碳循環:修订间差异
Uranus1781(留言 | 贡献) 小 回退115.164.57.249(討論)做出的1次編輯,到由顏嘉佑做出的最後修訂版本 |
ThomasYehYeh(留言 | 贡献) 我把Carbon cycle全部翻譯,用來補充原來碳循環內容過短的遺憾。我覺得這篇英文版說得清楚,言之有物,是好文章。敬請指教。 |
||
| 第1行: | 第1行: | ||
[[File:Carbon cycle.jpg|thumb|right|460px|此图显示每年有數十億噸的碳,在陆地大气和海洋之间中快速碳循环的运动。黄色数字是天然的通量,红色是人类的贡献,在每年數十億噸碳计。白色數字表示儲存的碳。]] |
|||
'''碳循環'''是一种[[生物地质化学循环]],指[[碳]]元素在地球上的[[生物圈]]、岩石圈、土壤圈、[[水圈]]及[[大氣]]中交換。碳的主要來源有四個,分別是大氣、陸上的生物圈(包括淡水系統及無生命的有機化合物)、海洋及沉積物。与氮循环和水循环一起,碳循环包含了一系列使地球能持续存在生命的关键过程和事件。碳循环描述了碳元素在地球上的回收和重复利用,包括碳沉淀。一个对湖泊的碳预算的测试可以检测这个湖泊是否起到了沉淀二氧化碳的作用。碳循环最早被[[约瑟夫·普利斯特里]]和[[安東萬-羅倫·德·拉瓦節]]发现,被[[汉弗里·戴维]]所推广。 |
|||
[[File:Carbon cycle.jpg|thumb|right|upright=2|地球於"快速碳循環"中,每年有幾吉噸(gigatons,十億噸)的碳在陸地、大氣與海洋之間移動。黃色數字代表自然產生的通量、紅色數字代表人類產生的排放,白色數字代表儲存的碳。"慢速碳循環"(也稱深層碳循環,如[[火山]]噴發與板塊活動所產生)的碳活動未包括在內。<ref name="nasacc">{{cite web|last1=Riebeek|first1=Holli|title=The Carbon Cycle|url=http://earthobservatory.nasa.gov/Features/CarbonCycle/?src=eoa-features|website=Earth Observatory|publisher=NASA|access-date=2018-04-05|date=2011-06-16|archive-url=https://web.archive.org/web/20160305010126/http://earthobservatory.nasa.gov/Features/CarbonCycle/?src=eoa-features|archive-date=2016-03-05|url-status=live|df=dmy-all}}</ref>]] |
|||
== 主要组成部分 == |
|||
{{碳循環側邊欄}} |
|||
'''碳循環'''({{lang-en|Carbon cycle}})是[[生物地球化學循環]]中的一支,地球的碳透過此循環在[[生物圈]]、[[土壤圈]]、{{le|地質圈|geosphere}}、[[水圈]]和[[地球大氣層|大氣層]]之間進行交換。其他主要的生物地球化學循環還包括[[氮循環]]和[[水循環]]。[[碳]]是生物化合物的主要成分,也是[[石灰石]]等許多礦物質的主要成分。碳循環由一連串事件組成,這些事件對於地球能維持生命存在有關鍵作用。碳循環描述碳在整個生物圈中[[資源回收|回收]]和[[重複使用]]期間的變動,以及[[碳截存|儲存]]及由[[碳匯]]釋放的漫長過程。 |
|||
為描述碳循環中的動態,可將之區分為快速循環和慢速循環兩種。快速碳循環也稱為生物碳循環,這類循環可在幾年內完成,由大氣轉移到生物圈,然後再返回大氣。慢速碳循環(即地質循環),也稱為{{le|深層碳循環|deep carbon cycle}},需花費數百萬年才能完成,其間碳穿過地殼,在岩石、土壤、海[[洋]]和大氣之間移動。<ref name="Libes2015">Libes, Susan M. (2015). [https://books.google.com/books?id=5tC9CgAAQBAJ&dq=%22blue+planet%22+libes&pg=PA89 Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change] {{Webarchive|url=https://web.archive.org/web/20230108232518/https://books.google.com/books?hl=en&lr=&id=5tC9CgAAQBAJ&oi=fnd&pg=PA89&dq=%22blue+planet%22+libes&ots=oesDSXq1NZ&sig=B7HrLG0Y6iE9p_AqfDfSVktQGN4#v=onepage&q=%22blue%20planet%22%20libes&f=false|date= 2023-01-08}} In: ''Routledge Handbook of Ocean Resources and Management'', Routledge, pages 89–107. {{isbn|9781136294822}}.</ref> |
|||
人類在過去幾個世紀中透過改變[[土地利用]],以及從地質圈中以工業化規模開採化石碳([[煤]]炭、[[石油]]和[[天然氣]],及製造[[水泥]]),擾亂快速碳循環的節奏。<ref name="nasacc" /><ref name="gcb19" />迄2020年,全球大氣中的[[二氧化碳]]含量比[[第一次工業革命]]開始前的平均水平增加近52%,經由太陽[[輻射強迫]]作用,導致大氣和地球表面溫度升高。<ref name="Prentice_etal_2001" /><ref name="noaagi">{{Cite web |url=https://www.esrl.noaa.gov/gmd/aggi/ |title=The NOAA Annual Greenhouse Gas Index (AGGI) - An Introduction |publisher=[[NOAA]] Global Monitoring Laboratory/Earth System Research Laboratories |access-date=2020-10-30 }}</ref>大氣中二氧化碳增加後也導致海洋[[pH值]]降低,而根本上將海洋化學改變。<ref>{{Cite web |url=https://oceanservice.noaa.gov/facts/acidification.html |title=What is Ocean Acidification? |publisher=National Ocean Service, [[National Oceanic and Atmospheric Administration]] |access-date=2020-10-30}}</ref><ref name="scor-int">{{cite web|url=http://www.scor-int.org/OBO2009/A&O_Report.pdf |archive-url=https://web.archive.org/web/20110715113115/http://www.scor-int.org/OBO2009/A&O_Report.pdf |archive-date=2011-07-15 |url-status=live|title=Report of the Ocean Acidification and Oxygen Working Group, SCOR Biological Observatories Workshop|publisher=International Council for Science's Scientific Committee on Ocean Research (SCOR) |website=scor-int.org/ |date=2009-09-30}}</ref>在過去的半個世紀中更有大量的化石碳被開採以及使用,且速率持續快速上升,加劇人為造成的{{le|氣候變化|Climate change}}。<ref>{{Cite journal |last=Heede|first=R. |title=Tracing anthropogenic carbon dioxide and methane emissions to fossil fuel and cement producers, 1854–2010 |journal=Climatic Change |volume=122 |pages=229–241 |year=2014 |issue=1–2 |doi=10.1007/s10584-013-0986-y|bibcode=2014ClCh..122..229H |doi-access=free }}</ref><ref>{{Cite journal |url=https://ourworldindata.org/emissions-by-fuel |title=CO₂ and Greenhouse Gas Emissions: CO₂ Emissions by Fuel |first1=Hannah|last1=Ritchie|first2=Max|last2=Roser |journal=Our World in Data |publisher=Published online at OurWorldInData.org. |year=2020 |access-date=2020-10-30}}</ref> |
|||
==主要碳庫== |
|||
碳循環的概念首先由[[法國]]化學家[[安托万-洛朗·德·拉瓦锡|安托萬-羅倫·德·拉瓦節]]和[[英國]]化學家[[約瑟夫·普利斯特里]]於十九世紀中葉後期提出,隨後由英國化學家[[漢弗里·戴維]]推廣。<ref name="AOW">Holmes, Richard (2008). "The Age Of Wonder", Pantheon Books. {{ISBN|978-0-375-42222-5}}.</ref>目前碳循環透過交換路徑於以下幾個主要碳庫(即碳匯)間連接:<ref>{{cite book|last1=Archer|first1=David|title=The global carbon cycle|date=2010|publisher=Princeton University Press|location=Princeton|isbn=9781400837076}}</ref>{{rp|5–6}} |
|||
*大氣層 |
|||
*陸地生物圈 |
|||
*海洋,包括溶解無機碳(DIC),以及溶解有機碳(DOC) |
|||
*[[沉積物]],包括[[化石燃料]]、淡水系統和死亡有機材料。 |
|||
*地球內部({{le|地函|Mantle (geology)}}和[[地殼]])。存於此處的碳透過地質過程與其他成分相互作用。 |
|||
碳庫之間的碳交換是各種化學、物理、地質和生物過程的結果。海洋是地表最大的活性碳庫。<ref name=GlobalCarbonCycle/>碳在大氣、海洋、陸地的[[生態系|生態系統]]和沈積物之間以相當平衡的方式自然流動,在沒人類影響的情況下,碳水平將大致維持穩定。<ref name=Prentice_etal_2001>{{cite book |last=Prentice |first=I.C. |hdl=10067/381670151162165141 |chapter=The carbon cycle and atmospheric carbon dioxide |title=Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change |editor1-last=Houghton |editor1-first=J.T. |year=2001 }}</ref><ref name=U>{{cite web|title=An Introduction to the Global Carbon Cycle|publisher=University of New Hampshire|url=http://globecarboncycle.unh.edu/CarbonCycleBackground.pdf|year=2009|access-date=2016-02-06|archive-url=https://web.archive.org/web/20161008110835/http://globecarboncycle.unh.edu/CarbonCycleBackground.pdf|archive-date=2016-10-08|url-status=live|df=dmy-all}}</ref> |
|||
===大氣層=== |
|||
{{main|{{le|大氣碳循環|Atmosferic carbon cycle}}}} |
|||
[[File:NASA - A Year in the Life of Earth's CO2 x1SgmFa0r04.webm|thumb|upright=1.2|left|{{center|[[美國國家航空暨太空總署|NASA]]電腦模型顯示大氣中二氧化碳如何在一年的週期內於全球移動。<ref>[https://svs.gsfc.nasa.gov/cgi-bin/details.cgi?aid=11719 A Year In The Life Of Earth's CO2] {{Webarchive|url=https://web.archive.org/web/20211226063515/https://svs.gsfc.nasa.gov/cgi-bin/details.cgi?aid=11719 |date= 2021-12-26 }} ''[[NASA]]: [[Goddard Space Flight Center]]'', 2014-11-17.</ref>}}]] |
|||
大氣層中的碳有兩種主要形式:二氧化碳和[[甲烷]]。這兩種氣體會吸收來自太陽的熱量,並將其保留,是造成[[溫室效應]]的部分原因。<ref name=GlobalCarbonCycle>{{Cite journal |last1=Falkowski |first1=P. |last2=Scholes |first2=R. J. |last3=Boyle |first3=E. |last4=Canadell |first4=J. |last5=Canfield |first5=D. |last6=Elser |first6=J. |last7=Gruber |first7=N. |last8=Hibbard |first8=K. |last9=Högberg |first9=P. | last10 = Linder | first10 = S. |last11=MacKenzie |first11=F. T. |last12=Moore, III |first12=B. |last13=Pedersen |first13=T. |last14=Rosenthal |first14=Y. |last15=Seitzinger |first15=S. |last16=Smetacek |first16=V. |last17=Steffen |first17=W. |title=The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System |doi=10.1126/science.290.5490.291 |journal=Science |volume=290 |issue=5490 |pages=291–296 |year=2000 |pmid=11030643 |bibcode=2000Sci...290..291F}}</ref>同樣單位體積的甲烷會比二氧化碳產生更大的[[溫室效應]],但其在大氣層中的濃度要低很多,而且壽命更短。二氧化碳因此在全球溫室效應的影響比甲烷更大。<ref name=Forster2007>{{Cite journal |title=Changes in atmospheric constituents and in radiative forcing |year=2007 |journal=Climate Change 2007: The Physical Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change |last1=Forster |first1=P. |last2=Ramawamy |first2=V. |last3=Artaxo |first3=P. |last4=Berntsen |first4=T. |last5=Betts |first5=R. |last6=Fahey |first6=D.W. |last7=Haywood |first7=J. |last8=Lean |first8=J. |author8-link=Judith Lean |last9=Lowe |first9=D.C. | last10 = Myhre | first10 = G. |last11=Nganga |first11=J. |last12=Prinn |first12=R. |last13=Raga |first13=G. |last14=Schulz |first14=M. |last15=Van Dorland |first15=R. }}</ref> |
|||
大氣中的二氧化碳主要會受植物的[[光合作用]]移除,然後進入陸地和海洋生物圈。二氧化碳也會直接由大氣層溶入[[水體]](海洋、[[湖泊]]等)之中,以及溶於雨滴,隨[[降水]]下降到地表。當二氧化碳溶解與水時,會與水分子反應而形成[[碳酸]],最終導致[[海洋酸化]]。它可透過風化作用被岩石吸收,也會因酸化而腐蝕所接觸的其他表面,或被沖入海洋。<ref name=Planet>{{Cite journal |title=Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate |url=http://www.learner.org/courses/envsci/unit/text.php?unit=1&secNum=4 |journal=Many Planets, One Earth |volume=4 |access-date=2012-06-24 |archive-url=https://web.archive.org/web/20120417175417/http://www.learner.org/courses/envsci/unit/text.php?unit=1&secNum=4 |archive-date= 2012-04-17 |url-status=live |df=dmy-all }}</ref> |
|||
[[File:Carbon Dioxide 800kyr.svg|thumb|upright=1.35|根據鑽取的極地冰芯所測得地球於過去80萬年大氣中二氧化碳的濃度變化。顯示濃度自第一次工業革命後又開始增加,於20世紀時的增長開始加速。]] |
|||
過去兩個世紀中的人類活動已導致大氣中的碳含量增加近50%(迄2020年,主要以二氧化碳形式存在),不僅是透過改變生態系統從大氣中捕集二氧化碳的能力(例如[[森林砍伐]]),且又透過增加排放來實現(例如通過燃燒化石燃料和製造水泥)。<ref name="noaagi"/><ref name="GlobalCarbonCycle"/> |
|||
在遙遠的未來(20至30億年後),因[[太陽]]年紀增長而發生的變化(參見[[太陽系的形成和演化]]),導致土壤增快透過{{le|碳酸鹽-矽酸鹽循環|carbonate–silicate cycle}}吸收二氧化碳。預計屆時因太陽[[光度]]增加,會加快地表風化的速度,<ref name=swansong>{{cite journal |last1=O'Malley-James |first1=Jack T. |last2=Greaves |first2=Jane S. |last3=Raven |first3=John A. |last4=Cockell |first4=Charles S. |title=Swansong Biospheres: Refuges for life and novel microbial biospheres on terrestrial planets near the end of their habitable lifetimes |journal=[[International Journal of Astrobiology]] |date=2012 |volume=12 |issue=2 |pages=99–112 |arxiv=1210.5721 |bibcode=2013IJAsB..12...99O |doi=10.1017/S147355041200047X |s2cid=73722450 }}</ref>最終將導致大氣中的大部分二氧化碳會以[[碳酸鹽]]的形式被擠壓進入地殼。<ref>{{Cite journal|last1=Walker|first1=James C. G.|last2=Hays|first2=P. B.|last3=Kasting|first3=J. F.|date=1981|title=A negative feedback mechanism for the long-term stabilization of Earth's surface temperature|url=http://doi.wiley.com/10.1029/JC086iC10p09776|journal=Journal of Geophysical Research|language=en|volume=86|issue=C10|pages=9776|doi=10.1029/JC086iC10p09776|bibcode=1981JGR....86.9776W|issn=0148-0227}}</ref><ref name=":1">{{cite arXiv|last1=Heath|first1=Martin J.|last2=Doyle|first2=Laurance R.|date=2009-12-13|title=Circumstellar Habitable Zones to Ecodynamic Domains: A Preliminary Review and Suggested Future Directions|class=astro-ph.EP|eprint=0912.2482}}</ref><ref>{{Cite journal |last=Crockford |first=Peter W. |last2=Bar On |first2=Yinon M. |last3=Ward |first3=Luce M. |last4=Milo |first4=Ron |last5=Halevy |first5=Itay |date=November 2023 |title=The geologic history of primary productivity |url=https://linkinghub.elsevier.com/retrieve/pii/S0960982223012861 |journal=Current Biology |language=en |volume=33 |issue=21 |pages=4741–4750.e5 |doi=10.1016/j.cub.2023.09.040}}</ref>一旦大氣中二氧化碳的濃度降至約百萬分之50(50ppm)以下,C<sub>3</sub>類植物(約占當今地球植物[[生物質]]的95%)的[[C3類二氧化碳固定]]將不再發生。<ref name=":1" />不同模型的模擬結果有所不同,但一般預計此情況會在6億年後發生。<ref>{{Cite journal|last1=Lenton|first1=Timothy M.|last2=von Bloh|first2=Werner|date=2001-05-01|title=Biotic feedback extends the life span of the biosphere|journal=Geophysical Research Letters|language=en|volume=28|issue=9|pages=1715–1718|doi=10.1029/2000GL012198|bibcode=2001GeoRL..28.1715L|doi-access=free}}</ref> |
|||
