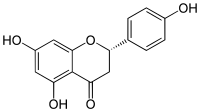
柚皮素
| 柚皮素 | |
|---|---|
| |
| IUPAC名 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one | |
| 别名 | Naringetol; Salipurol; Salipurpol; 4',5,7-Trihydroxyflavanone |
| 识别 | |
| CAS号 | 480-41-1 |
| PubChem | 439246 |
| ChemSpider | 388383 |
| SMILES |
|
| ChEBI | 50202 |
| DrugBank | DB03467 |
| 性质 | |
| 化学式 | C15H12O5 |
| 摩尔质量 | 272.25 g·mol−1 |
| 熔点 | 251 °C(524 K) |
| 溶解性(水) | 475 mg/L [1] |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
柚皮素是一种无味,颜色由白色至淡黄的黄烷酮,是一种类黄酮。它是葡萄柚中的主要黄烷酮,[2]并存在于多种水果与草药中。 [3]
结构
[编辑]柚皮素具有黄烷酮的骨架结构,其4',5和7位碳原子处有三个羟基。柚皮素可以单体存在,亦有糖苷形式即柚皮苷,其具有加入的二糖新橙皮糖与7位碳原子连接而成。
像大多数黄烷酮一样,柚皮素在碳2上有一个手性中心,尽管光学纯度是可变的。 [3] [4] S(-)-柚皮素的外消旋已显示相当快地发生。 [5]
来源和生物利用度
[编辑]柚皮素及其糖苷存在于多种草药和水果中,包括葡萄柚,[6]佛手柑,[7]酸橙,[8]酸樱桃,[9]西红柿,[10] [11]可可,[12] [11] [12]希腊牛至,[13]水薄荷,[14]以及豆类。[15]柚皮素与柚皮苷的比例因来源而异,对映体比例也是如此。 [4]
柚皮苷-7-葡萄糖苷形式的生物利用度似乎低于聚乙二醇形式。[16]
服用葡萄柚汁后,柚皮素血浆浓度比服用橙汁更高。 [17]在葡萄柚中还发现了相关的化合物山柰酚,其羟基紧挨着酮基。
柚皮素可以从煮熟的番茄酱中吸收。 每10克番茄酱中含有253毫克柚皮素。 [18]
生物合成与代谢
[编辑]它衍生自丙二酰基CoA和4-香豆酰基CoA 。后者衍生自苯丙氨酸。将所得丁烯酮通过作用于查耳酮合成酶,得到查耳酮。查尔酮随后经历闭环,生成柚皮素。 [19]
柚皮素-8-二甲基烯丙基转移酶使用二甲基烯丙基二磷酸和(−)- (2S)-柚皮素以产生二磷酸和8-戊基柚皮素 。雅致小克银汉霉(Cunninghamella elegans)是一种哺乳动物新陈代谢的真菌模型生物,它可用于研究柚皮素的硫酸化。 [20]
潜在的生物学影响
[编辑]抗菌,抗真菌和抗病毒
[编辑]柚皮素对表皮葡萄球菌,金黄色葡萄球菌,枯草芽孢杆菌,黄球菌和大肠杆菌具有抗菌作用。 [21]进一步的研究增加了对乳酸乳球菌,[22]嗜酸乳杆菌,内氏放线菌,口腔普雷沃菌[22]嗜酸乳杆菌,黑色素丙酸杆菌,牙龈卟啉单胞菌[23]以及白色念珠菌,热带和克柔念珠菌等抗菌药物的证据。 [24]尽管没有证明柚皮苷对微生物的脲酶活性有任何抑制作用,但有证据证明其对幽门螺杆菌具有抗菌作用。 [25]
柚皮素可以减少体外培养的HCV感染的肝细胞病毒的产生。这可能继发于柚皮素抑制极低密度脂蛋白分泌的作用。 [26]柚皮苷的抗病毒作用目前正在临床研究中。 [27]关于脊髓灰质炎病毒, HSV-1和HSV-2的抗病毒作用的报道也已经发表,尽管病毒的复制并未受到抑制。 [28] [29]
抗炎
[编辑]尽管有柚皮苷抗炎活性的证据, [30]但已观察到柚皮苷的抗炎活性很差或根本不存在。 [31] [32]
抗氧化剂
[编辑]柚皮素已被证明具有显着的抗氧化性能。 [33] [34]在体外和动物研究中已证明它可以减少DNA的氧化损伤。 [35] [36]
抗肿瘤
[编辑]研究指出,柚皮素在乳腺癌、胃癌、肝癌、宫颈癌、胰腺癌、结肠组织癌细胞中以及白血病细胞中都可诱导细胞毒性。[37]柚皮素抑制人类乳腺癌生长的机制已被证实,在此基础上提出了两种柚皮素抗癌的假说。[38][39] 第一种假说是柚皮素抑制芳香化酶,从而减少了肿瘤生长。 [40]第二种假说提出与雌激素受体的互作是其调节肿瘤生长的原因。 [41]柚皮苷的新衍生物对多药耐药的癌症具有活性。 [42]
补充阅读
[编辑]- 对人细胞色素P450同工型[[CYP1A2]]具有抑制作用,导致原本无害的物质致癌。 Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. April 1993, 35 (4): 431–6. PMC 1381556
 . PMID 8485024. doi:10.1111/j.1365-2125.1993.tb04162.x.
