白色至淡黄的黄烷酮,是一种类黄酮。它是葡萄柚中的主要黄烷酮,[2]并存在于多种水果与草药中。 [3]
结构
柚皮素具有黄烷酮的骨架结构,其4',5和7位碳原子处有三个羟基。柚皮素可以单体存在,亦有糖苷形式即柚皮苷,其具有加入的二糖新橙皮糖与7位碳原子连接而成。
像大多数黄烷酮一样,柚皮素在碳2上有一个手性中心,尽管光学纯度是可变的。 [3] [4] S(-)-柚皮素的外消旋已显示相当快地发生。 [5]
来源和生物利用度
柚皮素及其糖苷存在于多种草药和水果中,包括葡萄柚,[6]佛手柑,[7]酸橙,[8]酸樱桃,[9]西红柿,[10] [11]可可,[12] [13] [14]希腊牛至,[15]水薄荷,[16]以及豆类。[17]柚皮素与柚皮苷的比例因来源而异,对映体比例也是如此。 [4]
柚皮苷-7-葡萄糖苷形式的生物利用度似乎低于聚乙二醇形式。[18]
服用葡萄柚汁后,柚皮素血浆浓度比服用橙汁更高。 [19]在葡萄柚中还发现了相关的化合物山柰酚,其羟基紧挨着酮基。
柚皮素可以从煮熟的番茄酱中吸收。 每10克番茄酱中含有253毫克柚皮素。 [20]
生物合成与代谢
它衍生自丙二酰基CoA和4-香豆酰基CoA 。后者衍生自苯丙氨酸。将所得丁烯酮通过作用于查耳酮合成酶,得到查耳酮。查尔酮随后经历闭环,生成柚皮素。 [21]
柚皮素-8-二甲基烯丙基转移酶使用二甲基烯丙基二磷酸和(−)- (2S)-柚皮素以产生二磷酸和8-戊基柚皮素 。雅致小克银汉霉(Cunninghamella elegans)是一种哺乳动物新陈代谢的真菌模型生物,它可用于研究柚皮素的硫酸化。 [22]
潜在的生物学影响
抗菌,抗真菌和抗病毒
柚皮素对表皮葡萄球菌,金黄色葡萄球菌,枯草芽孢杆菌,黄球菌和大肠杆菌具有抗菌作用。 [23]进一步的研究增加了对乳酸乳球菌,[24]嗜酸乳杆菌,内氏放线菌,口腔普雷沃菌[25]嗜酸乳杆菌,黑色素丙酸杆菌,牙龈卟啉单胞菌[26]以及白色念珠菌,热带和克柔念珠菌等抗菌药物的证据。 [27]尽管没有证明柚皮苷对微生物的脲酶活性有任何抑制作用,但有证据证明其对幽门螺杆菌具有抗菌作用。 [28]
柚皮素可以减少体外培养的HCV感染的肝细胞病毒的产生。这可能继发于柚皮素抑制极低密度脂蛋白分泌的作用。 [29]柚皮苷的抗病毒作用目前正在临床研究中。 [30]关于脊髓灰质炎病毒, HSV-1和HSV-2的抗病毒作用的报道也已经发表,尽管病毒的复制并未受到抑制。 [31] [32]
抗炎
尽管有柚皮苷抗炎活性的证据, [33]但已观察到柚皮苷的抗炎活性很差或根本不存在。 [34] [35]
抗氧化剂
柚皮素已被证明具有显着的抗氧化性能。 [36] [37]在体外和动物研究中已证明它可以减少DNA的氧化损伤。 [38] [39]
抗肿瘤
据报道,柚皮素在乳腺癌,胃癌,肝癌,宫颈癌,胰腺癌和结肠组织癌细胞中以及白血病细胞中都可诱导细胞毒性。[40]柚皮素抑制人类乳腺癌生长的机制已被证实,在此基础上提出了两种柚皮素抗癌的假说。[41]第一种假说是柚皮素抑制芳香化酶,从而减少了肿瘤生长。 [42]第二种假说提出与雌激素受体的互作是其调节肿瘤生长的原因。 [43]柚皮苷的新衍生物对多药耐药的癌症具有活性。 [44]
补充阅读
- 对人细胞色素P450同工型[[CYP1A2]]具有抑制作用,导致原本无害的物质致癌。 Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. April 1993, 35 (4): 431–6. PMC 1381556
 . PMID 8485024. doi:10.1111/j.1365-2125.1993.tb04162.x.
