蒎烯
外觀
| 蒎烯 | |
|---|---|
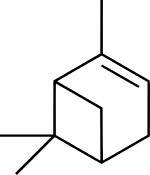
| |
| IUPAC名 (1S,5S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (1S,5S)-6,6-dimethyl-2-methylenebicyclo[3.1.1]heptane | |
| 識別 | |
| CAS號 | 80-56-8((±)-α) 7785-70-8(1R,5R-α) 7785-26-4(1S,5S-α) 2437-95-8(來源未指明) 127-91-3((±)-β) 18172-67-3(1S,5S-β) |
| 性質 | |
| 化學式 | C10H16 |
| 摩爾質量 | 136.24 g·mol⁻¹ |
| 外觀 | 液體 |
| 密度 | 0.86 g·cm−3 (α, 15 °C)[1][2] |
| 熔點 | -62 ~ -55 °C |
| 沸點 | 155 ~ 156 °C |
| 溶解性(水) | 幾乎不溶於水 |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
蒎烯(Pinene)是一類具有相同骨架結構的天然有機化合物,屬於雙環單萜,分子式C10H16。
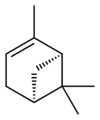
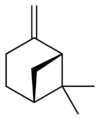
自然界存在α-蒎烯和β-蒎烯,它們之間屬雙鍵的位置異構。這兩種蒎烯是松節油的主要成分(蒎烯英文名即出自松樹),也存在於其它松柏門植物中。在針葉植物以外的種類中也發現其存在,比如一種菊科植物Heterotheca[3]以及大鼠尾草(三齒蒿)。
異構體
[編輯]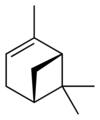 |
 |
 |
 | |
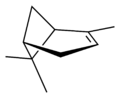 |
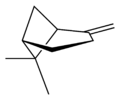 | |||
 |
 | |||
生物合成
[編輯]生物體內,α-蒎烯和β-蒎烯都是由香葉基焦磷酸酯轉化生成的。反應機理經過酯基異構化,首先得芳樟醇焦磷酸酯,接着環化重排,然後從碳正離子失去一個質子得到產物。

分佈
[編輯]α-蒎烯是自然界中最廣泛分佈的萜烯之一,[4]具有強烈的驅蟲性。[5]
α-蒎烯主要存在於針葉樹等植物。[6]也是毒馬草屬和鼠尾草屬植物精油的主要成分。[7][8]大麻屬也含α-蒎烯。[6] 圓柄黃連木的樹脂富含蒎烯。松子中也存在。[6]
應用
[編輯]化學工業上,蒎烯的催化氧化得到各種人工香料,如馬鞭烯酮等。[10]

參考資料
[編輯]- ^ Record of alpha-Pinen in the GESTIS Substance Database from the IFA, accessed on 07-January-2016
- ^ Record of beta-Pinen in the GESTIS Substance Database from the IFA, accessed on 07-January-2016
- ^ Lincoln, D.E., B.M. Lawrence. 1984. The volatile constituents of camphorweed, Heterotheca subaxillaris. Phytochemistry 23(4):933-934
- ^ Noma Y, Asakawa Y (2010). Biotransformation of monoterpenoids by microorganisms, insects, and mammals. In: Baser KHC, Buchbauer G (eds). Handbook of Essential Oils: Science, Technology, and Applications. CRC Press: Boca Raton, FL, pp. 585–736.
- ^ Nerio LS, Olivero-Verbel J, Stashenko E. Repellent activity of essential oils: a review. Bioresour Technol. 2010, 101 (1): 372–378. PMID 19729299. doi:10.1016/j.biortech.2009.07.048.
- ^ 6.0 6.1 6.2 Russo, E. B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British Journal of Pharmacology. 2011, 163 (7): 1344–1364 [2017-05-12]. PMC 3165946
 . PMID 21749363. doi:10.1111/j.1476-5381.2011.01238.x. (原始內容存檔於2018-02-22).
. PMID 21749363. doi:10.1111/j.1476-5381.2011.01238.x. (原始內容存檔於2018-02-22).
- ^ Kose EO, Deniz IG, Sarikurkcu C, Aktas O, Yavuz M (2010). Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr. (var. erythrantha and var. cedretorum P.H. Davis) endemic in Turkey. Food Chem Toxicol 48: 2960–2965.
- ^ Ozek G, Demirci F, Ozek T, Tabanca N, Wedge DE, Khan SI et al. (2010). Gas chromatographic-mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J Chromatog 1217: 741–748.
- ^ Kasuan, Nurhani. Extraction of Citrus hystrix D.C. (Kaffir Lime) Essential Oil Using Automated Steam Distillation Process: Analysis of Volatile Compounds (PDF). Malyasian Journal of Analytical Sciences. 2013, 17 (3): 359–369 [2017-05-12]. (原始內容存檔 (PDF)於2020-08-06).
- ^ U. Neuenschwander, Mechanism of the Aerobic Oxidation of α-Pinene, ChemSusChem. 2010, 3 (1): pp. 75–84, (德文)
- Mann, J.; Davidson, R. S.; Hobbs, J. B.; Banthorpe, D. V.; Harborne, J. B. Natural Products. Harlow, UK: Addison Wesley Longman Ltd. 1994: 309–311. ISBN 0-582-06009-5.