. PMID 8485024. doi:10.1111/j.1365-2125.1993.tb04162.x. - Wistuba, Dorothee; Trapp, Oliver; Gel-Moreto, Nuria; Galensa, Rudolf; Schurig, Volker. Stereoisomeric Separation of Flavanones and Flavanone-7-O-glycosides by Capillary Electrophoresis and Determination of Interconversion Barriers. Analytical Chemistry. 2006-05-01, 78 (10): 3424–3433. ISSN 0003-2700. PMID 16689546. doi:10.1021/ac0600499.
- Krause, Martin; Galensa, Rudolf. High-performance liquid chromatography of diastereomeric flavanone glycosides in Citrus on a β-cyclodextrin-bonded stationary phase (Cyclobond I). Journal of Chromatography A. 1991, 588 (1–2): 41–45. doi:10.1016/0021-9673(91)85005-z (英语).
- Gaggeri, Raffaella; Rossi, Daniela; Collina, Simona; Mannucci, Barbara; Baierl, Marcel; Juza, Markus. Quick development of an analytical enantioselective high performance liquid chromatography separation and preparative scale-up for the flavonoid Naringenin. Journal of Chromatography A. 2011-08-12, 1218 (32): 5414–5422. PMID 21397238. doi:10.1016/j.chroma.2011.02.038.
- Wan, Lili; Sun, Xipeng; Li, Yan; Yu, Qi; Guo, Cheng; Wang, Xiangwei. A Stereospecific HPLC Method and Its Application in Determination of Pharmacokinetics Profile of Two Enantiomers of Naringenin in Rats. Journal of Chromatographic Science. 2011-04-01, 49 (4): 316–320. ISSN 0021-9665. PMID 21439124. doi:10.1093/chrsci/49.4.316.
- 柚皮苷还在小鼠中产生BDNF依赖性抗抑郁剂样作用。 BDNF signaling is necessary for the antidepressant-like effect of naringenin. Prog. Neuropsychopharmacol. Biol. Psychiatry. October 2013, 48C: 135–141. PMID 24121063. doi:10.1016/j.pnpbp.2013.10.002.
- Gao, K; Henning, S; Niu, Y; Youssefian, A; Seeram, N; Xu, A; Heber, D. The citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells. The Journal of Nutritional Biochemistry. 2006, 17 (2): 89–95. PMID 16111881. doi:10.1016/j.jnutbio.2005.05.009. </ ref>
- Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships. J. Nat. Prod. August 2007, 70 (8): 1278–82. PMC 2265593
 . PMID 17685652. doi:10.1021/np070194x.
. PMID 17685652. doi:10.1021/np070194x. - 据报道,柚皮素可诱导前脂肪细胞凋亡。Hsu, Chin-Lin; Huang, Shih-Li; Yen, Gow-Chin. Inhibitory Effect of Phenolic Acids on the Proliferation of 3T3-L1 Preadipocytes in Relation to Their Antioxidant Activity. Journal of Agricultural and Food Chemistry. 2006-06-01, 54 (12): 4191–4197. ISSN 0021-8561. PMID 16756346. doi:10.1021/jf0609882.
