莫米松
| 此條目可参照英語維基百科相應條目来扩充。 (2023年12月13日) |
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | Nasonex, Asmanex, Elocon, othersMometasone Use During Pregnancy. Drugs.com. 14 February 2020 [1 April 2020]. (原始内容存档于2020-10-26).</ref> |
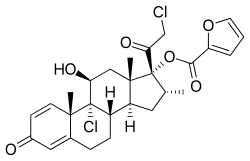
| 其他名稱 | LAS-41002, 9α,21-Dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17α-(2-furoate) |
| 给药途径 | Topical, inhalation (nasal spray) |
| 藥物類別 | Corticosteroid; Glucocorticoid |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | Nasal spray is virtually undetectable in plasma; but systemic availability is comparable to fluticasone[3] |
| 血漿蛋白結合率 | 98% to 99% |
| 药物代谢 | Liver |
| 生物半衰期 | 5.8 hours |
| 识别信息 | |
| |
| CAS号 | 105102-22-5 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.600 |
| 化学信息 | |
| 化学式 | C22H28Cl2O4 for mometasone C27H30O6Cl2 as furoate |
| 3D模型(JSmol) | |
| |
| |
莫米松(Mometasone),是一种糖皮质激素,用于治疗某些皮肤病、花粉热和哮喘[4] [5] [6],具体用于预防哮喘发作(非治疗)。[4]可应用于皮肤、吸入或鼻部。[4][5][6][7]
莫米松用于哮喘的常见副作用包括鹅口疮等[4],因此建议使用后漱口,[4]长期使用可能会增加患青光眼和白内障的风险。[4]应用于鼻部的常见副作用包括上呼吸道感染和流鼻血。[6]应用于皮肤时的常见副作用包括痤疮、皮肤萎缩和瘙痒。[5]它通过减少炎症起作用。[4]
糠酸莫米松于1981年获得专利,并于1987年开始医用。[8]它在世界卫生组织的基本药物清单上[9],可作为通用药物使用。 [10]2017年,它是美国第197种最常用的处方药,共开出超过200万张处方。[11] [12]
参考资料[编辑]
- ^ Nasonex- mometasone furoate spray, metered. DailyMed. 26 January 2011 [19 June 2022]. (原始内容存档于2022-11-05).
- ^ https://www.ema.europa.eu/documents/psusa/mometasone-list-nationally-authorised-medicinal-products-psusa/00002085/202005_en.pdf [裸網址]
- ^ Tayab ZR, Fardon TC, Lee DK, Haggart K, McFarlane LC, Lipworth BJ, Hochhaus G. Pharmacokinetic/pharmacodynamic evaluation of urinary cortisol suppression after inhalation of fluticasone propionate and mometasone furoate. British Journal of Clinical Pharmacology. November 2007, 64 (5): 698–705. PMC 2203259
 . PMID 17509041. doi:10.1111/j.1365-2125.2007.02919.x.
. PMID 17509041. doi:10.1111/j.1365-2125.2007.02919.x.
- ^ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Mometasone Furoate Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [11 March 2019]. (原始内容存档于2016-10-07) (英语).
- ^ 5.0 5.1 5.2 Mometasone Furoate topical Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [11 March 2019]. (原始内容存档于2020-08-05) (英语).
- ^ 6.0 6.1 6.2 Mometasone Furoate eent Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [11 March 2019]. (原始内容存档于2016-04-30).
- ^ Mometasone. DrugBank. [30 April 2020]. (原始内容存档于2019-06-29).
- ^ Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 488. ISBN 9783527607495.
- ^ World Health Organization. World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ^ British national formulary : BNF 76 76. Pharmaceutical Press. 2018: 265. ISBN 9780857113382.
- ^ The Top 300 of 2020. ClinCalc. [11 April 2020]. (原始内容存档于2020-03-18).
- ^ Mometasone - Drug Usage Statistics. ClinCalc. [11 April 2020]. (原始内容存档于2021-01-27).