. PMID 8485024. doi:10.1111/j.1365-2125.1993.tb04162.x. - Wistuba, Dorothee; Trapp, Oliver; Gel-Moreto, Nuria; Galensa, Rudolf; Schurig, Volker. Stereoisomeric Separation of Flavanones and Flavanone-7-O-glycosides by Capillary Electrophoresis and Determination of Interconversion Barriers. Analytical Chemistry. 2006-05-01, 78 (10): 3424–3433. ISSN 0003-2700. PMID 16689546. doi:10.1021/ac0600499.
- Krause, Martin; Galensa, Rudolf. High-performance liquid chromatography of diastereomeric flavanone glycosides in Citrus on a β-cyclodextrin-bonded stationary phase (Cyclobond I). Journal of Chromatography A. 1991, 588 (1–2): 41–45. doi:10.1016/0021-9673(91)85005-z (英语).
- Gaggeri, Raffaella; Rossi, Daniela; Collina, Simona; Mannucci, Barbara; Baierl, Marcel; Juza, Markus. Quick development of an analytical enantioselective high performance liquid chromatography separation and preparative scale-up for the flavonoid Naringenin. Journal of Chromatography A. 2011-08-12, 1218 (32): 5414–5422. PMID 21397238. doi:10.1016/j.chroma.2011.02.038.
- Wan, Lili; Sun, Xipeng; Li, Yan; Yu, Qi; Guo, Cheng; Wang, Xiangwei. A Stereospecific HPLC Method and Its Application in Determination of Pharmacokinetics Profile of Two Enantiomers of Naringenin in Rats. Journal of Chromatographic Science. 2011-04-01, 49 (4): 316–320. ISSN 0021-9665. PMID 21439124. doi:10.1093/chrsci/49.4.316. 无效
|subscription=free(帮助) - 柚皮苷还在小鼠中产生BDNF依赖性抗抑郁剂样作用。 BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog. Neuropsychopharmacol. Biol. Psychiatry. October 2013, 48C: 135–141. PMID 24121063. doi:10.1016/j.pnpbp.2013.10.002.
- Gao, K; Henning, S; Niu, Y; Youssefian, A; Seeram, N; Xu, A; Heber, D. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. The Journal of Nutritional Biochemistry. 2006, 17 (2): 89–95. PMID 16111881. doi:10.1016/j.jnutbio.2005.05.009. </ ref>
- Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships. J. Nat. Prod. August 2007, 70 (8): 1278–82. PMC 2265593
 . PMID 17685652. doi:10.1021/np070194x.
. PMID 17685652. doi:10.1021/np070194x. - 据报道,柚皮素可诱导前脂肪细胞凋亡。Hsu, Chin-Lin; Huang, Shih-Li; Yen, Gow-Chin. Inhibitory Effect of Phenolic Acids on the Proliferation of 3T3-L1 Preadipocytes in Relation to Their Antioxidant Activity. Journal of Agricultural and Food Chemistry. 2006-06-01, 54 (12): 4191–4197. ISSN 0021-8561. PMID 16756346. doi:10.1021/jf0609882.
- 柚皮素似乎可以保护LDLR缺陷型小鼠免受高脂饮食的肥胖影响。 Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. October 2009, 58 (10): 2198–210. PMC 2750228
 . PMID 19592617. doi:10.2337/db09-0634.
. PMID 19592617. doi:10.2337/db09-0634. - 柚皮素通过抑制高胆固醇饮食的大鼠中的HMG-CoA还原酶和ACAT降低血浆和肝胆固醇的浓度。 Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A:cholesterol acyltransferase in rats. Ann. Nutr. Metab. 1999, 43 (3): 173–80. PMID 10545673. doi:10.1159/000012783.