一旦海洋中的水分在大約11億年後蒸發完畢,<ref name="swansong"/>板塊構造移動很可能會因為缺乏水作潤滑劑而停止,而因缺乏火山噴出二氧化碳,將導致碳循環在未來10億至20億年後結束。<ref>{{cite book | last1=Brownlee | first1=Donald E. | date=2010 | chapter=Planetary habitability on astronomical time scales | title=Heliophysics: Evolving Solar Activity and the Climates of Space and Earth | editor1-first=Carolus J. | editor1-last=Schrijver | editor2-first=George L. | editor2-last=Siscoe | editor2-link=George Siscoe | chapter-url=https://books.google.com/books?id=M8NwTYEl0ngC&pg=PA94 | publisher=Cambridge University Press | isbn=978-0-521-11294-9 | page=94}}</ref> |
|||
===陸地生物圈=== |
|||
[[File:Carbon stored in ecosystems.png|thumb|right|upright=1.35|儲存於陸地上不同生態系統中的碳(單位:吉噸)。<ref name="janow">{{cite journal |doi=10.2737/WO-GTR-95 |doi-access=free |title=Considering Forest and Grassland Carbon in Land Management |journal=General Technical Report |date=2017 |last1=Janowiak |first1=M. |last2=Connelly |first2=W.J. |last3=Dante-Wood |first3=K. |last4=Domke |first4=G.M. |last5=Giardina |first5=C. |last6=Kayler |first6=Z. |last7=Marcinkowski |first7=K. |last8=Ontl |first8=T. |last9=Rodriguez-Franco |first9=C. |last10=Swanston |first10=C. |last11=Woodall |first11=C.W. |last12=Buford |first12=M. |display-authors=3 |publisher=United States Department of Agriculture, Forest Service |pages=1–68 }}</ref>]] |
|||
{{main|{{le|陸地生物碳循環|terrestrial biological carbon cycle}}}} |
|||
陸地生物圈囊括所有陸地[[生物]](無論是活的或是死的)中的有機碳,以及儲存於土壤中的碳。大約有500吉噸(Gt,十億噸)的碳儲存在地面上的植物和其他生物體中,<ref name=Prentice_etal_2001/>而土壤中約存有1,500吉噸的碳。<ref>{{cite journal|last1=Rice|first1=Charles W.|title=Storing carbon in soil: Why and how?|journal=Geotimes|date=January 2002|volume=47|issue=1|pages=14–17|url=http://www.geotimes.org/jan02/feature_carbon.html|access-date=2018-04-05|archive-url=https://web.archive.org/web/20180405153123/http://www.geotimes.org/jan02/feature_carbon.html|archive-date=2018-04-05|url-status=live|df=dmy-all}}</ref>陸地生物圈中的大部分碳是有機碳,<ref>{{cite journal|doi=10.1111/gcbb.12401|title=Investigating the biochar effects on C-mineralization and sequestration of carbon in soil compared with conventional amendments using the stable isotope (δ<sup>13</sup>C) approach|journal=GCB Bioenergy|volume=9|issue=6|pages=1085–1099|year=2016|last1=Yousaf|first1=Balal|last2=Liu|first2=Guijian|last3=Wang|first3=Ruwei|last4=Abbas|first4=Qumber|last5=Imtiaz|first5=Muhammad|last6=Liu|first6=Ruijia|doi-access=free}}</ref>而大約三分之一的土壤碳以無機形式存在(例如[[碳酸鈣]])。<ref name=Lal-2008>{{Cite journal |doi=10.1039/b809492f |title=Sequestration of atmospheric CO<sub>2</sub> in global carbon pools |last=Lal |first=Rattan |journal=Energy and Environmental Science |volume=1 |pages=86–100 |year=2008}}</ref>有機碳是地球上所有生物的主要成分。[[自營生物]]以二氧化碳的形式從空氣中捕集碳,將其轉化為有機碳,而[[異營生物]]則透過攝取其他生物體來獲取。 |
|||
由於陸地生物圈吸收碳的方式取決於生物因素,因此會循晝夜和季節循環而進行。在測量二氧化碳之時,此一特徵可由[[基林曲線]]清楚顯現。此情況在[[北半球]]最強,因為北半球比[[南半球]]有更多的陸地面積,導致有更大的空間供生態系統吸收和排放碳。 |
|||
[[File:SRS1000 being used to measure soil respiration in the field..jpg|thumb|upright=1.2|left|手提式裝置,用於測量土壤的二氧化碳排放通量。]] |
|||
碳以多種方式和不同的時間尺度離開陸地生物圈。透過燃燒或是呼吸會將有機碳迅速釋放到大氣中。它也透過河流攜帶進入海洋或以惰性碳的形式保留在土壤中。<ref>{{cite journal |doi=10.1016/j.ecolind.2017.04.049 |title=The carbon flux of global rivers: A re-evaluation of amount and spatial patterns |journal=Ecological Indicators |volume=80 |pages=40–51 |year=2017 |last1=Li |first1=Mingxu |last2=Peng |first2=Changhui |last3=Wang |first3=Meng |last4=Xue |first4=Wei |last5=Zhang |first5=Kerou |last6=Wang |first6=Kefeng |last7=Shi |first7=Guohua |last8=Zhu |first8=Qiuan }}</ref>儲存在土壤中的碳可保留數千年,然後經侵蝕作用受沖刷進入河流,或透過[[土壤呼吸]]釋放進入大氣。於1989年至2008年期間,土壤呼吸作用每年增加的速率約為0.1%。<ref>{{cite journal |doi=10.1038/nature08930 |pmid=20336143 |title=Temperature-associated increases in the global soil respiration record |journal=Nature |volume=464 |issue=7288 |pages=579–582 |year=2010 |last1=Bond-Lamberty |first1=Ben |last2=Thomson |first2=Allison |bibcode=2010Natur.464..579B |s2cid=4412623 }}</ref>於2008年,全球土壤呼吸釋放的二氧化碳總量約為980億噸,<ref>{{cite web name=”World Agriculture”| url =http://www.world-agriculture.net/article/the-functions-and-sizes-of-the-five-carbon-sinks-on-planet-earth-and-their-relation-to-climate-change-part-i-their-present-sizes| title =The functions and sizes of the five carbon sinks on planet Earth and their relation to climate change Part I Their present sizes and locations| publisher =World Agriculture | date =2016-09-25 | authors=Dr. David Frape|accessdate = 2023-12-06 }}</ref>大約是人類現在每年透過燃燒化石燃料,排放到大氣中碳量的3倍。{{cite web name=”World Agriculture”/>對於這種趨勢有一些合理的解釋,其中最可能的是氣溫升高,而加速土壤有機質的分解速度,繼而增加二氧化碳的流量。土壤中儲存碳的時間長度取決於當地的氣候條件以及氣候變化過程中的變化。<ref name="Varney">{{Cite journal|last1=Varney|first1=Rebecca M.|last2=Chadburn|first2=Sarah E.|last3=Friedlingstein|first3=Pierre|last4=Burke|first4=Eleanor J.|last5=Koven|first5=Charles D.|last6=Hugelius|first6=Gustaf|last7=Cox|first7=Peter M.|date=2020-11-02|title=A spatial emergent constraint on the sensitivity of soil carbon turnover to global warming|url= |journal=Nature Communications|language=en|volume=11|issue=1|pages=5544|doi=10.1038/s41467-020-19208-8|pmid=33139706|pmc=7608627|bibcode=2020NatCo..11.5544V|issn=2041-1723}}</ref> |
|||
{| class="wikitable" border="1" align="right" |
{| class="wikitable" border="1" align="right" |
||
|+ 在地球上的主要碳库。<ref name=GlobalCarbonCycle/> |
|||
|+ 在地球上的主要储集池的碳库。<ref name=GlobalCarbonCycle>{{cite journal|title=The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System|journal=Science|date=2000-10-13|doi=10.1126/science.290.5490.291|url=http://science.sciencemag.org/content/290/5490/291|language=en|volume=290|issue=5490|pages=291–296|issn=0036-8075|accessdate=2018-04-02|author=P. Falkowski, R. J. Scholes, E. Boyle, J. Canadell, D. Canfield, J. Elser, N. Gruber, K. Hibbard, P. Högberg, S. Linder, F. T. Mackenzie, B. Moore Iii, T. Pedersen, Y. Rosenthal, S. Seitzinger, V. Smetacek, W. Steffen|archive-date=2018-03-31|archive-url=https://web.archive.org/web/20180331215719/http://science.sciencemag.org/content/290/5490/291|dead-url=no}}</ref> |
|||
! 碳库 !! 数量 (gigatons/十億噸) |
! 碳库 !! 数量 (gigatons/十億噸) |
||
|- |
|- |
||
| 第44行: | 第86行: | ||
|} |
|} |
||
===海洋=== |
|||
全球碳循环通常分为以下几大储集池的碳相互关联的途径交换: |
|||
{{main|{{le|海洋碳循環|Oceanic carbon cycle}}}} |
|||
*[[大气]] |
|||
*陆地[[生物圈]] |
|||
*[[海]][[洋]],包括溶解[[无机碳]],活的和死的海洋生物 |
|||
*[[沉積物]],如土壤[[有机碳]]的沉積物,包括[[化石燃料]],淡水系统和非生物有机材料 |
|||
*地球的内部,碳来自于地球的[[地幔]]和[[地壳]]。这些碳存储通过地质过程与其他组成部分交互 |
|||
海洋在概念上可分為[[表面層]](其中水與大氣頻繁接觸(從每天到每年)),與深層(低於典型[[混合層]]的深度,達到幾百米或更小),此層與表面層接觸的時間間隔可能是幾個世紀。表面層中的溶解無機碳(dissolved inorganic carbon,DIC)與大氣快速交換,維持平衡。深層中含有更多的碳,部分原因是其DIC濃度較表面層高出約15%,<ref name=Sarmiento_and_Gruber_2006>{{cite book |last1=Sarmiento |first1=J.L. |last2=Gruber |first2=N. |title=Ocean Biogeochemical Dynamics |year=2006 |publisher=Princeton University Press, Princeton, New Jersey, US}}</ref>但主要是由於深層體積較大,是世界上最大的主動循環碳庫,其中碳含量是大氣的50倍,<ref name=GlobalCarbonCycle/>但與大氣達到平衡的時間尺度需要數百年(經由[[溫鹽環流]]驅動,兩海水層之間的碳交換很慢)。<ref name=GlobalCarbonCycle/> |
|||
碳储集之间的交流发生是各种化学,物理,地质和生物过程的结果。海洋是包含最大的地球的表面附近的碳的活跃储集池。<ref name=GlobalCarbonCycle/> |
|||
碳主經由大氣中二氧化碳溶解而進入海洋,其中一小部分轉化為碳酸鹽。它也可以溶解有機碳(DOC)形式通過河流進入海洋。碳透過光合作用被生物體轉化為有機碳,且可在整個[[食物鏈]]中交換,或者以死亡軟組織的形式沉澱到海洋更深、更富含碳的海水層中,或者以[[碳酸鈣]]的形式成為[[貝類]]的殼。碳會在此海水層中循環很長一段時間,然後成為沉積物,或是最終通過溫鹽循環返回表面層水域。<ref name=Prentice_etal_2001/> |
|||
== 從大氣中移走二氧化碳 == |
|||
=== 植物(生產者) === |
|||
[[光合作用]]會從[[空氣]]中吸入[[二氧化碳]]並轉化為[[碳水化合物]],例如[[葡萄糖]]和[[澱粉]],接著將[[碳水化合物]]轉化為[[蛋白質]]和[[脂肪]]。較新的樹林會轉化較多的二氧化碳,因為植物生長的速度較快。 |
|||
海水呈鹼性(目前pH值為8.1至8.2)。大氣中二氧化碳增加後,會導致海水的pH值趨向中性變動,此過程稱為海洋酸化。海洋吸收二氧化碳是碳截存作用最重要的形式之一。預計海水pH值降低的程度會降低海洋生物碳酸鈣沉澱,也會把海洋吸收二氧化碳的能力降低。<ref name=Klyepas1999>{{Cite journal |last1=Kleypas |first1=J. A. |last2=Buddemeier |first2=R. W. |last3=Archer |first3=D. |last4=Gattuso |first4=J. P. |last5=Langdon |first5=C. |last6=Opdyke |first6=B. N. |title=Geochemical Consequences of Increased Atmospheric Carbon Dioxide on Coral Reefs |doi=10.1126/science.284.5411.118 |journal=Science |volume=284 |issue=5411 |pages=118–120 |year=1999 |pmid=10102806 |bibcode=1999Sci...284..118K}}</ref><ref name=Langdon2000>{{Cite journal |last1=Langdon |first1=C. |last2=Takahashi |first2=T. |last3=Sweeney |first3=C. |last4=Chipman |first4=D. |last5=Goddard |first5=J. |last6=Marubini |first6=F. |last7=Aceves |first7=H. |last8=Barnett |first8=H. |last9=Atkinson |first9=M. J. |doi=10.1029/1999GB001195 |title=Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef |journal=Global Biogeochemical Cycles |volume=14 |issue=2 |pages=639 |year=2000 |bibcode=2000GBioC..14..639L|s2cid=128987509 |doi-access=free }}</ref> |
|||
=== 動物(消費者) === |
|||
[[動物]]會以[[植物]]為食物,並將[[植物]]組織的[[有機物]][[消化]]及[[轉化 (生物)|轉化]]為[[動物]]組織。 |
|||
=== |
===地質圈=== |
||
{{main|{{le|碳酸鹽-矽酸鹽循環|Carbonate–silicate cycle}}}} |
|||
[[生物]][[屍體]]埋在[[泥土]]下或壓縮在水底下,經過數萬年泥土或水的壓力及地下的高溫,就會生成[[化石燃料]]而儲存大量的[[碳]]。 |
|||
[[File:Global carbon stocks.png|thumb|left|upright=1.8|顯示地球迄2014年不同碳庫儲存碳量(單位:吉噸)的圖表,土地利用變化與燃燒化石燃料的累積排放也被列入,以供比較。<ref name="janow"/>]] |
|||
碳循環的地質部分與全球碳循環的其他部分相比,運作緩慢。此為大氣中碳含量以及全球氣溫最重要的決定因素之一。<ref name=NASA>{{Cite web |title=The Slow Carbon Cycle |url=http://earthobservatory.nasa.gov/Features/CarbonCycle/page2.php |publisher=NASA |access-date=2012-06-24 |archive-url=https://web.archive.org/web/20120616151904/http://earthobservatory.nasa.gov/Features/CarbonCycle/page2.php |archive-date= 2012-06-16 |url-status=live |df=dmy-all |date=2011-06-16 }}</ref> |
|||
=== 海中的动物 === |
|||
海中有壳的动物会利用海水中的碳去制造自己的壳。 |
|||
地球上大部分的碳以惰性方式儲存在地球的岩石圈中。<ref name=GlobalCarbonCycle/>在地函中大部分的碳是在地球形成時即已存在。<ref name=DiVenere2012>[http://www.columbia.edu/~vjd1/carbon.htm The Carbon Cycle and Earth's Climate] {{Webarchive|url=https://web.archive.org/web/20030623195122/http://www.columbia.edu/~vjd1/carbon.htm |date= 2003-06-23 }} Information sheet for Columbia University Summer Session 2012 Earth and Environmental Sciences Introduction to Earth Sciences I</ref>而有一些是以有機碳的形式從生物圈沉積而來。<ref name=Berner1999>{{cite journal |last1=Berner |first1=Robert A. |title=A New Look at the Long-term Carbon Cycle|journal=GSA Today |date=November 1999 |volume=9 |issue=11 |pages=1–6 |url=https://www.geosociety.org/gsatoday/archive/9/11/pdf/gt9911.pdf |archive-url=https://web.archive.org/web/20190213183546/https://www.geosociety.org/gsatoday/archive/9/11/pdf/gt9911.pdf |archive-date=2019-02-13 |url-status=live }}</ref>地質圈中儲存的碳中約80%是存於[[石灰石]]及其衍生物中,是由儲存在海洋生物殼中的碳酸鈣沉積形成。剩下的20%以[[油母質]]的形式存在,這些油母質是陸地生物質經過高溫與高壓的沉積和埋藏而形成。儲存在地質圈中的有機碳可保留數百萬年。<ref name=NASA/> |
|||
=== 其他 === |
|||
大氣中的二氧化碳,部分會與[[降雨]]的水結合成[[碳酸]],落至地面經滲透後,與地底的鈣質化合成[[碳酸鈣]]。此即為[[鐘乳石]]生成原理,但此作用極為緩慢。 |
|||
碳可透過多種方式離開地質圈。當碳酸鹽岩[[隱沒帶|隱沒]]到地函中時,會在[[變質作用]]過程中釋放二氧化碳。這些二氧化碳可透過火山噴發和熱點而釋放進入大氣和海洋。<ref name=DiVenere2012/>人類也可透過直接開採化石燃料,利用燃燒以釋放能量,並將其儲存的碳排放進入大氣。 |
|||
== 二氧化碳回歸大氣中 == |
|||
=== 呼吸作用 === |
|||
[[植物]]、[[動物]]、[[細菌]]和[[真菌]]皆會透過[[呼吸作用]]釋放[[二氧化碳]] |
|||
== |
==動力學類型== |
||
[[File:Rock cycle nps.PNG|thumb|upright=1.85| {{center|顯示慢速碳循環(深層碳循環)的繪圖,顯示碳如何在岩石中移動。]] |
|||
[[細菌]]和[[真菌]]會將[[生物]]的[[屍體]]分解並釋放[[二氧化碳]]返回[[大氣]]中。 |
|||
=== 燃燒 === |
|||
當燃燒[[化石燃料]]時,便會釋放大量[[二氧化碳]]。 |
|||
=== 海中的动物 === |
|||
当贝壳碎裂的时候、生物死亡被分解時二氧化碳會被釋放到海裡。 |
|||
碳循環有快有慢。快速循環在生物圈中運行,慢速循環在岩石中運行。快速循環可在幾年內完成,將碳從大氣轉移到生物圈,然後返回大氣。慢速循環會延伸到地函深處,可能需要數百萬年才會完成,碳會穿過地殼,在岩石、土壤、海[[洋]]和大氣之間移動。<ref name="Libes2015" /> |
|||
===其他=== |
|||
[[碳酸鈣]]遇到[[酸雨]]([[鹽酸]]、[[亞硫酸]]),將導致碳酸鈣之中封存的二氧化碳釋出到大氣。 |