- 柚皮素似乎可以保护LDLR缺陷型小鼠免受高脂饮食的肥胖影响。 Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. October 2009, 58 (10): 2198–210. PMC 2750228
 . PMID 19592617. doi:10.2337/db09-0634.
. PMID 19592617. doi:10.2337/db09-0634. - 柚皮素通过抑制高胆固醇饮食的大鼠中的HMG-CoA还原酶和ACAT降低血浆和肝胆固醇的浓度。 Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A:cholesterol acyltransferase in rats. Ann. Nutr. Metab. 1999, 43 (3): 173–80. PMID 10545673. doi:10.1159/000012783.
- 在一项使用阿尔茨海默氏病小鼠模型的研究中,柚皮苷已被证明可以改善记忆力并减少淀粉样蛋白和tau蛋白。Ghofraniab, Saeed; Joghataei, Mohammad-Taghi; Mohsenia, Simin; Baluchnejadmojaradd, Tourandokht; Bagheriac, Maryam; Khamsee, Safoura; Roghani, Mehrdad. Naringenin improves learning and memory in an Alzheimer's disease rat model: Insights into the underlying mechanisms. European Journal of Pharmacology. 5 October 2015, 764: 195–201. PMID 26148826. doi:10.1016/j.ejphar.2015.07.001.Yang, Zhiyou; Kuboyama, Tomoharu; Tohda, Chihiro. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytotherapy Research. 2019-02-15, 33 (4): 1114–1121. ISSN 1099-1573. PMID 30768735. doi:10.1002/ptr.6305.Yang, Zhiyou; Kuboyama, Tomoharu; Tohda, Chihiro. A Systematic Strategy for Discovering a Therapeutic Drug for Alzheimer's Disease and Its Target Molecule. Frontiers in Pharmacology. 19 June 2017, 8: 340. PMC 5474478
 . PMID 28674493. doi:10.3389/fphar.2017.00340.
. PMID 28674493. doi:10.3389/fphar.2017.00340.
参考文献
[编辑]- ^ Naringenin. ChemIDplus. (原始内容存档于2015-12-20).
- ^ Bioavailability of the flavanone naringenin and its glycosides in rats (PDF). Am. J. Physiol. Gastrointest. Liver Physiol. December 2000, 279 (6): G1148–54 [2020-09-29]. PMID 11093936. doi:10.1152/ajpgi.2000.279.6.G1148. (原始内容存档 (PDF)于2020-11-07).
- ^ 3.0 3.1 Yáñez, Jaime A.; Andrews, Preston K.; Davies, Neal M. Methods of analysis and separation of chiral flavonoids. Journal of Chromatography B. 2007-04-01, 848 (2): 159–181. PMID 17113835. doi:10.1016/j.jchromb.2006.10.052.
- ^ 4.0 4.1 Yáñez, Jaime A.; Remsberg, Connie M.; Miranda, Nicole D.; Vega-Villa, Karina R.; Andrews, Preston K.; Davies, Neal M. Pharmacokinetics of selected chiral flavonoids: hesperetin, naringenin and eriodictyol in rats and their content in fruit juices. Biopharmaceutics & Drug Disposition. 2008-01-01, 29 (2): 63–82. ISSN 1099-081X. PMID 18058792. doi:10.1002/bdd.588 (英语).
- ^ Krause, M.; Galensa, R. Analysis of enantiomeric flavanones in plant extracts by high-performance liquid chromatography on a cellulose triacetate based chiral stationary phase. Chromatographia. 1991-07-01, 32 (1–2): 69–72. ISSN 0009-5893. doi:10.1007/BF02262470 (英语).
- ^ Ho, Ping C; Saville, Dorothy J; Coville, Peter F; Wanwimolruk, Sompon. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharmaceutica Acta Helvetiae. 2000-04-01, 74 (4): 379–385. PMID 10812937. doi:10.1016/S0031-6865(99)00062-X.
- ^ Gattuso, Giuseppe; Barreca, Davide; Gargiulli, Claudia; Leuzzi, Ugo; Caristi, Corrado. Flavonoid Composition of Citrus Juices. Molecules. 2007-08-03, 12 (8): 1641–1673. PMC 6149096
 . PMID 17960080. doi:10.3390/12081641 (英语).