- 在一项使用阿尔茨海默氏病小鼠模型的研究中,柚皮苷已被证明可以改善记忆力并减少淀粉样蛋白和tau蛋白。Ghofraniab, Saeed; Joghataei, Mohammad-Taghi; Mohsenia, Simin; Baluchnejadmojaradd, Tourandokht; Bagheriac, Maryam; Khamsee, Safoura; Roghani, Mehrdad. Naringenin improves learning and memory in an Alzheimer's disease rat model: Insights into the underlying mechanisms. European Journal of Pharmacology. 5 October 2015, 764: 195–201. PMID 26148826. doi:10.1016/j.ejphar.2015.07.001.Yang, Zhiyou; Kuboyama, Tomoharu; Tohda, Chihiro. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytotherapy Research. 2019-02-15, 33 (4): 1114–1121. ISSN 1099-1573. PMID 30768735. doi:10.1002/ptr.6305.Yang, Zhiyou; Kuboyama, Tomoharu; Tohda, Chihiro. A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer's Disease and Its Target Molecule. Frontiers in Pharmacology. 19 June 2017, 8: 340. PMC 5474478
 . PMID 28674493. doi:10.3389/fphar.2017.00340.
. PMID 28674493. doi:10.3389/fphar.2017.00340.
参考文献
- ^ 引用错误:没有为名为
chemidplus的参考文献提供内容 - ^ Bioavailability of the flavanone naringenin and its glycosides in rats (PDF). Am. J. Physiol. Gastrointest. Liver Physiol. December 2000, 279 (6): G1148–54. PMID 11093936. doi:10.1152/ajpgi.2000.279.6.G1148.
- ^ 3.0 3.1 Yáñez, Jaime A.; Andrews, Preston K.; Davies, Neal M. Methods of analysis and separation of chiral flavonoids. Journal of Chromatography B. 2007-04-01, 848 (2): 159–181. PMID 17113835. doi:10.1016/j.jchromb.2006.10.052.
- ^ 4.0 4.1 Yáñez, Jaime A.; Remsberg, Connie M.; Miranda, Nicole D.; Vega-Villa, Karina R.; Andrews, Preston K.; Davies, Neal M. Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharmaceutics & Drug Disposition. 2008-01-01, 29 (2): 63–82. ISSN 1099-081X. PMID 18058792. doi:10.1002/bdd.588 (英语).
- ^ Krause, M.; Galensa, R. Analysis of enantiomeric flavanones in plant extracts by high-performance liquid chromatography on a cellulose triacetate based chiral stationary phase. Chromatographia. 1991-07-01, 32 (1–2): 69–72. ISSN 0009-5893. doi:10.1007/BF02262470 (英语).
- ^ Ho, Ping C; Saville, Dorothy J; Coville, Peter F; Wanwimolruk, Sompon. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharmaceutica Acta Helvetiae. 2000-04-01, 74 (4): 379–385. PMID 10812937. doi:10.1016/S0031-6865(99)00062-X.
- ^ Gattuso, Giuseppe; Barreca, Davide; Gargiulli, Claudia; Leuzzi, Ugo; Caristi, Corrado. Flavonoid Composition of Citrus Juices. Molecules. 2007-08-03, 12 (8): 1641–1673. PMC 6149096
 . PMID 17960080. doi:10.3390/12081641 (英语). 无效
. PMID 17960080. doi:10.3390/12081641 (英语). 无效|subscription=free(帮助) - ^ Gel-Moreto, Nuria; Streich, René; Galensa, Rudolf. Chiral separation of diastereomeric flavanone-7-O-glycosides in citrus by capillary electrophoresis. Electrophoresis. 2003-08-01, 24 (15): 2716–2722. ISSN 0173-0835. PMID 12900888. doi:10.1002/elps.200305486.
- ^ Wang, H.; Nair, M. G.; Strasburg, G. M.; Booren, A. M.; Gray, J. I. Antioxidant polyphenols from tart cherries (Prunus cerasus). Journal of Agricultural and Food Chemistry. 1999-03-01, 47 (3): 840–844. ISSN 0021-8561. PMID 10552377. doi:10.1021/jf980936f.