|||
快速循環涉及環境和生物圈中的生物體之間相對短期的生物地球化學過程(參見文章開頭的圖表)。包括大氣與陸地和海洋生態系統以及土壤和海底沉積物之間的碳移動。快速循環包括涉及光合作用的年度循環以及涉及營養生長和分解的代際循環。快速循環對人類活動的反應將決定氣候變化所產生的許多更直接的影響。<ref name="Bush2020">{{cite book |last1=Bush |first1=Martin J. |chapter=The Carbon Cycle |chapter-url=https://books.google.com/books?id=h_60DwAAQBAJ&q=%22Climate+Change+and+Renewable+Energy%22+%22The+Carbon+Cycle%22chapter+%3D+The+Carbon+Cycle&pg=PA109 |title=Climate Change and Renewable Energy |year=2020 |isbn=978-3-030-15423-3 |pages=109–141 |doi=10.1007/978-3-030-15424-0_3 |s2cid=210305910}}</ref><ref name=NASAfast>NASA Earth Observatory ( 2011-06-16). "The Fast Carbon Cycle". [https://earthobservatory.nasa.gov/features/CarbonCycle/page3.php Archive]. {{PD-notice}}</ref><ref>{{cite journal |last1=Rothman |first1=D. H. |year=2002 |title=Atmospheric carbon dioxide levels for the last 500 million years |journal=Proceedings of the National Academy of Sciences |volume=99 |issue=7 |pages=4167–4171 |bibcode=2002PNAS...99.4167R |doi=10.1073/pnas.022055499 |pmc=123620 |pmid=11904360 |doi-access=free}}</ref><ref name="Carpinteri2019">{{cite journal |last1=Carpinteri |first1=Alberto |last2=Niccolini |first2=Gianni |year=2019 |title=Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution |journal=Sci |volume=1 |page=17 |doi=10.3390/sci1010017 |doi-access=free}} [[File:CC-BY_icon.svg|50x50px]] Material was copied from this source, which is available under a [[creativecommons:by/4.0/|Creative Commons Attribution 4.0 International License]] {{Cite web |url=https://creativecommons.org/licenses/by/4.0/ |title=Archived copy |access-date= 2020-07-05 |archive-date= 2017-10-16 |archive-url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |url-status=bot: unknown }}.</ref><ref>{{Cite journal |last=Rothman |first=Daniel |date=January 2015 |title=Earth's carbon cycle: A mathematical perspective |url=https://www.ams.org/bull/2015-52-01/S0273-0979-2014-01471-5/ |journal=Bulletin of the American Mathematical Society |language=en |volume=52 |issue=1 |pages=47–64 |doi=10.1090/S0273-0979-2014-01471-5 |issn=0273-0979 |hdl-access=free |hdl=1721.1/97900}}</ref> |
|||
== 參看 == |
|||
* [[生物碳]]({{lang-en|Biochar}}) |
|||
* [[碳足印]]({{lang-en|Carbon footprint}}) |
|||
* [[初级生产]]({{lang-en|Primary production}}) |
|||
* [[雪球地球]]和慢速“碳循環” |
|||
* [[卡尔文循环]] |
|||
* [[生物地質化學循環]] |
|||
慢速(或是深層)碳循環涉及屬於岩石循環的中長期地球化學過程(參見附圖)。海洋和大氣之間的交換需要幾個世紀,岩石的風化需要數百萬年。海洋中的碳沉澱到海底,形成[[沉積岩]]並隱沒到地函中。[[山脈形成|造山過程]]導致地質碳返回地表,同時岩石受到風化,碳透過{{le|脫氣|degassing}}返回大氣,也透過河流攜帶進入海洋。其他地質碳則透過鈣離子的[[熱液系統]]排放返回海洋。在任何一年中皆會有1千萬至1億噸碳沿著這個緩慢的循環移動,也包括火山將地質碳以二氧化碳的形式直接送回大氣。然而這些二氧化碳的數量還不到燃燒化石燃料排放到大氣中的百分之一。<ref name="Libes2015" /><ref name="Bush2020" /><ref name=NASAslow>NASA Earth Observatory (2011-06-16). "The Slow Carbon Cycle". [https://earthobservatory.nasa.gov/features/CarbonCycle/page2.php Archive]. {{PD-notice}}</ref> |
|||
== 參考資料 == |
|||
* Basic Principles in Biology Book 1 -Y.K.TO. Chapter 6.6 Cycling of Materials |
|||
{{reflist}} |
|||
==快速碳循環中的子過程== |
|||
{{生物地質化學循環}} |
|||
===水循環中的陸地碳=== |
|||
[[File:Where carbon goes when water flows.jpg|thumb|upright=2|河流可將土地上的碳移往各處。<ref name=Ward2017>{{cite journal |doi = 10.3389/fmars.2017.00007|title = Where Carbon Goes when Water Flows: Carbon Cycling across the Aquatic Continuum|year = 2017|last1 = Ward|first1 = Nicholas D.|last2 = Bianchi|first2 = Thomas S.|last3 = Medeiros|first3 = Patricia M.|last4 = Seidel|first4 = Michael|last5 = Richey|first5 = Jeffrey E.|last6 = Keil|first6 = Richard G.|last7 = Sawakuchi|first7 = Henrique O.|journal = Frontiers in Marine Science|volume = 4|doi-access = free}} [[File:CC-BY icon.svg|50px]] Modified material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date= 2017-10-16 }}.</ref>}}]] |
|||
陸地碳在[[水循環]]中的移動如右圖所示,解釋如下:<ref name=Ward2017 /> |
|||
#大氣中[[懸浮微粒]]擔任[[雲凝結核]],促進雲形成。<ref name=Kerminen2000>{{cite journal |doi = 10.1029/1999JD901203|title = Secondary organics and atmospheric cloud condensation nuclei production|year = 2000|last1 = Kerminen|first1 = Veli-Matti|last2 = Virkkula|first2 = Aki|last3 = Hillamo|first3 = Risto|last4 = Wexler|first4 = Anthony S.|last5 = Kulmala|first5 = Markku|journal = Journal of Geophysical Research: Atmospheres|volume = 105|issue = D7|pages = 9255–9264|bibcode = 2000JGR...105.9255K|doi-access = free}}</ref><ref name=Riipinen2011>{{cite journal |doi = 10.5194/acp-11-3865-2011|title = Organic condensation: A vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations|year = 2011|last1 = Riipinen|first1 = I.|last2 = Pierce|first2 = J. R.|last3 = Yli-Juuti|first3 = T.|last4 = Nieminen|first4 = T.|last5 = Häkkinen|first5 = S.|last6 = Ehn|first6 = M.|last7 = Junninen|first7 = H.|last8 = Lehtipalo|first8 = K.|last9 = Petäjä|first9 = T.|last10 = Slowik|first10 = J.|last11 = Chang|first11 = R.|last12 = Shantz|first12 = N. C.|last13 = Abbatt|first13 = J.|last14 = Leaitch|first14 = W. R.|last15 = Kerminen|first15 = V.-M.|last16 = Worsnop|first16 = D. R.|last17 = Pandis|first17 = S. N.|last18 = Donahue|first18 = N. M.|last19 = Kulmala|first19 = M.|journal = Atmospheric Chemistry and Physics|volume = 11|issue = 8|pages = 3865–3878|bibcode = 2011ACP....11.3865R|doi-access = free}}</ref> |
|||
#雨滴於降落途中,透過顆粒和有機蒸氣吸附作用,吸收有機和無機碳。<ref name=Waterloo2006>{{cite journal |doi = 10.1002/hyp.6217|title = Export of organic carbon in run-off from an Amazonian rainforest blackwater catchment|year = 2006|last1 = Waterloo|first1 = Maarten J.|last2 = Oliveira|first2 = Sylvia M.|last3 = Drucker|first3 = Debora P.|last4 = Nobre|first4 = Antonio D.|last5 = Cuartas|first5 = Luz A.|last6 = Hodnett|first6 = Martin G.|last7 = Langedijk|first7 = Ivar|last8 = Jans|first8 = Wilma W. P.|last9 = Tomasella|first9 = Javier|last10 = De Araújo|first10 = Alessandro C.|last11 = Pimentel|first11 = Tania P.|last12 = Múnera Estrada|first12 = Juan C.|journal = Hydrological Processes|volume = 20|issue = 12|pages = 2581–2597|bibcode = 2006HyPr...20.2581W|s2cid = 129377411}}</ref><ref name=Neu2016>{{cite journal |doi = 10.3389/fmars.2016.00114|title = Dissolved Organic and Inorganic Carbon Flow Paths in an Amazonian Transitional Forest|year = 2016|last1 = Neu|first1 = Vania|last2 = Ward|first2 = Nicholas D.|last3 = Krusche|first3 = Alex V.|last4 = Neill|first4 = Christopher|journal = Frontiers in Marine Science|volume = 3|s2cid = 41290209|doi-access = free}}</ref> |
|||
#燃燒和火山爆發產生高度濃縮的[[多環芳香烴|多環芳香化合物]]分子(即[[黑碳]]),並與二氧化碳等溫室氣體一起進入大氣。<ref name=Baldock2004>{{cite journal |doi = 10.1016/j.marchem.2004.06.016|title = Cycling and composition of organic matter in terrestrial and marine ecosystems|year = 2004|last1 = Baldock|first1 = J.A.|last2 = Masiello|first2 = C.A.|last3 = Gélinas|first3 = Y.|last4 = Hedges|first4 = J.I.|journal = Marine Chemistry|volume = 92|issue = 1–4|pages = 39–64| bibcode=2004MarCh..92...39B }}</ref><ref name=Myers-Pigg2016>{{cite journal |doi = 10.1021/acs.est.6b02132|title = Signatures of Biomass Burning Aerosols in the Plume of a Saltmarsh Wildfire in South Texas|year = 2016|last1 = Myers-Pigg|first1 = Allison N.|last2 = Griffin|first2 = Robert J.|last3 = Louchouarn|first3 = Patrick|last4 = Norwood|first4 = Matthew J.|last5 = Sterne|first5 = Amanda|last6 = Cevik|first6 = Basak Karakurt|journal = Environmental Science & Technology|volume = 50|issue = 17|pages = 9308–9314|pmid = 27462728|bibcode = 2016EnST...50.9308M}}</ref> |
|||
#陸地植物透過光合作用將大氣中的二氧化碳固定,並透過呼吸作用將一小部分返還大氣。<ref name=Field1998>{{cite journal |doi = 10.1126/science.281.5374.237|title = Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components|year = 1998|last1 = Field|first1 = C. B.|last2 = Behrenfeld|first2 = M. J.|last3 = Randerson|first3 = J. T.|last4 = Falkowski|first4 = P.|journal = Science|volume = 281|issue = 5374|pages = 237–240|pmid = 9657713|bibcode = 1998Sci...281..237F|url = https://escholarship.org/uc/item/9gm7074q}}</ref>[[木質素]]和[[纖維素]]佔森林中有機碳中的80%,和牧場有機碳中的60%。<ref name=Martens2004>{{cite journal |doi = 10.1046/j.1529-8817.2003.00722.x|title = Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements|year = 2004|last1 = Martens|first1 = Dean A.|last2 = Reedy|first2 = Thomas E.|last3 = Lewis|first3 = David T.|journal = Global Change Biology|volume = 10|issue = 1|pages = 65–78|bibcode = 2004GCBio..10...65M|s2cid = 5019983|url = https://digitalcommons.unl.edu/agronomyfacpub/124}}</ref><ref name=Bose2009>{{cite journal |doi = 10.1016/j.biortech.2008.08.046|title = Lignin content versus syringyl to guaiacyl ratio amongst poplars|year = 2009|last1 = Bose|first1 = Samar K.|last2 = Francis|first2 = Raymond C.|last3 = Govender|first3 = Mark|last4 = Bush|first4 = Tamara|last5 = Spark|first5 = Andrew|journal = Bioresource Technology|volume = 100|issue = 4|pages = 1628–1633|pmid = 18954979}}</ref> |
|||
#植物[[凋落物]]和根系有機碳與沉積物混合形成有機土壤,其中植物來源的有機碳和石化有機碳透過微生物和真菌活動而被儲存與轉化。<ref name=Schlesinger2000>{{cite journal |doi = 10.1023/A:1006247623877|year = 2000|last1 = Schlesinger|first1 = William H.|last2 = Andrews|first2 = Jeffrey A.|title = Soil respiration and the global carbon cycle |journal = Biogeochemistry|volume = 48|pages = 7–20|s2cid = 94252768}}</ref><ref name=Schmidt2011>{{cite journal |doi = 10.1038/nature10386|title = Persistence of soil organic matter as an ecosystem property|year = 2011|last1 = Schmidt|first1 = Michael W. I.|last2 = Torn|first2 = Margaret S.|last3 = Abiven|first3 = Samuel|last4 = Dittmar|first4 = Thorsten|last5 = Guggenberger|first5 = Georg|last6 = Janssens|first6 = Ivan A.|last7 = Kleber|first7 = Markus|last8 = Kögel-Knabner|first8 = Ingrid|last9 = Lehmann|first9 = Johannes|last10 = Manning|first10 = David A. C.|last11 = Nannipieri|first11 = Paolo|last12 = Rasse|first12 = Daniel P.|last13 = Weiner|first13 = Steve|last14 = Trumbore|first14 = Susan E.|author14-link=Susan Trumbore |journal = Nature|volume = 478|issue = 7367|pages = 49–56|pmid = 21979045|bibcode = 2011Natur.478...49S|s2cid = 3461265|url = https://digital.library.unt.edu/ark:/67531/metadc844476/}}</ref><ref name=Lehmann2015>{{cite journal |doi = 10.1038/nature16069|title = The contentious nature of soil organic matter|year = 2015|last1 = Lehmann|first1 = Johannes|last2 = Kleber|first2 = Markus|journal = Nature|volume = 528|issue = 7580|pages = 60–68|pmid = 26595271|bibcode = 2015Natur.528...60L|s2cid = 205246638|doi-access = free}}</ref> |
|||