. PMID 17960080. doi:10.3390/12081641 (英语).
- ^ Gel-Moreto, Nuria; Streich, René; Galensa, Rudolf. Chiral separation of diastereomeric flavanone-7-O-glycosides in citrus by capillary electrophoresis. Electrophoresis. 2003-08-01, 24 (15): 2716–2722. ISSN 0173-0835. PMID 12900888. doi:10.1002/elps.200305486.
- ^ Wang, H.; Nair, M. G.; Strasburg, G. M.; Booren, A. M.; Gray, J. I. Antioxidant polyphenols from tart cherries (Prunus cerasus). Journal of Agricultural and Food Chemistry. 1999-03-01, 47 (3): 840–844. ISSN 0021-8561. PMID 10552377. doi:10.1021/jf980936f.
- ^ Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P. L.; Spigno, P.; Soressi, G. P. Polyphenol pattern and antioxidant activity of different tomato lines and cultivars. Annals of Nutrition & Metabolism. 2003-01-01, 47 (2): 64–69. ISSN 0250-6807. PMID 12652057. doi:10.1159/000069277.
- ^ 11.0 11.1 Vallverdú-Queralt, A; Odriozola-Serrano, I; Oms-Oliu, G; Lamuela-Raventós, RM; Elez-Martínez, P; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J Agric Food Chem. 2012, 60 (38): 9667–9672. PMID 22957841. doi:10.1021/jf302791k.
- ^ 12.0 12.1 Sánchez-Rabaneda, Ferran; Jáuregui, Olga; Casals, Isidre; Andrés-Lacueva, Cristina; Izquierdo-Pulido, Maria; Lamuela-Raventós, Rosa M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). Journal of Mass Spectrometry. 2003-01-01, 38 (1): 35–42. Bibcode:2003JMSp...38...35S. ISSN 1076-5174. PMID 12526004. doi:10.1002/jms.395.
- ^ Exarchou, Vassiliki; Godejohann, Markus; van Beek, Teris A.; Gerothanassis, Ioannis P.; Vervoort, Jacques. LC-UV-Solid-Phase Extraction-NMR-MS Combined with a Cryogenic Flow Probe and Its Application to the Identification of Compounds Present in Greek Oregano. Analytical Chemistry. 2003-11-01, 75 (22): 6288–6294. ISSN 0003-2700. PMID 14616013. doi:10.1021/ac0347819.
- ^ Olsen, Helle T.; Stafford, Gary I.; van Staden, Johannes; Christensen, Søren B.; Jäger, Anna K. Isolation of the MAO-inhibitor naringenin from Mentha aquatica L.. Journal of Ethnopharmacology. 2008-05-22, 117 (3): 500–502. PMID 18372132. doi:10.1016/j.jep.2008.02.015.
- ^ Hungria, M.; Johnston, A. W.; Phillips, D. A. Effects of flavonoids released naturally from bean (Phaseolus vulgaris) on nodD-regulated gene transcription in Rhizobium leguminosarum bv. phaseoli. Molecular Plant-Microbe Interactions. 1992-05-01, 5 (3): 199–203. ISSN 0894-0282. PMID 1421508. doi:10.1094/mpmi-5-199.
- ^ Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem. Biophys. Res. Commun. November 1999, 265 (2): 410–5. PMID 10558881. doi:10.1006/bbrc.1999.1695.
- ^ Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. February 2001, 131 (2): 235–41. PMID 11160539. doi:10.1093/jn/131.2.235.
- ^ Naringenin from cooked tomato paste is bioavailable in men. J. Nutr. November 2002, 132 (11): 3349–52. PMID 12421849. doi:10.1093/jn/132.11.3349.
- ^ Wang, Chuanhong; Zhi, Shuang; Liu, Changying; Xu, Fengxiang; Zhao, Aichun; Wang, Xiling; Ren, Yanhong; Li, Zhengang; Yu, Maode. Characterization of Stilbene Synthase Genes in Mulberry (Morus atropurpurea) and Metabolic Engineering for the Production of Resveratrol in Escherichia coli. Journal of Agricultural and Food Chemistry. 2017, 65 (8): 1659–1668. PMID 28168876. doi:10.1021/acs.jafc.6b05212.