- ^ Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P. L.; Spigno, P.; Soressi, G. P. Polyphenol pattern and antioxidant activity of different tomato lines and cultivars. Annals of Nutrition & Metabolism. 2003-01-01, 47 (2): 64–69. ISSN 0250-6807. PMID 12652057. doi:10.1159/000069277.
- ^ Vallverdú-Queralt, A; Odriozola-Serrano, I; Oms-Oliu, G; Lamuela-Raventós, RM; Elez-Martínez, P; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J Agric Food Chem. 2012, 60 (38): 9667–9672. PMID 22957841. doi:10.1021/jf302791k.
- ^ Sánchez-Rabaneda, Ferran; Jáuregui, Olga; Casals, Isidre; Andrés-Lacueva, Cristina; Izquierdo-Pulido, Maria; Lamuela-Raventós, Rosa M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). Journal of Mass Spectrometry. 2003-01-01, 38 (1): 35–42. Bibcode:2003JMSp...38...35S. ISSN 1076-5174. PMID 12526004. doi:10.1002/jms.395.
- ^ Vallverdú-Queralt, A; Odriozola-Serrano, I; Oms-Oliu, G; Lamuela-Raventós, RM; Elez-Martínez, P; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J Agric Food Chem. 2012, 60 (38): 9667–9672. PMID 22957841. doi:10.1021/jf302791k.
- ^ Sánchez-Rabaneda, Ferran; Jáuregui, Olga; Casals, Isidre; Andrés-Lacueva, Cristina; Izquierdo-Pulido, Maria; Lamuela-Raventós, Rosa M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). Journal of Mass Spectrometry. 2003-01-01, 38 (1): 35–42. Bibcode:2003JMSp...38...35S. ISSN 1076-5174. PMID 12526004. doi:10.1002/jms.395.
- ^ Exarchou, Vassiliki; Godejohann, Markus; van Beek, Teris A.; Gerothanassis, Ioannis P.; Vervoort, Jacques. LC-UV-Solid-Phase Extraction-NMR-MS Combined with a Cryogenic Flow Probe and Its Application to the Identification of Compounds Present in Greek Oregano. Analytical Chemistry. 2003-11-01, 75 (22): 6288–6294. ISSN 0003-2700. PMID 14616013. doi:10.1021/ac0347819.
- ^ Olsen, Helle T.; Stafford, Gary I.; van Staden, Johannes; Christensen, Søren B.; Jäger, Anna K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L.. Journal of Ethnopharmacology. 2008-05-22, 117 (3): 500–502. PMID 18372132. doi:10.1016/j.jep.2008.02.015.
- ^ Hungria, M.; Johnston, A. W.; Phillips, D. A. Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Molecular Plant-Microbe Interactions. 1992-05-01, 5 (3): 199–203. ISSN 0894-0282. PMID 1421508. doi:10.1094/mpmi-5-199.
- ^ Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem. Biophys. Res. Commun. November 1999, 265 (2): 410–5. PMID 10558881. doi:10.1006/bbrc.1999.1695.
- ^ Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. February 2001, 131 (2): 235–41. PMID 11160539. doi:10.1093/jn/131.2.235. 无效
|subscription=free(帮助) - ^ Naringenin from cooked tomato paste is bioavailable in men. J. Nutr. November 2002, 132 (11): 3349–52. PMID 12421849. doi:10.1093/jn/132.11.3349. 无效
|subscription=free(帮助) - ^ Wang, Chuanhong; Zhi, Shuang; Liu, Changying; Xu, Fengxiang; Zhao, Aichun; Wang, Xiling; Ren, Yanhong; Li, Zhengang; Yu, Maode. Characterization of Stilbene Synthase Genes in Mulberry (Morus atropurpurea) and Metabolic Engineering for the Production of Resveratrol in Escherichia coli. Journal of Agricultural and Food Chemistry. 2017, 65 (8): 1659–1668. PMID 28168876. doi:10.1021/acs.jafc.6b05212.