#當雨水穿過森林冠層(即{{le|樹冠穿透雨|throughfall}})和沿著植物樹幹/莖流下(即{{le|樹幹流|stemflow}})時,會吸收植物和沈降的[[氣膠]]衍生的溶解有機碳(DOC)和溶解無機碳(DIC)。<ref>{{cite journal |doi = 10.2136/sssaj1992.03615995005600020038x|title = Biodegradability of Dissolved Organic Matter in Forest Throughfall, Soil Solution, and Stream Water|year = 1992|last1 = Qualls|first1 = Robert G.|last2 = Haines|first2 = Bruce L.|journal = Soil Science Society of America Journal|volume = 56|issue = 2|pages = 578–586|bibcode = 1992SSASJ..56..578Q}}</ref>當水滲入土壤溶液和地下儲層時會發生生物地球化學轉變,<ref name=Grøn1992>{{cite journal |doi = 10.1016/0048-9697(92)90091-6|title = Biodegradability of dissolved organic carbon in groundwater from an unconfined aquifer|year = 1992|last1 = Grøn|first1 = Christian|last2 = Tørsløv|first2 = Jens|last3 = Albrechtsen|first3 = Hans-Jørgen|last4 = Jensen|first4 = Hanne Møller|journal = Science of the Total Environment|volume = 117-118|pages = 241–251|bibcode = 1992ScTEn.117..241G}}</ref><ref name=Pabich2001>{{cite journal |doi = 10.1023/A:1011842918260|title = Relationship between DOC concentration and vadose zone thickness and depth below water table in groundwater of Cape Cod, U.S.A.|year = 2001|last1 = Pabich|first1 = Wendy J.|last2 = Valiela|first2 = Ivan|last3 = Hemond|first3 = Harold F.|journal = Biogeochemistry|volume = 55|issue = 3|pages = 247–268|s2cid = 140536437}}</ref>當土壤中水分完全飽和後,<ref name=Linsley1975>{{Cite web|url=https://books.google.com/books?id=EopKHQAACAAJ&q=%22Hydrology+for+Engineers%22+1975|title=Solutions Manual to Accompany Hydrology for Engineers|last1=Linsley|first1=Ray K.|year=1975}}</ref>或降雨的速率快過土壤飽和的速度,<ref name=Horton1933>{{cite journal |doi = 10.1029/TR014i001p00446|title = The Rôle of infiltration in the hydrologic cycle|year = 1933|last1 = Horton|first1 = Robert E.|journal = Transactions, American Geophysical Union|volume = 14|issue = 1|page = 446|bibcode = 1933TrAGU..14..446H}}</ref>就會形成[[地表徑流]]。 |
|||
#來自陸地生物圈和[[in situ|原位]][[初級生產]]的有機碳被河流和溪流中的微生物群落分解以及物理分解(即{{le|光降解|Photodegradation}}),導致從河流排放到大氣中的二氧化碳通量與陸地生物圈每年固存的碳量相似。<ref name=Richey2002>{{cite journal |doi = 10.1038/416617a|title = Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2|year = 2002|last1 = Richey|first1 = Jeffrey E.|last2 = Melack|first2 = John M.|last3 = Aufdenkampe|first3 = Anthony K.|last4 = Ballester|first4 = Victoria M.|last5 = Hess|first5 = Laura L.|journal = Nature|volume = 416|issue = 6881|pages = 617–620|pmid = 11948346|bibcode = 2002Natur.416..617R|s2cid = 4345881}}</ref><ref name=Cole2007>{{cite journal |doi = 10.1007/s10021-006-9013-8|title = Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget|year = 2007|last1 = Cole|first1 = J. J.|last2 = Prairie|first2 = Y. T.|last3 = Caraco|first3 = N. F.|last4 = McDowell|first4 = W. H.|last5 = Tranvik|first5 = L. J.|last6 = Striegl|first6 = R. G.|last7 = Duarte|first7 = C. M.|last8 = Kortelainen|first8 = P.|last9 = Downing|first9 = J. A.|last10 = Middelburg|first10 = J. J.|last11 = Melack|first11 = J.|journal = Ecosystems|volume = 10|pages = 172–185|s2cid = 1728636}}</ref><ref name=Raymond2013>{{cite journal |doi = 10.1038/nature12760|title = Global carbon dioxide emissions from inland waters|year = 2013|last1 = Raymond|first1 = Peter A.|last2 = Hartmann|first2 = Jens|last3 = Lauerwald|first3 = Ronny|last4 = Sobek|first4 = Sebastian|last5 = McDonald|first5 = Cory|last6 = Hoover|first6 = Mark|last7 = Butman|first7 = David|last8 = Striegl|first8 = Robert|last9 = Mayorga|first9 = Emilio|last10 = Humborg|first10 = Christoph|last11 = Kortelainen|first11 = Pirkko|last12 = Dürr|first12 = Hans|last13 = Meybeck|first13 = Michel|last14 = Ciais|first14 = Philippe|last15 = Guth|first15 = Peter|journal = Nature|volume = 503|issue = 7476|pages = 355–359|pmid = 24256802|bibcode = 2013Natur.503..355R|s2cid = 4460910|url = http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-213816}}</ref>木質素<ref name=Ward2013>{{cite journal |doi = 10.1038/ngeo1817|title = Degradation of terrestrially derived macromolecules in the Amazon River|year = 2013|last1 = Ward|first1 = Nicholas D.|last2 = Keil|first2 = Richard G.|last3 = Medeiros|first3 = Patricia M.|last4 = Brito|first4 = Daimio C.|last5 = Cunha|first5 = Alan C.|last6 = Dittmar|first6 = Thorsten|last7 = Yager|first7 = Patricia L.|last8 = Krusche|first8 = Alex V.|last9 = Richey|first9 = Jeffrey E.|journal = Nature Geoscience|volume = 6|issue = 7|pages = 530–533|bibcode = 2013NatGe...6..530W}}</ref>和黑碳<ref name=Myers-Pigg2015>{{cite journal |doi = 10.1002/2014GL062762|title = Labile pyrogenic dissolved organic carbon in major Siberian Arctic rivers: Implications for wildfire-stream metabolic linkages|year = 2015|last1 = Myers-Pigg|first1 = Allison N.|last2 = Louchouarn|first2 = Patrick|last3 = Amon|first3 = Rainer M. W.|last4 = Prokushkin|first4 = Anatoly|last5 = Pierce|first5 = Kayce|last6 = Rubtsov|first6 = Alexey|journal = Geophysical Research Letters|volume = 42|issue = 2|pages = 377–385|bibcode = 2015GeoRL..42..377M|doi-access = free}}</ref>等陸地來源的大分子被分解成較小的成分和[[單體]],最終轉化為二氧化碳、代謝中間體或生物質。 |
|||
#湖泊、水庫和[[河漫灘|洪氾區]]通常儲存大量有機碳和沈積物,但在水體中也經歷淨[[異營生物|異營]]作用,導致進入大氣的二氧化碳淨通量大約比河流的少一個數量級。<ref name=Tranvik2009>{{cite journal |doi = 10.4319/lo.2009.54.6_part_2.2298|title = Lakes and reservoirs as regulators of carbon cycling and climate|year = 2009|last1 = Tranvik|first1 = Lars J.|last2 = Downing|first2 = John A.|last3 = Cotner|first3 = James B.|last4 = Loiselle|first4 = Steven A.|last5 = Striegl|first5 = Robert G.|last6 = Ballatore|first6 = Thomas J.|last7 = Dillon|first7 = Peter|last8 = Finlay|first8 = Kerri|last9 = Fortino|first9 = Kenneth|last10 = Knoll|first10 = Lesley B.|last11 = Kortelainen|first11 = Pirkko L.|last12 = Kutser|first12 = Tiit|last13 = Larsen|first13 = Soren.|last14 = Laurion|first14 = Isabelle|last15 = Leech|first15 = Dina M.|last16 = McCallister|first16 = S. Leigh|last17 = McKnight|first17 = Diane M.|last18 = Melack|first18 = John M.|last19 = Overholt|first19 = Erin|last20 = Porter|first20 = Jason A.|last21 = Prairie|first21 = Yves|last22 = Renwick|first22 = William H.|last23 = Roland|first23 = Fabio|last24 = Sherman|first24 = Bradford S.|last25 = Schindler|first25 = David W.|last26 = Sobek|first26 = Sebastian|last27 = Tremblay|first27 = Alain|last28 = Vanni|first28 = Michael J.|last29 = Verschoor|first29 = Antonie M.|last30 = von Wachenfeldt|first30 = Eddie|journal = Limnology and Oceanography|volume = 54|issue = 6part2|pages = 2298–2314|bibcode = 2009LimOc..54.2298T|display-authors = 29|doi-access = free}}</ref><ref name=Raymond2013 />洪氾區、湖泊和水庫中[[缺氧水體]]沉積物中的甲烷產量通常也很高。<ref name=Bastviken2004>{{cite journal |doi = 10.1029/2004GB002238|title = Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate|year = 2004|last1 = Bastviken|first1 = David|last2 = Cole|first2 = Jonathan|last3 = Pace|first3 = Michael|last4 = Tranvik|first4 = Lars|journal = Global Biogeochemical Cycles|volume = 18|issue = 4|pages = n/a|bibcode = 2004GBioC..18.4009B|doi-access = free}}</ref> |
|||
#由於[[河流作用]]會輸出營養,{{le|河流羽流|river plumer}}中的初級生產通常因而得到增強。<ref name=Cooley2007>{{cite journal |doi = 10.1029/2006GB002831|title = Seasonal variations in the Amazon plume-related atmospheric carbon sink|year = 2007|last1 = Cooley|first1 = S. R.|last2 = Coles|first2 = V. J.|last3 = Subramaniam|first3 = A.|last4 = Yager|first4 = P. L.|journal = Global Biogeochemical Cycles|volume = 21|issue = 3|pages = n/a|bibcode = 2007GBioC..21.3014C|doi-access = free}}</ref><ref name=Subramaniam2008>{{cite journal |doi = 10.1073/pnas.0710279105|title = Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean|year = 2008|last1 = Subramaniam|first1 = A.|last2 = Yager|first2 = P. L.|last3 = Carpenter|first3 = E. J.|last4 = Mahaffey|first4 = C.|last5 = Bjorkman|first5 = K.|last6 = Cooley|first6 = S.|last7 = Kustka|first7 = A. B.|last8 = Montoya|first8 = J. P.|last9 = Sanudo-Wilhelmy|first9 = S. A.|last10 = Shipe|first10 = R.|last11 = Capone|first11 = D. G.|journal = Proceedings of the National Academy of Sciences|volume = 105|issue = 30|pages = 10460–10465|pmid = 18647838|pmc = 2480616|s2cid = 8889134|doi-access = free}}</ref>導致河口水域成為全球大氣中二氧化碳的來源。<ref name=Cai2011>{{cite journal |doi = 10.1146/annurev-marine-120709-142723|title = Estuarine and Coastal Ocean Carbon Paradox: CO2Sinks or Sites of Terrestrial Carbon Incineration?|year = 2011|last1 = Cai|first1 = Wei-Jun|journal = Annual Review of Marine Science|volume = 3|pages = 123–145|pmid = 21329201|bibcode = 2011ARMS....3..123C}}</ref> |
|||
#{{le|潮汐沼澤|tidal marsh}}既儲存又輸出{{le|藍碳|blue carbon}}。<ref name=Odum1979>{{Cite book|url=https://books.google.com/books?id=SHbdBwAAQBAJ&q=%22Factors+controlling+the+flux+of+particulate+organic+carbon+from+estuarine+wetlands%22&pg=PA69|title = Ecological Processes in Coastal and Marine Systems|isbn = 9781461591467|last1 = Livingston|first1 = R. J.|date = 2012-12-06| publisher=Springer }}</ref><ref name=Dittmar2001>{{cite journal |doi = 10.1016/s0304-4203(00)00110-9|title = River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters|year = 2001|last1 = Dittmar|first1 = Thorsten|last2 = Lara|first2 = Rubén José|last3 = Kattner|first3 = Gerhard|journal = Marine Chemistry|volume = 73|issue = 3–4|pages = 253–271| bibcode=2001MarCh..73..253D }}</ref><ref name=Moore2011>{{cite journal |doi = 10.1016/j.gca.2011.08.037|title = Radium-based pore water fluxes of silica, alkalinity, manganese, DOC, and uranium: A decade of studies in the German Wadden Sea|year = 2011|last1 = Moore|first1 = W.S.|last2 = Beck|first2 = M.|last3 = Riedel|first3 = T.|last4 = Rutgers Van Der Loeff|first4 = M.|last5 = Dellwig|first5 = O.|last6 = Shaw|first6 = T.J.|last7 = Schnetger|first7 = B.|last8 = Brumsack|first8 = H.-J.|journal = Geochimica et Cosmochimica Acta|volume = 75|issue = 21|pages = 6535–6555|bibcode = 2011GeCoA..75.6535M}}</ref>估計全球[[草澤]]和[[濕地]]向大氣排放的二氧化碳通量與河流相當。<ref name=Wehrli2013>{{cite journal |doi = 10.1038/503346a|title = Conduits of the carbon cycle|year = 2013|last1 = Wehrli|first1 = Bernhard|journal = Nature|volume = 503|issue = 7476|pages = 346–347|pmid = 24256800|s2cid = 205079291|doi-access = free}}</ref> |
|||
#[[大陸棚]]和開放海域通常會吸收大氣中的二氧化碳。<ref name=Cai2011 /> |
|||
#海洋[[生物泵]]吸收一小部分的二氧化碳(但很重要的一部分),形成存於{{le|海洋沉積物|marine sediment}}中的有機碳(見下文)。<ref name=Moran2016>{{cite journal |doi = 10.1073/pnas.1514645113|title = Deciphering ocean carbon in a changing world|year = 2016|last1 = Moran|first1 = Mary Ann|author-link1=Mary Ann Moran|last2 = Kujawinski|first2 = Elizabeth B.|author-link2=Elizabeth Kujawinski|last3 = Stubbins|first3 = Aron|last4 = Fatland|first4 = Rob|last5 = Aluwihare|first5 = Lihini I.|last6 = Buchan|first6 = Alison|last7 = Crump|first7 = Byron C.|last8 = Dorrestein|first8 = Pieter C.|last9 = Dyhrman|first9 = Sonya T.|author-link9=Sonya Dyhrman|last10 = Hess|first10 = Nancy J.|last11 = Howe|first11 = Bill|last12 = Longnecker|first12 = Krista|last13 = Medeiros|first13 = Patricia M.|last14 = Niggemann|first14 = Jutta|last15 = Obernosterer|first15 = Ingrid|last16 = Repeta|first16 = Daniel J.|last17 = Waldbauer|first17 = Jacob R.|journal = Proceedings of the National Academy of Sciences|volume = 113|issue = 12|pages = 3143–3151|pmid = 26951682|pmc = 4812754|bibcode = 2016PNAS..113.3143M|s2cid = 10255391|doi-access = free}}</ref><ref name=Ward2017 /> |
|||
===流入海洋的地表逕流=== |
|||
[[File:Terrestrial carbon escaping from inland waters.jpg|thumb|upright=2| {{center|'''碳如何被河流攜帶進入海洋'''}} 河流將植物等有機碳與無機碳攜帶進入海洋,及河流系統產生的碳排放進入大氣後,經二氧化碳交換而進入海洋等綜合因素所導致。<ref name="Gao2022">{{cite journal |last1=Gao |first1=Yang |last2=Jia |first2=Junjie |last3=Lu |first3=Yao |last4=Sun |first4=Kun |last5=Wang |first5=Jing |last6=Wang |first6=Shuoyue |year=2022 |title=Carbon transportation, transformation, and sedimentation processes at the land-river-estuary continuum |journal=Fundamental Research |publisher=Elsevier BV |doi=10.1016/j.fmre.2022.07.007 |issn=2667-3258 |doi-access=free |s2cid=251168582}} [[File:CC-BY_icon.svg|50x50px]] Modified material was copied from this source, which is available under a [[creativecommons:by/4.0/|Creative Commons Attribution 4.0 International License]].</ref>]] |