- ^ Ibrahim AR. Sulfation of naringenin by Cunninghamella elegans. Phytochemistry. January 2000, 53 (2): 209–12. PMID 10680173. doi:10.1016/S0031-9422(99)00487-2.
- ^ Rauha, Jussi-Pekka; Remes, Susanna; Heinonen, Marina; Hopia, Anu; Kähkönen, Marja; Kujala, Tytti; Pihlaja, Kalevi; Vuorela, Heikki; Vuorela, Pia. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. International Journal of Food Microbiology. 2000-05-25, 56 (1): 3–12. PMID 10857921. doi:10.1016/S0168-1605(00)00218-X.
- ^ 22.0 22.1 Mandalari, G.; Bennett, R. N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C. B.; Gasson, M. J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. Journal of Applied Microbiology. 2007-12-01, 103 (6): 2056–2064. ISSN 1364-5072. PMID 18045389. doi:10.1111/j.1365-2672.2007.03456.x.
- ^ Koru, Ozgur; Toksoy, Fulya; Acikel, Cengiz Han; Tunca, Yasar Meric; Baysallar, Mehmet; Uskudar Guclu, Aylin; Akca, Eralp; Ozkok Tuylu, Asli; Sorkun, Kadriye. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe. 2007-06-01, 13 (3–4): 140–145. ISSN 1075-9964. PMID 17475517. doi:10.1016/j.anaerobe.2007.02.001.
- ^ Uzel, Ataç; Sorkun, Kadri˙ye; Önçağ, Özant; Çoğulu, Dilşah; Gençay, Ömür; Sali˙h, Beki˙r. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiological Research. 2005-04-25, 160 (2): 189–195. PMID 15881836. doi:10.1016/j.micres.2005.01.002.
- ^ Bae, Eun-Ah; Han, Myung; Kim, Dong-Hyun. In vitroAnti-Helicobacter pylori Activity of Some Flavonoids and Their Metabolites. Planta Medica. 1999, 65 (5): 442–443. PMID 10454900. doi:10.1055/s-2006-960805.
- ^ Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. May 2008, 47 (5): 1437–45. PMC 4500072
 . PMID 18393287. doi:10.1002/hep.22197.
. PMID 18393287. doi:10.1002/hep.22197.
- ^ A Pilot Study of the Grapefruit Flavonoid Naringenin for HCV Infection - Full Text View - ClinicalTrials.gov. clinicaltrials.gov. (原始内容存档于2010-10-01).
- ^ Mucsi, I.; Prágai, B. M. Inhibition of virus multiplication and alteration of cyclic AMP level in cell cultures by flavonoids. Experientia. 1985-07-01, 41 (7): 930–931. ISSN 0014-4754. PMID 2989000. doi:10.1007/BF01970018 (英语).
- ^ Lyu, Su-Yun; Rhim, Jee-Young; Park, Won-Bong. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2)in vitro. Archives of Pharmacal Research. 2005-11-01, 28 (11): 1293–1301. ISSN 0253-6269. PMID 16350858. doi:10.1007/BF02978215 (英语).
- ^ Kawaguchi, K.; Kikuchi, S.; Hasunuma, R.; Maruyama, H.; Ryll, R.; Kumazawa, Y. Suppression of Infection-Induced Endotoxin Shock in Mice by aCitrusFlavanone Naringin. Planta Medica. 2004, 70 (1): 17–22. PMID 14765287. doi:10.1055/s-2004-815449.
- ^ Gutiérrez-Venegas, Gloria; Kawasaki-Cárdenas, Perla; Rita Arroyo-Cruz, Santa; Maldonado-Frías, Silvia. Luteolin inhibits lipopolysaccharide actions on human gingival fibroblasts. European Journal of Pharmacology. 2006-07-10, 541 (1–2): 95–105. PMID 16762341. doi:10.1016/j.ejphar.2006.03.069.
- ^ Olszanecki, R.; Gebska, A.; Kozlovski, V. I.; Gryglewski, R. J. Flavonoids and nitric oxide synthase. Journal of Physiology and Pharmacology. 2002-12-01, 53 (4 Pt 1): 571–584. ISSN 0867-5910. PMID 12512693.