- ^ Ibrahim AR. Sulfation of naringenin by Cunninghamella elegans. Phytochemistry. January 2000, 53 (2): 209–12. PMID 10680173. doi:10.1016/S0031-9422(99)00487-2.
- ^ Rauha, Jussi-Pekka; Remes, Susanna; Heinonen, Marina; Hopia, Anu; Kähkönen, Marja; Kujala, Tytti; Pihlaja, Kalevi; Vuorela, Heikki; Vuorela, Pia. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. 2000-05-25, 56 (1): 3–12. PMID 10857921. doi:10.1016/S0168-1605(00)00218-X.
- ^ Mandalari, G.; Bennett, R. N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C. B.; Gasson, M. J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. Journal of Applied Microbiology. 2007-12-01, 103 (6): 2056–2064. ISSN 1364-5072. PMID 18045389. doi:10.1111/j.1365-2672.2007.03456.x.
- ^ Mandalari, G.; Bennett, R. N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C. B.; Gasson, M. J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. Journal of Applied Microbiology. 2007-12-01, 103 (6): 2056–2064. ISSN 1364-5072. PMID 18045389. doi:10.1111/j.1365-2672.2007.03456.x.
- ^ Koru, Ozgur; Toksoy, Fulya; Acikel, Cengiz Han; Tunca, Yasar Meric; Baysallar, Mehmet; Uskudar Guclu, Aylin; Akca, Eralp; Ozkok Tuylu, Asli; Sorkun, Kadriye. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe. 2007-06-01, 13 (3–4): 140–145. ISSN 1075-9964. PMID 17475517. doi:10.1016/j.anaerobe.2007.02.001.
- ^ Uzel, Ataç; Sorkun, Kadri˙ye; Önçağ, Özant; Çoğulu, Dilşah; Gençay, Ömür; Sali˙h, Beki˙r. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiological Research. 2005-04-25, 160 (2): 189–195. PMID 15881836. doi:10.1016/j.micres.2005.01.002.
- ^ Bae, Eun-Ah; Han, Myung; Kim, Dong-Hyun. In vitroAnti-Helicobacter pylori Activity of Some Flavonoids and Their Metabolites. Planta Medica. 1999, 65 (5): 442–443. PMID 10454900. doi:10.1055/s-2006-960805.
- ^ Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. May 2008, 47 (5): 1437–45. PMC 4500072
 . PMID 18393287. doi:10.1002/hep.22197.
. PMID 18393287. doi:10.1002/hep.22197.
- ^ A Pilot Study of the Grapefruit Flavonoid Naringenin for HCV Infection - Full Text View - ClinicalTrials.gov. clinicaltrials.gov. (原始内容存档于2010-10-01).
- ^ Mucsi, I.; Prágai, B. M. Inhibition of virus multiplication and alteration of cyclic AMP level in cell cultures by flavonoids. Experientia. 1985-07-01, 41 (7): 930–931. ISSN 0014-4754. PMID 2989000. doi:10.1007/BF01970018 (英语).
- ^ Lyu, Su-Yun; Rhim, Jee-Young; Park, Won-Bong. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2)in vitro. Archives of Pharmacal Research. 2005-11-01, 28 (11): 1293–1301. ISSN 0253-6269. PMID 16350858. doi:10.1007/BF02978215 (英语).
- ^ Kawaguchi, K.; Kikuchi, S.; Hasunuma, R.; Maruyama, H.; Ryll, R.; Kumazawa, Y. Suppression of Infection-Induced Endotoxin Shock in Mice by aCitrusFlavanone Naringin. Planta Medica. 2004, 70 (1): 17–22. PMID 14765287. doi:10.1055/s-2004-815449.
- ^ Gutiérrez-Venegas, Gloria; Kawasaki-Cárdenas, Perla; Rita Arroyo-Cruz, Santa; Maldonado-Frías, Silvia. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. European Journal of Pharmacology. 2006-07-10, 541 (1–2): 95–105. PMID 16762341. doi:10.1016/j.ejphar.2006.03.069.