|||
陸地和海洋生態系統主要透過河流運輸而連結,河流是陸地侵蝕性物質進入海洋系統的主要通道。陸地生物圈和岩石圈之間的物質和能量交換,以及有機碳固定與氧化過程,共同調節生態系統中的碳和氧氣庫。<ref name="Gao2022" /> |
|||
河流運輸是這些碳庫的主要連結通道,發揮將淨初級生產(主要為溶解有機碳(DOC)和{{le|顆粒有機物質|Particulate organic matter|顆粒有機碳}}(POC)的形式)從陸地系統運輸到海洋系統的作用。<ref>{{cite journal |last1=Schlünz |first1=B. |last2=Schneider |first2=R. R. |date=2000-03-22 |title=Transport of terrestrial organic carbon to the oceans by rivers: re-estimating flux- and burial rates |journal=International Journal of Earth Sciences |publisher=Springer Science and Business Media LLC |volume=88 |issue=4 |pages=599–606 |bibcode=2000IJEaS..88..599S |doi=10.1007/s005310050290 |issn=1437-3254 |s2cid=128411658}}</ref>在運輸過程中,部分DOC會透過[[氧化還原反應]]迅速返回大氣,導致陸地-大氣儲存層之間發生"碳脫氣"。<ref>{{cite journal |last1=Blair |first1=Neal E. |last2=Leithold |first2=Elana L. |last3=Aller |first3=Robert C. |year=2004 |title=From bedrock to burial: The evolution of particulate organic carbon across coupled watershed-continental margin systems |journal=Marine Chemistry |volume=92 |issue=1–4 |pages=141–156 |doi=10.1016/j.marchem.2004.06.023|bibcode=2004MarCh..92..141B }}</ref><ref>{{cite journal |last1=Bouchez |first1=Julien |last2=Beyssac |first2=Olivier |last3=Galy |first3=Valier |last4=Gaillardet |first4=Jérôme |last5=France-Lanord |first5=Christian |last6=Maurice |first6=Laurence |last7=Moreira-Turcq |first7=Patricia |year=2010 |title=Oxidation of petrogenic organic carbon in the Amazon floodplain as a source of atmospheric CO2 |journal=Geology |publisher=Geological Society of America |volume=38 |issue=3 |pages=255–258 |bibcode=2010Geo....38..255B |doi=10.1130/g30608.1 |issn=1943-2682 |s2cid=53512466}}</ref>剩餘的DOC和溶解的無機碳(DIC)被攜帶進入海洋。<ref>{{cite journal |last1=Regnier |first1=Pierre |last2=Friedlingstein |first2=Pierre |last3=Ciais |first3=Philippe |last4=Mackenzie |first4=Fred T. |last5=Gruber |first5=Nicolas |last6=Janssens |first6=Ivan A. |last7=Laruelle |first7=Goulven G. |last8=Lauerwald |first8=Ronny |last9=Luyssaert |first9=Sebastiaan |last10=Andersson |first10=Andreas J. |last11=Arndt |first11=Sandra |last12=Arnosti |first12=Carol |last13=Borges |first13=Alberto V. |last14=Dale |first14=Andrew W. |last15=Gallego-Sala |first15=Angela |display-authors=4 |date=2013-06-09 |title=Anthropogenic perturbation of the carbon fluxes from land to ocean |url=https://archimer.ifremer.fr/doc/00264/37508/36764.pdf |journal=Nature Geoscience |publisher=Springer Science and Business Media LLC |volume=6 |issue=8 |pages=597–607 |bibcode=2013NatGe...6..597R |doi=10.1038/ngeo1830 |issn=1752-0894 |last16=Goddéris |first16=Yves |last17=Goossens |first17=Nicolas |last18=Hartmann |first18=Jens |last19=Heinze |first19=Christoph |last20=Ilyina |first20=Tatiana |last21=Joos |first21=Fortunat |last22=LaRowe |first22=Douglas E. |last23=Leifeld |first23=Jens |last24=Meysman |first24=Filip J. R. |last25=Munhoven |first25=Guy |last26=Raymond |first26=Peter A. |last27=Spahni |first27=Renato |last28=Suntharalingam |first28=Parvadha |last29=Thullner |first29=Martin |s2cid=53418968}}</ref><ref name="Bauer2013">{{cite journal |last1=Bauer |first1=James E. |last2=Cai |first2=Wei-Jun |last3=Raymond |first3=Peter A. |last4=Bianchi |first4=Thomas S. |last5=Hopkinson |first5=Charles S. |last6=Regnier |first6=Pierre A. G. |date=2013-12-04 |title=The changing carbon cycle of the coastal ocean |url=https://www.openaccessrepository.it/record/22208 |journal=Nature |publisher=Springer Science and Business Media LLC |volume=504 |issue=7478 |pages=61–70 |bibcode=2013Natur.504...61B |doi=10.1038/nature12857 |issn=0028-0836 |pmid=24305149 |s2cid=4399374}}</ref><ref>{{cite journal |last=Cai |first=Wei-Jun |date=2011-01-15 |title=Estuarine and Coastal Ocean Carbon Paradox: CO2 Sinks or Sites of Terrestrial Carbon Incineration? |journal=Annual Review of Marine Science |publisher=Annual Reviews |volume=3 |issue=1 |pages=123–145 |bibcode=2011ARMS....3..123C |doi=10.1146/annurev-marine-120709-142723 |issn=1941-1405 |pmid=21329201}}</ref>估計於2015年,全球河流的無機和有機碳輸出通量分別為0.50-0.70十億噸(Pg)碳/年和0.15-0.35十億噸碳/年。<ref name="Bauer2013" />另一方面,POC可埋藏在沉積物中很長時間,全球每年由陸地輸送到海洋的POC通量估計為0.20千噸(Gg)/年。<ref>{{cite journal |last1=Galy |first1=Valier |last2=Peucker-Ehrenbrink |first2=Bernhard |last3=Eglinton |first3=Timothy |year=2015 |title=Global carbon export from the terrestrial biosphere controlled by erosion |journal=Nature |publisher=Springer Science and Business Media LLC |volume=521 |issue=7551 |pages=204–207 |bibcode=2015Natur.521..204G |doi=10.1038/nature14400 |issn=0028-0836 |pmid=25971513 |s2cid=205243485}}</ref><ref name="Gao2022" /> |
|||
===海洋生物泵=== |
|||
[[File:Oceanic Food Web.jpg|thumb|upright=2| {{center|發生於開闊海洋中的碳循環}}]] |
|||
{{main|生物泵}} |
|||
海洋生物泵是由海洋生物從大氣和陸地徑流捕集碳,然後移往深海內部和海底沉積物的過程。<ref name="Sigman DM 2006. pp. 491-528">Sigman DM & GH Haug. 2006. The biological pump in the past. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 491–528</ref>生物泵並非一種單一過程,而是多個過程的總和,每個過程都會影響生物泵的結果。此種泵作用每年將約110億噸碳轉移到海洋內部。如果海洋中無生物泵作用,會導致大氣中的二氧化碳濃度比現在高出約400ppm。<ref>{{cite journal |doi = 10.1016/j.pocean.2014.05.005|title = The Biological Carbon Pump in the North Atlantic|year = 2014|last1 = Sanders|first1 = Richard|last2 = Henson|first2 = Stephanie A.|last3 = Koski|first3 = Marja|last4 = de la Rocha|first4 = Christina L.|last5 = Painter|first5 = Stuart C.|last6 = Poulton|first6 = Alex J.|last7 = Riley|first7 = Jennifer|last8 = Salihoglu|first8 = Baris|last9 = Visser|first9 = Andre|last10 = Yool|first10 = Andrew|last11 = Bellerby|first11 = Richard|last12 = Martin|first12 = Adrian P.|journal = Progress in Oceanography|volume = 129|pages = 200–218|bibcode = 2014PrOce.129..200S}}</ref><ref>{{cite journal |doi = 10.3389/fmars.2015.00077|title = Toward quantifying the response of the oceans' biological pump to climate change|year = 2015|last1 = Boyd|first1 = Philip W.|journal = Frontiers in Marine Science|volume = 2|s2cid = 16787695|doi-access = free}}</ref><ref name=Basu2018>{{cite journal |doi = 10.3390/su10030869|title = Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate|journal = Sustainability|year = 2018|volume = 10|issue = 3|page = 869|doi-access = free|last1 = Basu|first1 = Samarpita|last2 = MacKey|first2 = Katherine}}</ref> |
|||
有機和無機材料中的大部分碳是在海面形成,然後開始沉入海底。當這些物質由較高的{{le|海水水柱|Water column}}以[[海雪]]的形式下沉時,深海由此獲取大部分營養。這些物質由死亡或垂死的動物和微生物、動物[[糞便]]、沙粒和其他無機材料組成。<ref name=Steinberg2002>{{cite journal | last1=Steinberg | first1= Deborah |first2=Sarah|last2=Goldthwait |first3=Dennis|last3=Hansell |year=2002 | title=Zooplankton vertical migration and the active transport of dissolved organic and inorganic nitrogen in the Sargasso Sea| journal=Deep-Sea Research Part I| volume=49 | pages=1445–1461 | issn=0967-0637 | doi=10.1016/S0967-0637(02)00037-7 | issue=8 | citeseerx= 10.1.1.391.7622 | bibcode= 2002DSRI...49.1445S }}</ref> |
|||
生物泵將溶解的無機碳(DIC)轉化為有機生物質,並將其以顆粒或溶解形式泵入深海。浮游植物利用光合作用將無機養分和二氧化碳固定,同時釋放溶解有機物 (DOM),而被草食性[[浮游動物]]攝取。較大的浮游動物,例如[[橈足綱]]動物,排出糞便顆粒,可被重新攝入,或與其他有機碎屑一起下沉或聚集成更大、下沉更快的聚集體。 DOM部分被細菌消耗並釋放氣體,剩餘的難分解部分被海水攜帶,混入深海中。被攜帶到深海中的DOM和聚集體被消耗和釋放氣體,將有機碳返回到巨大的深海DIC庫中。<ref name=Ducklow2001 /> |
|||
單一[[浮游植物]]細胞的下沉速度約為每天一公尺。鑑於海洋的平均深度約為四公里,這些細胞可能需要十年以上才能到達海底。然而透過捕食者糞便顆粒的凝固和排出等過程,而形成聚集體,其下沉速度比單一細胞大幾個數量級,並在幾天內抵達深處。<ref name=Rocha2006>De La Rocha C.L. (2006) "The Biological Pump". In: ''Treatise on Geochemistry''; vol. 6, Pergamon Press, pp. 83–111.</ref> |
|||
由海洋表面下降的顆粒中大約有1%會到達海底並被消耗、釋放氣體或埋在沉積物中。這些過程最終是從表面去除有機形式的碳,並將其返回到更深的DIC,維持一個由海洋表面到深海的DIC梯度。溫鹽環流以千年的時間尺度將深海DIC送返大氣。埋藏在沉積物中的碳可隱沒進入地函並儲存數百萬年,形成一種緩慢碳循環中的形式(見下一節)。<ref name=Ducklow2001>Ducklow, H.W., Steinberg, D.K. and Buesseler, K.O. (2001) "Upper Ocean Carbon Export and the Biological Pump". ''Oceanography'', '''14'''(4): 50–58. {{doi|10.5670/oceanog.2001.06}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date= 2017-10-16 }}.</ref> |
|||
==慢速碳循環內的子過程== |
|||
[[File:Flux_of_crustal_material_in_the_mantle.jpg|thumb|upright=1.8| {{center|海底地球板塊移動,將碳移入地函中}}]] |
|||
{{main|{{le|深層碳循環|deep carbon cycle}}}} |
|||
慢速或深層碳循環為一重要過程,但它不像經由大氣、陸地生物圈、海洋和地質圈的相對快速碳循環那樣被充分理解。<ref name=Wong2019>{{cite journal |doi = 10.3389/feart.2019.00263|title = Deep Carbon Cycling over the Past 200 Million Years: A Review of Fluxes in Different Tectonic Settings|year = 2019|last1 = Wong|first1 = Kevin|last2 = Mason|first2 = Emily|last3 = Brune|first3 = Sascha|last4 = East|first4 = Madison|last5 = Edmonds|first5 = Marie|last6 = Zahirovic|first6 = Sabin|journal = Frontiers in Earth Science|volume = 7|page = 263|bibcode = 2019FrEaS...7..263W|s2cid = 204027259|doi-access = free}}</ref>深層碳循環與地球表面和大氣中的碳循環密切相關。如果沒此過程,碳將保留在大氣中,並在很長一段時間內累積到極高的濃度。<ref>{{Cite web|url=https://deepcarbon.net/feature/deep-carbon-cycle-and-our-habitable-planet|title=The Deep Carbon Cycle and our Habitable Planet {{!}} Deep Carbon Observatory|website=deepcarbon.net|access-date=2019-02-19|archive-date=2020-07-27|archive-url=https://web.archive.org/web/20200727084309/https://deepcarbon.net/feature/deep-carbon-cycle-and-our-habitable-planet|url-status=dead}}</ref>深層碳循環讓碳返回地球的作用,在維持陸地適合生命存在所需的條件方面具有有關鍵作用。 |
|||
此外,因為地球於此作用中可儲存大量的碳,過程本身就很重要。研究[[玄武岩]][[岩漿]]的成分並測量火山中的二氧化碳通量顯示地函中的碳含量實際上比地球表面的多一千倍。<ref name=":02">{{cite journal|last1=Wilson|first1=Mark|year=2003|title=Where do Carbon Atoms Reside within Earth's Mantle?|journal=Physics Today|volume=56|issue=10|pages=21–22|bibcode=2003PhT....56j..21W|doi=10.1063/1.1628990}}</ref>鑽探和物理觀測地球深部碳過程顯然極為困難,因為下地函和地核的深度分別為660公里至2,891公里和2,891公里至6,371公里。而導致人們對碳在地球深處的作用尚無太多確切的了解。雖然如此,一些證據(其中許多由模擬地球深處條件而取得)已顯示元素向下移動到下地函的機制,以及碳在該層的極端溫度和壓力下所具有的形式。此外,透過[[地震學]]等技術已讓我們對地核中碳的或有存在有更深入了解。 |
|||
===下地函中的碳=== |
|||
[[File:Carbon_Outgassing_(Dasgupta_2011).png|thumb|upright=1.8| {{center|可產生碳[[釋氣|釋放]](二氧化碳排放)的幾個途徑<ref>{{Cite web|url=http://www.deep-earth.org/postAGU2011/Dasgupta-cider-agu2011.ppt|title=From Magma Ocean to Crustal Recycling: Earth's Deep Carbon Cycle|last=Dasgupta|first=Rajdeep|date=2011-12-10|access-date= 2019-03-09|archive-url=https://web.archive.org/web/20160424031155/http://www.deep-earth.org/postAGU2011/Dasgupta-cider-agu2011.ppt|archive-date=2016-04-24|url-status=dead}}</ref>}}]] |
|||
碳主要是透過富含碳酸鹽沉積物,經由海洋底層的板塊邊緣,在持續隱沒過程中被拉入地函。我們目前對地函中的碳循環知之甚少,尤其是在地球深處,但許多研究可增強我們對其中元素運動和形式的理解。例如於2011年所做的一項研究,顯示碳循環一直延伸到下地函。該研究對[[巴西]][[茹伊納]]超深處挖掘而得的稀有鑽石進行研究,確定其中一些鑽石內含物整體成分與較低地函內溫度和壓力下玄武岩熔化和結晶的估計結果相匹配。<ref>{{Cite web|url=https://www.sciencedaily.com/releases/2011/09/110915141227.htm|title=Carbon cycle reaches Earth's lower mantle: Evidence of carbon cycle found in 'superdeep' diamonds From Brazil|website=ScienceDaily|access-date=2019-02-06}}</ref>調查結果表明海洋[[岩石圈]]玄武岩是將碳向地球深處傳輸的主要機制。這些隱沒的碳酸鹽可與下地函中矽酸鹽相互作用,最終形成像挖掘而得的這批超深鑽石。<ref>{{Cite journal|last1=Stagno|first1=V.|last2=Frost|first2=D. J.|last3=McCammon|first3=C. A.|author-link3=Catherine McCammon|last4=Mohseni|first4=H.|last5=Fei|first5=Y.|date=2015-02-05|title=The oxygen fugacity at which graphite or diamond forms from carbonate-bearing melts in eclogitic rocks|journal=Contributions to Mineralogy and Petrology|volume=169|issue=2|pages=16|bibcode=2015CoMP..169...16S|doi=10.1007/s00410-015-1111-1|issn=1432-0967|s2cid=129243867}}</ref> |