- ^ Gorinstein, Shela; Leontowicz, Hanna; Leontowicz, Maria; Krzeminski, Ryszard; Gralak, Mikolaj; Delgado-Licon, Efren; Martinez Ayala, Alma Leticia; Katrich, Elena; Trakhtenberg, Simon. Changes in Plasma Lipid and Antioxidant Activity in Rats as a Result of Naringin and Red Grapefruit Supplementation. Journal of Agricultural and Food Chemistry. 2005-04-01, 53 (8): 3223–3228. ISSN 0021-8561. PMID 15826081. doi:10.1021/jf058014h.
- ^ Yu, Jun; Wang, Limin; Walzem, Rosemary L.; Miller, Edward G.; Pike, Leonard M.; Patil, Bhimanagouda S. Antioxidant Activity of Citrus Limonoids, Flavonoids, and Coumarins. Journal of Agricultural and Food Chemistry. 2005-03-01, 53 (6): 2009–2014. ISSN 0021-8561. PMID 15769128. doi:10.1021/jf0484632.
- ^ Sumit Kumar & Ashu Bhan Tiku. Biochemical and Molecular Mechanisms of Radioprotective Effects of Naringenin, a Phytochemical from Citrus Fruits. J. Agric. Food Chem. 2016, 64 (8): 1676–1685. PMID 26881453. doi:10.1021/acs.jafc.5b05067.
- ^ Chandra Jagetia, Ganesh; Koti Reddy, Tiyyagura; Venkatesha, V. A; Kedlaya, Rajendra. Influence of naringin on ferric iron induced oxidative damage in vitro. Clinica Chimica Acta. 2004-09-01, 347 (1–2): 189–197. PMID 15313158. doi:10.1016/j.cccn.2004.04.022.
- ^ Kanno, Syu-ichi; Tomizawa, Ayako; Hiura, Takako; Osanai, Yuu; Shouji, Ai; Ujibe, Mayuko; Ohtake, Takaharu; Kimura, Katsuhiko; Ishikawa, Masaaki. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biological and Pharmaceutical Bulletin. 2005-01-01, 28 (3): 527–530. PMID 15744083. doi:10.1248/bpb.28.527.
- ^ So, Felicia V.; Guthrie, Najla; Chambers, Ann F.; Moussa, Madeleine; Carroll, Kenneth K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutrition and Cancer. 1996-01-01, 26 (2): 167–181. ISSN 0163-5581. PMID 8875554. doi:10.1080/01635589609514473.
- ^ Hermawan A, Ikawati M, Jenie RI, Khumaira A, Putri H, Nurhayati IP, Angraini SM, Muflikhasari HA. Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies (PDF). Saudi Pharmaceutical Journal. January 2021, 29 (1): 12–26. PMC 7873751
 . PMID 33603536. doi:10.1016/j.jsps.2020.12.002. (原始内容存档 (PDF)于2021-10-18).
. PMID 33603536. doi:10.1016/j.jsps.2020.12.002. (原始内容存档 (PDF)于2021-10-18).
- ^ van Meeuwen, J. A.; Korthagen, N.; de Jong, P. C.; Piersma, A. H.; van den Berg, M. (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicology and Applied Pharmacology. 2007-06-15, 221 (3): 372–383. PMID 17482226. doi:10.1016/j.taap.2007.03.016.
- ^ Harmon, Anne W.; Patel, Yashomati M. Naringenin Inhibits Glucose Uptake in MCF-7 Breast Cancer Cells: A Mechanism for Impaired Cellular Proliferation. Breast Cancer Research and Treatment. 2004-05-01, 85 (2): 103–110. ISSN 0167-6806. PMID 15111768. doi:10.1023/B:BREA.0000025397.56192.e2 (英语).
- ^ Ferreira, Ricardo J; Baptista, Rafael; Moreno, Alexis; Madeira, Patricia G; Khonkarn, Ruttiros; Baubichon-Cortay, Hélène; Santos, Daniel JVA dos; Falson, Pierre; Ferreira, Maria-José U. Optimizing the flavanone core toward new selective nitrogen-containing modulators of ABC transporters. Future Medicinal Chemistry. 2018-03-23, 10 (7): 725–741. PMID 29570361. doi:10.4155/fmc-2017-0228 (英语).