- ^ Olszanecki, R.; Gebska, A.; Kozlovski, V. I.; Gryglewski, R. J. Flavonoids and nitric oxide synthase. Journal of Physiology and Pharmacology. 2002-12-01, 53 (4 Pt 1): 571–584. ISSN 0867-5910. PMID 12512693.
- ^ Gorinstein, Shela; Leontowicz, Hanna; Leontowicz, Maria; Krzeminski, Ryszard; Gralak, Mikolaj; Delgado-Licon, Efren; Martinez Ayala, Alma Leticia; Katrich, Elena; Trakhtenberg, Simon. Changes in Plasma Lipid and Antioxidant Activity in Rats as a Result of Naringin and Red Grapefruit Supplementation. Journal of Agricultural and Food Chemistry. 2005-04-01, 53 (8): 3223–3228. ISSN 0021-8561. PMID 15826081. doi:10.1021/jf058014h.
- ^ Yu, Jun; Wang, Limin; Walzem, Rosemary L.; Miller, Edward G.; Pike, Leonard M.; Patil, Bhimanagouda S. Antioxidant Activity of Citrus Limonoids, Flavonoids, and Coumarins. Journal of Agricultural and Food Chemistry. 2005-03-01, 53 (6): 2009–2014. ISSN 0021-8561. PMID 15769128. doi:10.1021/jf0484632.
- ^ Sumit Kumar & Ashu Bhan Tiku. Biochemical and Molecular Mechanisms of Radioprotective Effects of Naringenin, a Phytochemical from Citrus Fruits. J. Agric. Food Chem. 2016, 64 (8): 1676–1685. PMID 26881453. doi:10.1021/acs.jafc.5b05067.
- ^ Chandra Jagetia, Ganesh; Koti Reddy, Tiyyagura; Venkatesha, V. A; Kedlaya, Rajendra. Influence of naringin on ferric iron induced oxidative damage in vitro. Clinica Chimica Acta. 2004-09-01, 347 (1–2): 189–197. PMID 15313158. doi:10.1016/j.cccn.2004.04.022.
- ^ Kanno, Syu-ichi; Tomizawa, Ayako; Hiura, Takako; Osanai, Yuu; Shouji, Ai; Ujibe, Mayuko; Ohtake, Takaharu; Kimura, Katsuhiko; Ishikawa, Masaaki. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biological and Pharmaceutical Bulletin. 2005-01-01, 28 (3): 527–530. PMID 15744083. doi:10.1248/bpb.28.527.
- ^ So, Felicia V.; Guthrie, Najla; Chambers, Ann F.; Moussa, Madeleine; Carroll, Kenneth K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutrition and Cancer. 1996-01-01, 26 (2): 167–181. ISSN 0163-5581. PMID 8875554. doi:10.1080/01635589609514473.
- ^ van Meeuwen, J. A.; Korthagen, N.; de Jong, P. C.; Piersma, A. H.; van den Berg, M. (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicology and Applied Pharmacology. 2007-06-15, 221 (3): 372–383. PMID 17482226. doi:10.1016/j.taap.2007.03.016.
- ^ Harmon, Anne W.; Patel, Yashomati M. Naringenin Inhibits Glucose Uptake in MCF-7 Breast Cancer Cells: A Mechanism for Impaired Cellular Proliferation. Breast Cancer Research and Treatment. 2004-05-01, 85 (2): 103–110. ISSN 0167-6806. PMID 15111768. doi:10.1023/B:BREA.0000025397.56192.e2 (英语).
- ^ Ferreira, Ricardo J; Baptista, Rafael; Moreno, Alexis; Madeira, Patricia G; Khonkarn, Ruttiros; Baubichon-Cortay, Hélène; Santos, Daniel JVA dos; Falson, Pierre; Ferreira, Maria-José U. Optimizing the flavanone core toward new selective nitrogen-containing modulators of ABC transporters. Future Medicinal Chemistry. 2018-03-23, 10 (7): 725–741. PMID 29570361. doi:10.4155/fmc-2017-0228 (英语).