|||
但進入下地函的碳酸鹽除會形成鑽石之外,還會形成其他的物質。有項於2011年進行的實驗,將碳酸鹽岩置於與下地函類似的深達1,800公里處,而形成[[菱鎂礦]]、[[菱鐵礦]]和多種[[石墨]]。<ref name="Fiquet 5184–5187">{{Cite journal|last1=Fiquet|first1=Guillaume|last2=Guyot|first2=François|last3=Perrillat|first3=Jean-Philippe|last4=Auzende|first4=Anne-Line|last5=Antonangeli|first5=Daniele|last6=Corgne|first6=Alexandre|last7=Gloter|first7=Alexandre|last8=Boulard|first8=Eglantine|date=2011-03-29|title=New host for carbon in the deep Earth|journal=Proceedings of the National Academy of Sciences|volume=108|issue=13|pages=5184–5187|doi=10.1073/pnas.1016934108|issn=0027-8424|pmid=21402927|pmc=3069163|bibcode=2011PNAS..108.5184B|doi-access=free}}</ref>其他實驗以及岩石學觀察也支持此一說法,顯示菱鎂礦實際上是地函中大部分地區中最穩定的碳酸鹽相,主要是由於其可忍受較高的熔化溫度。<ref>{{Cite journal|last1=Dorfman|first1=Susannah M.|last2=Badro|first2=James|last3=Nabiei|first3=Farhang|last4=Prakapenka|first4=Vitali B.|last5=Cantoni|first5=Marco|last6=Gillet|first6=Philippe|date=2018-05-01|title=Carbonate stability in the reduced lower mantle|journal=Earth and Planetary Science Letters|volume=489|pages=84–91|doi=10.1016/j.epsl.2018.02.035|issn=0012-821X|bibcode=2018E&PSL.489...84D|osti=1426861|s2cid=134119145 |url=https://hal.archives-ouvertes.fr/hal-02363561/file/document-1.pdf |archive-url=https://web.archive.org/web/20210718171229/https://hal.archives-ouvertes.fr/hal-02363561/file/document-1.pdf |archive-date=2021-07-18 |url-status=live}}</ref>科學家們得到的結論是碳酸鹽在下降到地函時會發生氧化還原反應,然後在低氧[[逸度]]環境下在深處穩定下來。鎂、鐵和其他金屬化合物在整個過程中充當緩衝劑。<ref>{{Cite journal|last1=Kelley|first1=Katherine A.|last2=Cottrell|first2=Elizabeth|date=2013-06-14|title=Redox Heterogeneity in Mid-Ocean Ridge Basalts as a Function of Mantle Source|journal=Science|volume=340|issue=6138|pages=1314–1317|doi=10.1126/science.1233299|issn=0036-8075|pmid=23641060|bibcode=2013Sci...340.1314C|s2cid=39125834|doi-access=free}}</ref>如碳(如石墨)的還原元素形式,其存在顯示碳化合物在進入地函時被還原。 |
|||
[[File:Carbon tetrahedral oxygen.png|thumb|upright=0.8|left|碳原子與氧原子結合成為[[四面體形分子構型]]。]] |
|||
[[同質異形體]]現象改變碳酸鹽化合物在地球不同深度的穩定性。為說明這一點,實驗室模擬和[[密度泛函理論]]計算顯示[[四面體形分子構型]]碳酸鹽在接近[[核幔邊界|核函邊界]]的深度最為穩定。<ref>{{Cite book|url=https://www.sciencedirect.com/book/9780128113011/magmas-under-pressure|title=Magmas Under Pressure | ScienceDirect|isbn=9780128113011 |access-date=2019-02-07 |last1=Kono |first1=Yoshio |last2=Sanloup |first2=Chrystèle |date= 2018-04-10 |publisher=Elsevier Science }}</ref><ref name="Fiquet 5184–5187"/>於2015年所做的一項研究顯示下地函的高壓導致碳鍵從sp2[[混成軌域]]轉變為sp3混成軌域,導致碳與氧以四面體形分子構型鍵合。<ref>{{Cite journal|last1=Mao|first1=Wendy L.|author-link=Wendy Mao|last2=Liu|first2=Zhenxian|last3=Galli|first3=Giulia|last4=Pan|first4=Ding|last5=Boulard|first5=Eglantine|date=2015-02-18|title=Tetrahedrally coordinated carbonates in Earth's lower mantle|journal=Nature Communications|volume=6|pages=6311|arxiv=1503.03538|bibcode=2015NatCo...6.6311B|doi=10.1038/ncomms7311|issn=2041-1723|pmid=25692448|s2cid=205335268}}</ref>CO<sub>3</sub>三角形團(也稱三角形碳酸鹽團)不能形成可聚合網絡,而四面體CO<sub></sub>可以,表示碳[[配位數]]增加,而下地函中碳酸鹽化合物的性質也因而發生巨大變化。例如,初步理論研究顯示高壓會導致碳酸鹽熔體黏度增加,之後熔體的流動性較低,導致大量碳沉積到地函深處。<ref>{{Cite journal|last1=Carmody|first1=Laura|last2=Genge|first2=Matthew|last3=Jones|first3=Adrian P.|s2cid=49365059|date=2013-01-01|title=Carbonate Melts and Carbonatites|journal=Reviews in Mineralogy and Geochemistry|volume=75|issue=1|pages=289–322|doi=10.2138/rmg.2013.75.10|issn=1529-6466|bibcode=2013RvMG...75..289J}}</ref> |
|||
碳因而可在下地函中保留很長一段時間,但大量的碳經常會返回岩石圈。這個過程被稱為碳[[釋氣]],是碳化地函經歷減壓熔融以及地函羽流攜帶碳化合物向上到達地殼的結果。<ref>{{Cite journal|last1=Dasgupta|first1=Rajdeep|author-link2=Marc M. Hirschmann|last2=Hirschmann|first2=Marc M.|date=2010-09-15|title=The deep carbon cycle and melting in Earth's interior|journal=Earth and Planetary Science Letters|volume=298|issue=1|pages=1–13|doi=10.1016/j.epsl.2010.06.039|issn=0012-821X|bibcode=2010E&PSL.298....1D}}</ref>碳在上升到火山熱點時受到氧化,然後以二氧化碳的形式釋放。發生這種情况是為讓碳原子的氧化態與在這些地區噴發的玄武岩的氧化態相匹配。<ref>{{Cite journal|doi=10.1146/annurev.earth.36.031207.124322|title=The Redox State of Earth's Mantle|journal=Annual Review of Earth and Planetary Sciences|volume=36|pages=389–420|year=2008|last1=Frost|first1=Daniel J.|last2=McCammon|first2=Catherine A.|bibcode=2008AREPS..36..389F}}</ref> |
|||
[[File:Speeds_of_seismic_waves.svg|thumb|透過分析[[s波]]通過的速度可推斷地核中可能有碳的存在。]] |
|||
===地核中的碳=== |
|||
雖然地核中碳的存在受到很大的限制,但最近的研究顯示該區域可儲存大量的碳。透過[[s波]]地震測試,s波通過的速度僅為通過多數富鐵合金所需的一半。<ref>{{Cite web|url=https://deepcarbon.net/feature/does-earths-core-host-deep-carbon-reservoir|title=Does Earth's Core Host a Deep Carbon Reservoir? {{!}} Deep Carbon Observatory|website=deepcarbon.net|access-date=2019-03-09|archive-date=27 July 2020|archive-url=https://web.archive.org/web/20200727092747/https://deepcarbon.net/feature/does-earths-core-host-deep-carbon-reservoir|url-status=dead}}</ref>由於核心的成分以往被認為是結晶鐵和少量鎳的合金,因此這種地震結果異常現象顯示地核中存在包括碳在內的輕元素。事實上,使用[[金剛石壓砧]]複製地核高壓條件的研究顯示[[雪明碳鐵]] (Fe7C3) 與內核的波速和密度相符。因此這種實驗模型可作為地核含有多達地球碳67%的證據。<ref>{{Cite journal|last1=Li|first1=Jie|last2=Chow|first2=Paul|last3=Xiao|first3=Yuming|last4=Alp|first4=E. Ercan|last5=Bi|first5=Wenli|last6=Zhao|first6=Jiyong|last7=Hu|first7=Michael Y.|last8=Liu|first8=Jiachao|last9=Zhang|first9=Dongzhou|date=2014-12-16|title=Hidden carbon in Earth's inner core revealed by shear softening in dense Fe<sub>7</sub>C<sub>3</sub>|journal=Proceedings of the National Academy of Sciences|volume=111|issue=50|pages=17755–17758|doi=10.1073/pnas.1411154111|issn=0027-8424|pmid=25453077|pmc=4273394|bibcode=2014PNAS..11117755C|doi-access=free}}</ref>而在另一項研究中發現在地球內核的壓力和溫度條件下,有碳溶解在鐵中,並形成具有與Fe7C3相同成分的穩定相,但結構與前面提到的不同。<ref>{{Cite journal|last1=Li|first1=Jie|last2=Chow|first2=Paul|last3=Xiao|first3=Yuming|last4=Alp|first4=E. Ercan|last5=Bi|first5=Wenli|last6=Zhao|first6=Jiyong|last7=Hu|first7=Michael Y.|last8=Liu|first8=Jiachao|last9=Zhang|first9=Dongzhou|date=2014-12-16|title=Hidden carbon in Earth's inner core revealed by shear softening in dense Fe<sub>7</sub>C<sub>3</sub>|journal=Proceedings of the National Academy of Sciences|volume=111|issue=50|pages=17755–17758|doi=10.1073/pnas.1411154111|issn=0027-8424|pmid=25453077|pmc=4273394|bibcode=2014PNAS..11117755C|doi-access=free}}</ref>總之,雖然地核中潛在儲存的碳量尚不清楚,但最近的研究顯示雪明碳鐵的存在可解釋一些地球物理觀測結果。 |
|||
==人類對快速碳循環的影響== |
|||
{{multiple image |
|||
| align = right |
|||
| direction = horizontal |
|||
| header = 二氧化碳排放與分類 |
|||
| header_align = |
|||
| header_background = |
|||
| footer = |
|||
| footer_align = |
|||
| footer_background = |
|||
| background color = |
|||
| width1 = 282 |
|||
| image1 = CO2 Emissions by Source Since 1880.svg |
|||
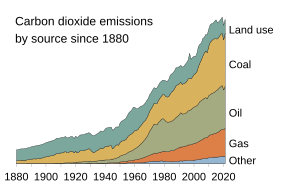
| caption1 = 各種二氧化碳來源競相增加排放(資料來源:{{le|全球碳計畫|Global Carbon Project}}) |
|||
| width2 = 257 |
|||
| image2 = Carbon Dioxide Partitioning.svg |
|||
| caption2 = 大部分的二氧化碳排放由不同的碳庫 - 植物、土壤與海洋所捕集(資料來源:全球碳計畫從事的全球碳預算研究活動(Global Carbon Budget)) |
|||
}} |
|||
[[File:Anthropogenic changes in the global carbon cycle.png|thumb|upright=1.7|right|圖表,顯示人類活動於2010年至2019年期間造成的平均碳排放,對於全球碳循環造成的擾動。]] |
|||
自第一次工業革命以來,特別是在[[第二次世界大戰]]結束後,人類活動將地質圈中大量的碳重新分配,嚴重擾亂全球碳循環。<ref name="nasacc" />人類也因改變植被和其他土地利用,不斷改變陸地生物圈的自然功能。<ref name=GlobalCarbonCycle/>並透過涉及與大量製造人造(合成)碳化合物,然後以[[污染物]]的形式在空氣、水和沈積物中存在,持續數十年至數千年。<ref>{{cite web |url=https://www.epa.gov/ghgemissions/overview-greenhouse-gases |title=Overview of greenhouse gases |date=2015-12-23 |publisher=U.S. Environmental Protection Agency |access-date=2020-11-02}}</ref><ref name="plaspol">{{cite news |title=The known unknowns of plastic pollution |url=https://www.economist.com/news/international/21737498-so-far-it-seems-less-bad-other-kinds-pollution-about-which-less-fuss-made |access-date= 2018-06-17 |newspaper=The Economist |date= 2018-03-03}}</ref>由於各種正[[回饋]]和負回饋,造成的氣候變化正加劇並迫使人類進一步間接改變碳循環。<ref>{{cite web |url=https://www.epa.gov/ghgemissions/overview-greenhouse-gases |title=Overview of greenhouse gases |date= 2015-12-23 |publisher=U.S. Environmental Protection Agency |access-date=2020-11-02}}</ref><ref name="plaspol"/> |
|||
===氣候變化=== |
|||
{{main|氣候變化反饋|氣候變化對海洋的影響}} |
|||
[[File:Climate–carbon cycle feedbacks and state variables.png|thumb|upright=2|right| {{center|'''氣候–碳循環回饋與狀態變量<br />簡式圖形表達'''}} <ref name="Donges2018">{{cite journal |last1=Lade |first1=Steven J. |last2=Donges |first2=Jonathan F. |last3=Fetzer |first3=Ingo |last4=Anderies |first4=John M. |last5=Beer |first5=Christian |last6=Cornell |first6=Sarah E. |last7=Gasser |first7=Thomas |last8=Norberg |first8=Jon |last9=Richardson |first9=Katherine |last10=Rockström |first10=Johan |last11=Steffen |first11=Will |year=2018 |title=Analytically tractable climate–carbon cycle feedbacks under 21st century anthropogenic forcing |journal=Earth System Dynamics |volume=9 |issue=2 |pages=507–523 |bibcode=2018ESD.....9..507L |doi=10.5194/esd-9-507-2018 |doi-access=free}} [[File:CC-BY_icon.svg|50x50px]] Material was copied from this source, which is available under a [[creativecommons:by/4.0/|Creative Commons Attribution 4.0 International License]] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/|date=2017-10-16}}.</ref>]] |
|||
當前的氣候變化導致海洋溫度與酸度升高,將海洋生態系統改變。<ref>{{cite journal |last1=Takahashi |first1=Taro |last2=Sutherland |first2=Stewart C. |last3=Sweeney |first3=Colm |last4=Poisson |first4=Alain |last5=Metzl |first5=Nicolas |last6=Tilbrook |first6=Bronte |last7=Bates |first7=Nicolas |last8=Wanninkhof |first8=Rik |last9=Feely |first9=Richard A. |last10=Sabine |first10=Christopher |last11=Olafsson |first11=Jon |last12=Nojiri |first12=Yukihiro |year=2002 |title=Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects |journal=Deep Sea Research Part II: Topical Studies in Oceanography |volume=49 |issue=9–10 |pages=1601–1622 |bibcode=2002DSRII..49.1601T |doi=10.1016/S0967-0645(02)00003-6}}</ref>此外,[[酸雨]]和農業和工業所產生的污染,經過徑流攜帶入海,也將海洋的化學成分改變。這種變化會對[[珊瑚礁]]等具高敏感度的生態系統產生巨大影響,<ref>{{cite journal |last1=Orr |first1=James C. |last2=Fabry |first2=Victoria J. |last3=Aumont |first3=Olivier |last4=Bopp |first4=Laurent |last5=Doney |first5=Scott C. |author-link5=Scott Doney |last6=Feely |first6=Richard A. |last7=Gnanadesikan |first7=Anand |last8=Gruber |first8=Nicolas |last9=Ishida |first9=Akio |last10=Joos |first10=Fortunat |last11=Key |first11=Robert M. |last12=Lindsay |first12=Keith |last13=Maier-Reimer |first13=Ernst |last14=Matear |first14=Richard |last15=Monfray |first15=Patrick |year=2005 |title=Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms |url=https://epic.awi.de/id/eprint/13479/1/Orr2005a.pdf |url-status=live |journal=Nature |volume=437 |issue=7059 |pages=681–686 |bibcode=2005Natur.437..681O |doi=10.1038/nature04095 |pmid=16193043 |s2cid=4306199 |archive-url=https://web.archive.org/web/20190426081745/https://epic.awi.de/id/eprint/13479/1/Orr2005a.pdf |archive-date=2019-04-26 |last16=Mouchet |first16=Anne |last17=Najjar |first17=Raymond G. |last18=Plattner |first18=Gian-Kasper |last19=Rodgers |first19=Keith B. |last20=Sabine |first20=Christopher L. |last21=Sarmiento |first21=Jorge L. |last22=Schlitzer |first22=Reiner |last23=Slater |first23=Richard D. |last24=Totterdell |first24=Ian J. |last25=Weirig |first25=Marie-France |last26=Yamanaka |first26=Yasuhiro |last27=Yool |first27=Andrew}}</ref>而限制海洋在區域範圍內由大氣吸收碳的能力,並減少全球海洋[[生物多樣性]]。 |
