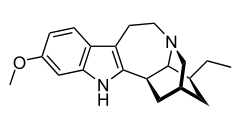
馬山茶鹼
外觀
 | |
 | |
| 臨床資料 | |
|---|---|
| ATC碼 |
|
| 識別資訊 | |
| |
| CAS號 | 83-94-3 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
| 化學式 | C20H26N2O |
| 摩爾質量 | 310.44 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
馬山茶鹼(英語:Tabernanthine)是一種在鵝花樹中發現的生物鹼。[1]它已被用於實驗室實驗,以研究成癮如何影響大腦。[2]馬山茶鹼可持續減少大鼠可卡因和嗎啡的自我給藥。[3]
藥理學
[編輯]它是κ-阿片激動劑(Ki=0.15μM)和NMDA受體(Ki=10.5μM)拮抗劑。[4][5]與伊波加因相比,它與σ1和σ2受體的結合較弱。[5]
參見
[編輯]參考資料
[編輯]- ^ Bartlett, M. F.; Dickel, D. F.; Taylor, W. I. The Alkaloids of Tabernanthe iboga. Part IV.1 The Structures of Ibogamine, Ibogaine, Tabernanthine and Voacangine. Journal of the American Chemical Society. 1958, 80: 126–136. doi:10.1021/ja01534a036.
- ^ Levi MS, Borne RF. A review of chemical agents in the pharmacotherapy of addiction. Curr. Med. Chem. October 2002, 9 (20): 1807–18. PMID 12369879. doi:10.2174/0929867023368980.
- ^ Glick SD, Kuehne ME, Raucci J, Wilson TE, Larson D, Keller RW, Carlson JN. Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum. Brain Research. September 1994, 657 (1–2): 14–22. PMID 7820611. S2CID 1940631. doi:10.1016/0006-8993(94)90948-2.
- ^ Deecher DC, Teitler M, Soderlund DM, Bornmann WG, Kuehne ME, Glick SD. Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies. Brain Research. February 1992, 571 (2): 242–7. PMID 1377086. S2CID 17159661. doi:10.1016/0006-8993(92)90661-r.
- ^ 5.0 5.1 Christophe Wiart. Lead Compounds from Medicinal Plants for the Treatment of Neurodegenerative Diseases. Academic Press. 16 December 2013: 67–69, 73. ISBN 978-0-12-398383-1.