|||
大氣與地球系統其他組成部分之間的碳交換,統稱為碳循環,人為碳排放對氣候變化的影響構成重要的負(抑制)回饋。目前陸地和海洋每年各可捕集人為碳排放量的四分之一。<ref>{{cite journal |last1=Le Quéré |first1=Corinne |last2=Andrew |first2=Robbie M. |last3=Canadell |first3=Josep G. |last4=Sitch |first4=Stephen |last5=Korsbakken |first5=Jan Ivar |last6=Peters |first6=Glen P. |last7=Manning |first7=Andrew C. |last8=Boden |first8=Thomas A. |last9=Tans |first9=Pieter P. |last10=Houghton |first10=Richard A. |last11=Keeling |first11=Ralph F. |last12=Alin |first12=Simone |last13=Andrews |first13=Oliver D. |last14=Anthoni |first14=Peter |last15=Barbero |first15=Leticia |display-authors=29 |year=2016 |title=Global Carbon Budget 2016 |journal=Earth System Science Data |volume=8 |issue=2 |pages=605–649 |bibcode=2016ESSD....8..605L |doi=10.5194/essd-8-605-2016 |doi-access=free |last16=Bopp |first16=Laurent |last17=Chevallier |first17=Frédéric |last18=Chini |first18=Louise P. |last19=Ciais |first19=Philippe |last20=Currie |first20=Kim |last21=Delire |first21=Christine |last22=Doney |first22=Scott C. |last23=Friedlingstein |first23=Pierre |last24=Gkritzalis |first24=Thanos |last25=Harris |first25=Ian |last26=Hauck |first26=Judith |last27=Haverd |first27=Vanessa |last28=Hoppema |first28=Mario |last29=Klein Goldewijk |first29=Kees |last30=Jain |first30=Atul K.}}</ref><ref name="Donges2018" /> |
|||
預計這些回饋能力在未來會減弱,而放大人為碳排放對氣候變化的影響。<ref>{{cite book |url=https://research-information.bris.ac.uk/en/publications/8d2134c0-3c0e-4e9b-b4d0-c52b6b8f2dfa |title=Climate Change 2013 - the Physical Science Basis |year=2014 |isbn=9781107415324 |editor1-last=Intergovernmental Panel On Climate Change |pages=465–570 |chapter=Carbon and Other Biogeochemical Cycles |publisher=Cambridge University Press |doi=10.1017/CBO9781107415324.015 |hdl=11858/00-001M-0000-0023-E34E-5}}</ref>但回饋能力減弱的程度是高度不確定,即使在相同的大氣濃度或排放情況下,地球系統模型所預測的陸地和海洋的碳吸收有很大的差異。<ref>{{cite journal |last1=Joos |first1=F. |last2=Roth |first2=R. |last3=Fuglestvedt |first3=J. S. |last4=Peters |first4=G. P. |last5=Enting |first5=I. G. |last6=von Bloh |first6=W. |last7=Brovkin |first7=V. |last8=Burke |first8=E. J. |last9=Eby |first9=M. |last10=Edwards |first10=N. R. |last11=Friedrich |first11=T. |last12=Frölicher |first12=T. L. |last13=Halloran |first13=P. R. |last14=Holden |first14=P. B. |last15=Jones |first15=C. |year=2013 |title=Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis |journal=Atmospheric Chemistry and Physics |volume=13 |issue=5 |pages=2793–2825 |bibcode=2013ACP....13.2793J |doi=10.5194/acp-13-2793-2013 |doi-access=free |last16=Kleinen |first16=T. |last17=MacKenzie |first17=F. T. |last18=Matsumoto |first18=K. |last19=Meinshausen |first19=M. |last20=Plattner |first20=G.-K. |last21=Reisinger |first21=A. |last22=Segschneider |first22=J. |last23=Shaffer |first23=G. |last24=Steinacher |first24=M. |last25=Strassmann |first25=K. |last26=Tanaka |first26=K. |last27=Timmermann |first27=A. |last28=Weaver |first28=A. J.}}</ref><ref name="Donges2018" /><ref>{{Cite web |last1=Hausfather |first1=Zeke |last2=Betts |first2=Richard |date=2020-04-14 |title=Analysis: How 'carbon-cycle feedbacks' could make global warming worse |url=https://www.carbonbrief.org/analysis-how-carbon-cycle-feedbacks-could-make-global-warming-worse |url-status=live |archive-url=https://web.archive.org/web/20200416095707/https://www.carbonbrief.org/analysis-how-carbon-cycle-feedbacks-could-make-global-warming-worse |archive-date= 2020-04-16 |access-date=2022-01-04 |website=Carbon Brief |language=en}}</ref>人為氣候變化間接引起的北極甲烷排放也會影響碳循環並導致進一步暖化。 |
|||
====化石碳開採與燃燒==== |
|||
{{see also|煤炭開採|{{le|石油開採|Extraction of petroleum}}}} |
|||
[[File:Anthropogenic carbon flows 1850-2018.png|thumb|upright=1.2|right|於1850年至2018年期間,人為產生的二氧化碳,與各碳庫捕集數量(吉噸)累計(圖左部分),於2009年至2018年期間全球平均每年平均排放量(圖右部分)。斜虛線部分為不確定值,紅色部分為根據資料加總後失衡的部分。最大的排放源為開採與燃燒石化燃料,其次為土地利用改變。 <ref name="gcb19">Friedlingstein, P., Jones, M., O'Sullivan, M., Andrew, R., Hauck, J., Peters, G., Peters, W., Pongratz, J., Sitch, S., Le Quéré, C. and 66 others (2019) "Global carbon budget 2019". ''Earth System Science Data'', '''11'''(4): 1783–1838. {{doi|10.5194/essd-11-1783-2019}}. [[File:CC-BY icon.svg|50px]] Material was copied from this source, which is available under a [https://creativecommons.org/licenses/by/4.0/ Creative Commons Attribution 4.0 International License] {{Webarchive|url=https://web.archive.org/web/20171016050101/https://creativecommons.org/licenses/by/4.0/ |date= 2017-10-16 }}.</ref>]] |
|||
人類對碳循環和生物圈最大且成長最快的影響之一是開採與燃燒化石燃料,直接將碳從地質圈轉移到大氣中。在生產水泥的[[前體]] - {{le|熟料|Cement clinker }}時需煅燒石灰石,過程中也會釋放大量二氧化碳。<ref>IPCC (2007) [https://www.ipcc.ch/publications_and_data/ar4/wg3/en/ch7s7-4-5.html 7.4.5 Minerals] {{Webarchive|url=https://web.archive.org/web/20160525042327/http://www.ipcc.ch/publications_and_data/ar4/wg3/en/ch7s7-4-5.html|date= 2016-05-25}} in ''Climate Change 2007'': Working Group III: Mitigation of Climate Change,</ref> |
|||
截至2020年,全球總共已開採約450億噸化石碳,接近地球上所有陸地生物量中所含碳的數量。<ref name="gcb19" />全球最近直接排放到大氣中的碳數量已超過植被和海洋的吸收能力。<ref name="NASA-20151112-ab">{{cite web |last1=Buis |first1=Alan |last2=Ramsayer |first2=Kate |last3=Rasmussen |first3=Carol |date= 2015-11-12 |title=A Breathing Planet, Off Balance |url=http://www.jpl.nasa.gov/news/news.php?feature=4769 |url-status=live |archive-url=https://web.archive.org/web/20151114055636/http://www.jpl.nasa.gov/news/news.php?feature=4769 |archive-date= 2015-11-14 |access-date= 2015-11-13 |website=[[NASA]] |df=dmy-all}}</ref><ref name="NASA-20151112b">{{cite web |date=2015-11-12 |title=Audio (66:01) - NASA News Conference - Carbon & Climate Telecon |url=http://www.ustream.tv/recorded/77531778 |url-status=live |archive-url=https://web.archive.org/web/20151117033437/http://www.ustream.tv/recorded/77531778 |archive-date= 2015-11-17 |access-date= 2015-11-12 |website=[[NASA]] |df=dmy-all}}</ref><ref name="NYT-20151110">{{cite news |last=St. Fleur |first=Nicholas |date= 2015-11-10 |title=Atmospheric Greenhouse Gas Levels Hit Record, Report Says |work=[[The New York Times]] |url=https://www.nytimes.com/2015/11/11/science/atmospheric-greenhouse-gas-levels-hit-record-report-says.html |url-status=live |access-date=2015-11-11 |archive-url=https://web.archive.org/web/20151111074131/http://www.nytimes.com/2015/11/11/science/atmospheric-greenhouse-gas-levels-hit-record-report-says.html |archive-date= 2015-11-11 |df=dmy-all}}</ref><ref name="AP-20151109">{{cite news |last=Ritter |first=Karl |date= 2015-11-09 |title=UK: In 1st, global temps average could be 1 degree C higher |work=[[AP News]] |url=http://apnews.excite.com/article/20151109/climate_countdown-greenhouse_gases-d8a21f0397.html |url-status=live |access-date= 2015-11-11 |archive-url=https://web.archive.org/web/20151117021206/http://apnews.excite.com/article/20151109/climate_countdown-greenhouse_gases-d8a21f0397.html |archive-date=2015-11-17 |df=dmy-all}}</ref>預計且觀察到這些碳庫將在約一個世紀內只能將新增大氣碳中的一半移除。<ref name="gcb19" /><ref name=":0" /><ref>{{cite book |title=Intergovernmental Panel on Climate Change Fifth Assessment Report |page=8SM-16 |chapter=Figure 8.SM.4 |chapter-url=https://www.ipcc.ch/site/assets/uploads/2018/07/WGI_AR5.Chap_.8_SM.pdf |archive-url=https://web.archive.org/web/20190313233759/https://www.ipcc.ch/site/assets/uploads/2018/07/WGI_AR5.Chap_.8_SM.pdf |archive-date=2019-03-13 |url-status=live}}</ref>但像海洋這樣的碳庫具有不斷變化的飽和特性,且預計新增的碳的很大部分(20-35%,根據{{le|耦合氣候模式比對專案|Coupled model intercomparison project}}模擬結果)會在大氣中保留幾個世紀到幾千年。<ref>{{cite journal |last=Archer |first=David |year=2009 |title=Atmospheric lifetime of fossil fuel carbon dioxide |url=https://orbi.uliege.be/handle/2268/12933 |journal=Annual Review of Earth and Planetary Sciences |volume=37 |issue=1 |pages=117–34 |bibcode=2009AREPS..37..117A |doi=10.1146/annurev.earth.031208.100206 |hdl=2268/12933}}</ref><ref>{{Cite journal |last1=Joos |first1=F. |last2=Roth |first2=R. |last3=Fuglestvedt |first3=J.D. |display-authors=etal |year=2013 |title=Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis |url=https://www.atmos-chem-phys.net/13/2793/2013/ |journal=Atmospheric Chemistry and Physics |volume=13 |issue=5 |pages=2793–2825 |doi=10.5194/acpd-12-19799-2012 |doi-access=free|hdl=20.500.11850/58316 |hdl-access=free }}</ref> |
|||
====有機鹵化物==== |
|||
{{see also|有機鹵化物|{{le|氟化氣體|Fluorinated gases}}}} |
|||
有機鹵化物是為多種工業用途而開發且產量較低的化合物,通常用於[[溶劑]]和[[冷媒]]。雖然其中如[[氯氟烴|氯氟碳化合物]](CFCs)、[[氫氟烴|氫氟碳化合物]](HFCs)和[[碳氟化合物]](PFCs)氣體於大氣中的濃度較低(僅兆分之幾),效果卻約佔所有長壽溫室氣體總直接[[輻射強迫]]的10%(2019年)。<ref>{{Cite web |last1=Butler |first1=J. |last2=Montzka |first2=S. |year=2020 |title=The NOAA Annual Greenhouse Gas Index (AGGI) |url=https://www.esrl.noaa.gov/gmd/aggi/aggi.html |publisher=[[NOAA]] Global Monitoring Laboratory/Earth System Research Laboratories}}</ref>氯氟碳化合物也會導致平流層中{{le|臭氧耗損|Ozone depletion}}。國際社會正根據《[[蒙特婁議定書]]》和《[[京都議定書]]》持續努力控制這些對環境有害的氣體的製造和使用。目前已開發出,並逐步引入更良性的替代品(例如[[氫氟烯烴|氫氟烯碳化合物]](HFO))。<ref>{{cite news |last1=Sciance |first1=Fred |date=2013-10-29 |title=The Transition from HFC- 134a to a Low -GWP Refrigerant in Mobile Air Conditioners HFO -1234yf |work=General Motors Public Policy Center |url=https://www.epa.gov/sites/production/files/2014-09/documents/sciance.pdf |url-status=live |access-date=2018-08-01 |archive-url=https://web.archive.org/web/20151015181041/http://www2.epa.gov/sites/production/files/2014-09/documents/sciance.pdf |archive-date=2015-10-15}}</ref> |
|||
===土地利用變化=== |
|||
{{main|農業|森林砍伐}} |
|||
自人類開始進行農業活動以來,已改變陸地生物圈中的植被組合,在長達一個世紀的時間尺度上直接並逐漸影響碳循環。<ref name=":0">{{cite book |doi=10.1016/S0070-4571(08)70338-8 |chapter=Chapter 9 the Current Carbon Cycle and Human Impact |title=Geochemistry of Sedimentary Carbonates |volume=48 |pages=447–510 |series=Developments in Sedimentology |year=1990 |isbn=9780444873910 |last1=Morse |first1=John W. |last2=Morse |first2=John W. Autor |last3=Morse |first3=John W. |last4=MacKenzie |first4=F. T. |last5=MacKenzie |first5=Fred T. }}</ref>在過去的幾個世紀中,直接和間接由人類引起的土地利用和土地覆蓋變化(LUCC)導致{{le|生物多樣性喪失|Biodiversity loss}},降低生態系統對環境壓力的韌性,並降低其從大氣中移除碳的能力,即經常導致陸地生態系中的碳釋放進入大氣。 |
|||
出於農業目的而砍伐森林,等於移除含有大量碳的碳庫,開闢出來的土地除用於農業外,也用以建立城市。農地與城市所能儲存的碳量相對較少,最終的結果是有更多的碳留在大氣中。但此類對大氣和整體碳循環的影響可透過[[林地復育]]來改善。 |
|||
== 參見 == |
|||
{{div col}} |
|||
* [[生物地球化學循環]] |
|||
* [[氣候變化緩解]] |
|||
* {{le|地球大氣中的二氧化碳|Carbon dioxide in Earth's atmosphere}} |
|||
*[[碳截存]] |
|||
* {{le|碳酸鹽-矽酸鹽循環|Carbonate–silicate cycle}} |
|||
* [[海洋酸化]] |
|||
* {{le|永久凍土碳循環|Permafrost carbon cycle}} |
|||
{{div col end}} |
|||
== 參考文獻 == |
|||
{{reflist|2}} |
|||
==外部連結== |
|||
{{commons category|lcfirst=yes}} |
|||
* [https://web.archive.org/web/20040602163557/http://www.carboncyclescience.gov/ Carbon Cycle Science Program] – an interagency partnership. |
|||
* [http://www.esrl.noaa.gov/gmd/ccgg/index.html NOAA's Carbon Cycle Greenhouse Gases Group] |
|||
* [http://www.globalcarbonproject.org/ Global Carbon Project – initiative of the Earth System Science Partnership] |
|||
* [http://www.grida.no/climate/vital/13.htm UNEP – The present carbon cycle – Climate Change] {{Webarchive|url=https://web.archive.org/web/20080915231431/http://www.grida.no/climate/vital/13.htm |date= 2008-09-15 }} carbon levels and flows |
|||
* [http://oco.jpl.nasa.gov/ NASA's Orbiting Carbon Observatory] {{Webarchive|url=https://web.archive.org/web/20180909072113/https://oco.jpl.nasa.gov/ |date= 2018-09-09 }} |
|||
{{模板:生物地質化學循環}} |
|||
{{全球變暖}} |
|||
{{Authority control}} |
{{Authority control}} |
||
[[Category:地球化学]] |
|||
{{DEFAULTSORT:Carbon Cycle}} |
|||
[[Category:化学海洋学]] |
|||
[[Category:光合作用]] |
|||
[[分類:化學海洋學]] |
|||
[[Category:土壤生物学]] |
|||
[[分類:光合作用]] |
|||
[[Category:土壤化学]] |
|||
[[分類:土壤生物學]] |
|||
[[Category:碳]] |
|||
[[分類:土壤化學]] |
|||
[[Category:生物地理学]] |
|||
[[分類:數值氣候與天氣模型]] |
|||
[[Category:生物地球化学循环]] |
|||
[[分類:氣候變遷效應]] |
|||
[[Category:Carbon cycle]] |
|||
2024年1月28日 (日) 03:53的版本

| 系列之一 |
| 碳循環 |
|---|
 |
碳循環(英語:Carbon cycle)是生物地球化學循環中的一支,地球的碳透過此循環在生物圈、土壤圈、地質圈、水圈和大氣層之間進行交換。其他主要的生物地球化學循環還包括氮循環和水循環。碳是生物化合物的主要成分,也是石灰石等許多礦物質的主要成分。碳循環由一連串事件組成,這些事件對於地球能維持生命存在有關鍵作用。碳循環描述碳在整個生物圈中回收和重複使用期間的變動,以及儲存及由碳匯釋放的漫長過程。
為描述碳循環中的動態,可將之區分為快速循環和慢速循環兩種。快速碳循環也稱為生物碳循環,這類循環可在幾年內完成,由大氣轉移到生物圈,然後再返回大氣。慢速碳循環(即地質循環),也稱為深層碳循環,需花費數百萬年才能完成,其間碳穿過地殼,在岩石、土壤、海洋和大氣之間移動。[2]
人類在過去幾個世紀中透過改變土地利用,以及從地質圈中以工業化規模開採化石碳(煤炭、石油和天然氣,及製造水泥),擾亂快速碳循環的節奏。[1][3]迄2020年,全球大氣中的二氧化碳含量比第一次工業革命開始前的平均水平增加近52%,經由太陽輻射強迫作用,導致大氣和地球表面溫度升高。[4][5]大氣中二氧化碳增加後也導致海洋pH值降低,而根本上將海洋化學改變。[6][7]在過去的半個世紀中更有大量的化石碳被開採以及使用,且速率持續快速上升,加劇人為造成的氣候變化。[8][9]
主要碳庫
碳循環的概念首先由法國化學家安托萬-羅倫·德·拉瓦節和英國化學家約瑟夫·普利斯特里於十九世紀中葉後期提出,隨後由英國化學家漢弗里·戴維推廣。[10]目前碳循環透過交換路徑於以下幾個主要碳庫(即碳匯)間連接:[11]:5–6
碳庫之間的碳交換是各種化學、物理、地質和生物過程的結果。海洋是地表最大的活性碳庫。[12]碳在大氣、海洋、陸地的生態系統和沈積物之間以相當平衡的方式自然流動,在沒人類影響的情況下,碳水平將大致維持穩定。[4][13]
大氣層
大氣層中的碳有兩種主要形式:二氧化碳和甲烷。這兩種氣體會吸收來自太陽的熱量,並將其保留,是造成溫室效應的部分原因。[12]同樣單位體積的甲烷會比二氧化碳產生更大的溫室效應,但其在大氣層中的濃度要低很多,而且壽命更短。二氧化碳因此在全球溫室效應的影響比甲烷更大。[15]
大氣中的二氧化碳主要會受植物的光合作用移除,然後進入陸地和海洋生物圈。二氧化碳也會直接由大氣層溶入水體(海洋、湖泊等)之中,以及溶於雨滴,隨降水下降到地表。當二氧化碳溶解與水時,會與水分子反應而形成碳酸,最終導致海洋酸化。它可透過風化作用被岩石吸收,也會因酸化而腐蝕所接觸的其他表面,或被沖入海洋。[16]
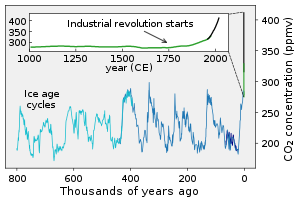
過去兩個世紀中的人類活動已導致大氣中的碳含量增加近50%(迄2020年,主要以二氧化碳形式存在),不僅是透過改變生態系統從大氣中捕集二氧化碳的能力(例如森林砍伐),且又透過增加排放來實現(例如通過燃燒化石燃料和製造水泥)。[5][12]
在遙遠的未來(20至30億年後),因太陽年紀增長而發生的變化(參見太陽系的形成和演化),導致土壤增快透過碳酸鹽-矽酸鹽循環吸收二氧化碳。預計屆時因太陽光度增加,會加快地表風化的速度,[17]最終將導致大氣中的大部分二氧化碳會以碳酸鹽的形式被擠壓進入地殼。[18][19][20]一旦大氣中二氧化碳的濃度降至約百萬分之50(50ppm)以下,C3類植物(約占當今地球植物生物質的95%)的C3類二氧化碳固定將不再發生。[19]不同模型的模擬結果有所不同,但一般預計此情況會在6億年後發生。[21]
一旦海洋中的水分在大約11億年後蒸發完畢,[17]板塊構造移動很可能會因為缺乏水作潤滑劑而停止,而因缺乏火山噴出二氧化碳,將導致碳循環在未來10億至20億年後結束。[22]
陸地生物圈
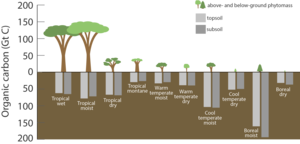
陸地生物圈囊括所有陸地生物(無論是活的或是死的)中的有機碳,以及儲存於土壤中的碳。大約有500吉噸(Gt,十億噸)的碳儲存在地面上的植物和其他生物體中,[4]而土壤中約存有1,500吉噸的碳。[24]陸地生物圈中的大部分碳是有機碳,[25]而大約三分之一的土壤碳以無機形式存在(例如碳酸鈣)。[26]有機碳是地球上所有生物的主要成分。自營生物以二氧化碳的形式從空氣中捕集碳,將其轉化為有機碳,而異營生物則透過攝取其他生物體來獲取。
由於陸地生物圈吸收碳的方式取決於生物因素,因此會循晝夜和季節循環而進行。在測量二氧化碳之時,此一特徵可由基林曲線清楚顯現。此情況在北半球最強,因為北半球比南半球有更多的陸地面積,導致有更大的空間供生態系統吸收和排放碳。

碳以多種方式和不同的時間尺度離開陸地生物圈。透過燃燒或是呼吸會將有機碳迅速釋放到大氣中。它也透過河流攜帶進入海洋或以惰性碳的形式保留在土壤中。[27]儲存在土壤中的碳可保留數千年,然後經侵蝕作用受沖刷進入河流,或透過土壤呼吸釋放進入大氣。於1989年至2008年期間,土壤呼吸作用每年增加的速率約為0.1%。[28]於2008年,全球土壤呼吸釋放的二氧化碳總量約為980億噸,[29]大約是人類現在每年透過燃燒化石燃料,排放到大氣中碳量的3倍。{{cite web name=”World Agriculture”/>對於這種趨勢有一些合理的解釋,其中最可能的是氣溫升高,而加速土壤有機質的分解速度,繼而增加二氧化碳的流量。土壤中儲存碳的時間長度取決於當地的氣候條件以及氣候變化過程中的變化。[30]
| 碳库 | 数量 (gigatons/十億噸) |
|---|---|
| 大气 | 720 |
| 海洋 (总共) | 38,400 |
| 总无机碳 | 37,400 |
| 总有机碳 | 1,000 |
| 表面层 | 670 |
| 深层 | 36,730 |
| 岩石圈 | |
| 沉积碳酸盐 | > 60,000,000 |
| 油母質 | 15,000,000 |
| 陆地生物圈 (总共) | 2,000 |
| 活的生物质 | 600 - 1,000 |
| 死的生物质 | 1,200 |
| 水生生物圈 | 1 - 2 |
| 化石燃料 (总共) | 4,130 |
| 煤炭 | 3,510 |
| 石油 | 230 |
| 天然气 | 140 |
| 其他 (泥炭) | 250 |
海洋
海洋在概念上可分為表面層(其中水與大氣頻繁接觸(從每天到每年)),與深層(低於典型混合層的深度,達到幾百米或更小),此層與表面層接觸的時間間隔可能是幾個世紀。表面層中的溶解無機碳(dissolved inorganic carbon,DIC)與大氣快速交換,維持平衡。深層中含有更多的碳,部分原因是其DIC濃度較表面層高出約15%,[31]但主要是由於深層體積較大,是世界上最大的主動循環碳庫,其中碳含量是大氣的50倍,[12]但與大氣達到平衡的時間尺度需要數百年(經由溫鹽環流驅動,兩海水層之間的碳交換很慢)。[12]
碳主經由大氣中二氧化碳溶解而進入海洋,其中一小部分轉化為碳酸鹽。它也可以溶解有機碳(DOC)形式通過河流進入海洋。碳透過光合作用被生物體轉化為有機碳,且可在整個食物鏈中交換,或者以死亡軟組織的形式沉澱到海洋更深、更富含碳的海水層中,或者以碳酸鈣的形式成為貝類的殼。碳會在此海水層中循環很長一段時間,然後成為沉積物,或是最終通過溫鹽循環返回表面層水域。[4]
海水呈鹼性(目前pH值為8.1至8.2)。大氣中二氧化碳增加後,會導致海水的pH值趨向中性變動,此過程稱為海洋酸化。海洋吸收二氧化碳是碳截存作用最重要的形式之一。預計海水pH值降低的程度會降低海洋生物碳酸鈣沉澱,也會把海洋吸收二氧化碳的能力降低。[32][33]
地質圈

碳循環的地質部分與全球碳循環的其他部分相比,運作緩慢。此為大氣中碳含量以及全球氣溫最重要的決定因素之一。[34]
地球上大部分的碳以惰性方式儲存在地球的岩石圈中。[12]在地函中大部分的碳是在地球形成時即已存在。[35]而有一些是以有機碳的形式從生物圈沉積而來。[36]地質圈中儲存的碳中約80%是存於石灰石及其衍生物中,是由儲存在海洋生物殼中的碳酸鈣沉積形成。剩下的20%以油母質的形式存在,這些油母質是陸地生物質經過高溫與高壓的沉積和埋藏而形成。儲存在地質圈中的有機碳可保留數百萬年。[34]
碳可透過多種方式離開地質圈。當碳酸鹽岩隱沒到地函中時,會在變質作用過程中釋放二氧化碳。這些二氧化碳可透過火山噴發和熱點而釋放進入大氣和海洋。[35]人類也可透過直接開採化石燃料,利用燃燒以釋放能量,並將其儲存的碳排放進入大氣。
動力學類型

碳循環有快有慢。快速循環在生物圈中運行,慢速循環在岩石中運行。快速循環可在幾年內完成,將碳從大氣轉移到生物圈,然後返回大氣。慢速循環會延伸到地函深處,可能需要數百萬年才會完成,碳會穿過地殼,在岩石、土壤、海洋和大氣之間移動。[2]
快速循環涉及環境和生物圈中的生物體之間相對短期的生物地球化學過程(參見文章開頭的圖表)。包括大氣與陸地和海洋生態系統以及土壤和海底沉積物之間的碳移動。快速循環包括涉及光合作用的年度循環以及涉及營養生長和分解的代際循環。快速循環對人類活動的反應將決定氣候變化所產生的許多更直接的影響。[37][38][39][40][41]
慢速(或是深層)碳循環涉及屬於岩石循環的中長期地球化學過程(參見附圖)。海洋和大氣之間的交換需要幾個世紀,岩石的風化需要數百萬年。海洋中的碳沉澱到海底,形成沉積岩並隱沒到地函中。造山過程導致地質碳返回地表,同時岩石受到風化,碳透過脫氣返回大氣,也透過河流攜帶進入海洋。其他地質碳則透過鈣離子的熱液系統排放返回海洋。在任何一年中皆會有1千萬至1億噸碳沿著這個緩慢的循環移動,也包括火山將地質碳以二氧化碳的形式直接送回大氣。然而這些二氧化碳的數量還不到燃燒化石燃料排放到大氣中的百分之一。[2][37][42]
快速碳循環中的子過程
水循環中的陸地碳

- 大氣中懸浮微粒擔任雲凝結核,促進雲形成。[44][45]
- 雨滴於降落途中,透過顆粒和有機蒸氣吸附作用,吸收有機和無機碳。[46][47]
- 燃燒和火山爆發產生高度濃縮的多環芳香化合物分子(即黑碳),並與二氧化碳等溫室氣體一起進入大氣。[48][49]
- 陸地植物透過光合作用將大氣中的二氧化碳固定,並透過呼吸作用將一小部分返還大氣。[50]木質素和纖維素佔森林中有機碳中的80%,和牧場有機碳中的60%。[51][52]
- 植物凋落物和根系有機碳與沉積物混合形成有機土壤,其中植物來源的有機碳和石化有機碳透過微生物和真菌活動而被儲存與轉化。[53][54][55]
- 當雨水穿過森林冠層(即樹冠穿透雨)和沿著植物樹幹/莖流下(即樹幹流)時,會吸收植物和沈降的氣膠衍生的溶解有機碳(DOC)和溶解無機碳(DIC)。[56]當水滲入土壤溶液和地下儲層時會發生生物地球化學轉變,[57][58]當土壤中水分完全飽和後,[59]或降雨的速率快過土壤飽和的速度,[60]就會形成地表徑流。
- 來自陸地生物圈和原位初級生產的有機碳被河流和溪流中的微生物群落分解以及物理分解(即光降解),導致從河流排放到大氣中的二氧化碳通量與陸地生物圈每年固存的碳量相似。[61][62][63]木質素[64]和黑碳[65]等陸地來源的大分子被分解成較小的成分和單體,最終轉化為二氧化碳、代謝中間體或生物質。
- 湖泊、水庫和洪氾區通常儲存大量有機碳和沈積物,但在水體中也經歷淨異營作用,導致進入大氣的二氧化碳淨通量大約比河流的少一個數量級。[66][63]洪氾區、湖泊和水庫中缺氧水體沉積物中的甲烷產量通常也很高。[67]
- 由於河流作用會輸出營養,河流羽流中的初級生產通常因而得到增強。[68][69]導致河口水域成為全球大氣中二氧化碳的來源。[70]
- 潮汐沼澤既儲存又輸出藍碳。[71][72][73]估計全球草澤和濕地向大氣排放的二氧化碳通量與河流相當。[74]
- 大陸棚和開放海域通常會吸收大氣中的二氧化碳。[70]
- 海洋生物泵吸收一小部分的二氧化碳(但很重要的一部分),形成存於海洋沉積物中的有機碳(見下文)。[75][43]
流入海洋的地表逕流

陸地和海洋生態系統主要透過河流運輸而連結,河流是陸地侵蝕性物質進入海洋系統的主要通道。陸地生物圈和岩石圈之間的物質和能量交換,以及有機碳固定與氧化過程,共同調節生態系統中的碳和氧氣庫。[76]
河流運輸是這些碳庫的主要連結通道,發揮將淨初級生產(主要為溶解有機碳(DOC)和顆粒有機碳(POC)的形式)從陸地系統運輸到海洋系統的作用。[77]在運輸過程中,部分DOC會透過氧化還原反應迅速返回大氣,導致陸地-大氣儲存層之間發生"碳脫氣"。[78][79]剩餘的DOC和溶解的無機碳(DIC)被攜帶進入海洋。[80][81][82]估計於2015年,全球河流的無機和有機碳輸出通量分別為0.50-0.70十億噸(Pg)碳/年和0.15-0.35十億噸碳/年。[81]另一方面,POC可埋藏在沉積物中很長時間,全球每年由陸地輸送到海洋的POC通量估計為0.20千噸(Gg)/年。[83][76]
海洋生物泵

海洋生物泵是由海洋生物從大氣和陸地徑流捕集碳,然後移往深海內部和海底沉積物的過程。[84]生物泵並非一種單一過程,而是多個過程的總和,每個過程都會影響生物泵的結果。此種泵作用每年將約110億噸碳轉移到海洋內部。如果海洋中無生物泵作用,會導致大氣中的二氧化碳濃度比現在高出約400ppm。[85][86][87]
有機和無機材料中的大部分碳是在海面形成,然後開始沉入海底。當這些物質由較高的海水水柱以海雪的形式下沉時,深海由此獲取大部分營養。這些物質由死亡或垂死的動物和微生物、動物糞便、沙粒和其他無機材料組成。[88]
生物泵將溶解的無機碳(DIC)轉化為有機生物質,並將其以顆粒或溶解形式泵入深海。浮游植物利用光合作用將無機養分和二氧化碳固定,同時釋放溶解有機物 (DOM),而被草食性浮游動物攝取。較大的浮游動物,例如橈足綱動物,排出糞便顆粒,可被重新攝入,或與其他有機碎屑一起下沉或聚集成更大、下沉更快的聚集體。 DOM部分被細菌消耗並釋放氣體,剩餘的難分解部分被海水攜帶,混入深海中。被攜帶到深海中的DOM和聚集體被消耗和釋放氣體,將有機碳返回到巨大的深海DIC庫中。[89]
單一浮游植物細胞的下沉速度約為每天一公尺。鑑於海洋的平均深度約為四公里,這些細胞可能需要十年以上才能到達海底。然而透過捕食者糞便顆粒的凝固和排出等過程,而形成聚集體,其下沉速度比單一細胞大幾個數量級,並在幾天內抵達深處。[90]
由海洋表面下降的顆粒中大約有1%會到達海底並被消耗、釋放氣體或埋在沉積物中。這些過程最終是從表面去除有機形式的碳,並將其返回到更深的DIC,維持一個由海洋表面到深海的DIC梯度。溫鹽環流以千年的時間尺度將深海DIC送返大氣。埋藏在沉積物中的碳可隱沒進入地函並儲存數百萬年,形成一種緩慢碳循環中的形式(見下一節)。[89]
慢速碳循環內的子過程

慢速或深層碳循環為一重要過程,但它不像經由大氣、陸地生物圈、海洋和地質圈的相對快速碳循環那樣被充分理解。[91]深層碳循環與地球表面和大氣中的碳循環密切相關。如果沒此過程,碳將保留在大氣中,並在很長一段時間內累積到極高的濃度。[92]深層碳循環讓碳返回地球的作用,在維持陸地適合生命存在所需的條件方面具有有關鍵作用。
此外,因為地球於此作用中可儲存大量的碳,過程本身就很重要。研究玄武岩岩漿的成分並測量火山中的二氧化碳通量顯示地函中的碳含量實際上比地球表面的多一千倍。[93]鑽探和物理觀測地球深部碳過程顯然極為困難,因為下地函和地核的深度分別為660公里至2,891公里和2,891公里至6,371公里。而導致人們對碳在地球深處的作用尚無太多確切的了解。雖然如此,一些證據(其中許多由模擬地球深處條件而取得)已顯示元素向下移動到下地函的機制,以及碳在該層的極端溫度和壓力下所具有的形式。此外,透過地震學等技術已讓我們對地核中碳的或有存在有更深入了解。
下地函中的碳

碳主要是透過富含碳酸鹽沉積物,經由海洋底層的板塊邊緣,在持續隱沒過程中被拉入地函。我們目前對地函中的碳循環知之甚少,尤其是在地球深處,但許多研究可增強我們對其中元素運動和形式的理解。例如於2011年所做的一項研究,顯示碳循環一直延伸到下地函。該研究對巴西茹伊納超深處挖掘而得的稀有鑽石進行研究,確定其中一些鑽石內含物整體成分與較低地函內溫度和壓力下玄武岩熔化和結晶的估計結果相匹配。[95]調查結果表明海洋岩石圈玄武岩是將碳向地球深處傳輸的主要機制。這些隱沒的碳酸鹽可與下地函中矽酸鹽相互作用,最終形成像挖掘而得的這批超深鑽石。[96]
但進入下地函的碳酸鹽除會形成鑽石之外,還會形成其他的物質。有項於2011年進行的實驗,將碳酸鹽岩置於與下地函類似的深達1,800公里處,而形成菱鎂礦、菱鐵礦和多種石墨。[97]其他實驗以及岩石學觀察也支持此一說法,顯示菱鎂礦實際上是地函中大部分地區中最穩定的碳酸鹽相,主要是由於其可忍受較高的熔化溫度。[98]科學家們得到的結論是碳酸鹽在下降到地函時會發生氧化還原反應,然後在低氧逸度環境下在深處穩定下來。鎂、鐵和其他金屬化合物在整個過程中充當緩衝劑。[99]如碳(如石墨)的還原元素形式,其存在顯示碳化合物在進入地函時被還原。

同質異形體現象改變碳酸鹽化合物在地球不同深度的穩定性。為說明這一點,實驗室模擬和密度泛函理論計算顯示四面體形分子構型碳酸鹽在接近核函邊界的深度最為穩定。[100][97]於2015年所做的一項研究顯示下地函的高壓導致碳鍵從sp2混成軌域轉變為sp3混成軌域,導致碳與氧以四面體形分子構型鍵合。[101]CO3三角形團(也稱三角形碳酸鹽團)不能形成可聚合網絡,而四面體CO可以,表示碳配位數增加,而下地函中碳酸鹽化合物的性質也因而發生巨大變化。例如,初步理論研究顯示高壓會導致碳酸鹽熔體黏度增加,之後熔體的流動性較低,導致大量碳沉積到地函深處。[102]
碳因而可在下地函中保留很長一段時間,但大量的碳經常會返回岩石圈。這個過程被稱為碳釋氣,是碳化地函經歷減壓熔融以及地函羽流攜帶碳化合物向上到達地殼的結果。[103]碳在上升到火山熱點時受到氧化,然後以二氧化碳的形式釋放。發生這種情况是為讓碳原子的氧化態與在這些地區噴發的玄武岩的氧化態相匹配。[104]

地核中的碳
雖然地核中碳的存在受到很大的限制,但最近的研究顯示該區域可儲存大量的碳。透過s波地震測試,s波通過的速度僅為通過多數富鐵合金所需的一半。[105]由於核心的成分以往被認為是結晶鐵和少量鎳的合金,因此這種地震結果異常現象顯示地核中存在包括碳在內的輕元素。事實上,使用金剛石壓砧複製地核高壓條件的研究顯示雪明碳鐵 (Fe7C3) 與內核的波速和密度相符。因此這種實驗模型可作為地核含有多達地球碳67%的證據。[106]而在另一項研究中發現在地球內核的壓力和溫度條件下,有碳溶解在鐵中,並形成具有與Fe7C3相同成分的穩定相,但結構與前面提到的不同。[107]總之,雖然地核中潛在儲存的碳量尚不清楚,但最近的研究顯示雪明碳鐵的存在可解釋一些地球物理觀測結果。
人類對快速碳循環的影響

自第一次工業革命以來,特別是在第二次世界大戰結束後,人類活動將地質圈中大量的碳重新分配,嚴重擾亂全球碳循環。[1]人類也因改變植被和其他土地利用,不斷改變陸地生物圈的自然功能。[12]並透過涉及與大量製造人造(合成)碳化合物,然後以污染物的形式在空氣、水和沈積物中存在,持續數十年至數千年。[108][109]由於各種正回饋和負回饋,造成的氣候變化正加劇並迫使人類進一步間接改變碳循環。[110][109]
氣候變化

簡式圖形表達
當前的氣候變化導致海洋溫度與酸度升高,將海洋生態系統改變。[112]此外,酸雨和農業和工業所產生的污染,經過徑流攜帶入海,也將海洋的化學成分改變。這種變化會對珊瑚礁等具高敏感度的生態系統產生巨大影響,[113]而限制海洋在區域範圍內由大氣吸收碳的能力,並減少全球海洋生物多樣性。
大氣與地球系統其他組成部分之間的碳交換,統稱為碳循環,人為碳排放對氣候變化的影響構成重要的負(抑制)回饋。目前陸地和海洋每年各可捕集人為碳排放量的四分之一。[114][111]
預計這些回饋能力在未來會減弱,而放大人為碳排放對氣候變化的影響。[115]但回饋能力減弱的程度是高度不確定,即使在相同的大氣濃度或排放情況下,地球系統模型所預測的陸地和海洋的碳吸收有很大的差異。[116][111][117]人為氣候變化間接引起的北極甲烷排放也會影響碳循環並導致進一步暖化。
化石碳開採與燃燒

人類對碳循環和生物圈最大且成長最快的影響之一是開採與燃燒化石燃料,直接將碳從地質圈轉移到大氣中。在生產水泥的前體 - 熟料時需煅燒石灰石,過程中也會釋放大量二氧化碳。[118]
截至2020年,全球總共已開採約450億噸化石碳,接近地球上所有陸地生物量中所含碳的數量。[3]全球最近直接排放到大氣中的碳數量已超過植被和海洋的吸收能力。[119][120][121][122]預計且觀察到這些碳庫將在約一個世紀內只能將新增大氣碳中的一半移除。[3][123][124]但像海洋這樣的碳庫具有不斷變化的飽和特性,且預計新增的碳的很大部分(20-35%,根據耦合氣候模式比對專案模擬結果)會在大氣中保留幾個世紀到幾千年。[125][126]
有機鹵化物
有機鹵化物是為多種工業用途而開發且產量較低的化合物,通常用於溶劑和冷媒。雖然其中如氯氟碳化合物(CFCs)、氫氟碳化合物(HFCs)和碳氟化合物(PFCs)氣體於大氣中的濃度較低(僅兆分之幾),效果卻約佔所有長壽溫室氣體總直接輻射強迫的10%(2019年)。[127]氯氟碳化合物也會導致平流層中臭氧耗損。國際社會正根據《蒙特婁議定書》和《京都議定書》持續努力控制這些對環境有害的氣體的製造和使用。目前已開發出,並逐步引入更良性的替代品(例如氫氟烯碳化合物(HFO))。[128]
土地利用變化
自人類開始進行農業活動以來,已改變陸地生物圈中的植被組合,在長達一個世紀的時間尺度上直接並逐漸影響碳循環。[123]在過去的幾個世紀中,直接和間接由人類引起的土地利用和土地覆蓋變化(LUCC)導致生物多樣性喪失,降低生態系統對環境壓力的韌性,並降低其從大氣中移除碳的能力,即經常導致陸地生態系中的碳釋放進入大氣。
出於農業目的而砍伐森林,等於移除含有大量碳的碳庫,開闢出來的土地除用於農業外,也用以建立城市。農地與城市所能儲存的碳量相對較少,最終的結果是有更多的碳留在大氣中。但此類對大氣和整體碳循環的影響可透過林地復育來改善。
參見
參考文獻
- ^ 1.0 1.1 1.2 Riebeek, Holli. The Carbon Cycle. Earth Observatory. NASA. 2011-06-16 [2018-04-05]. (原始内容存档于2016-03-05). 已忽略未知参数
|df=(帮助) - ^ 2.0 2.1 2.2 Libes, Susan M. (2015). Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change 互联网档案馆的存檔,存档日期2023-01-08. In: Routledge Handbook of Ocean Resources and Management, Routledge, pages 89–107. ISBN 9781136294822.
- ^ 3.0 3.1 3.2 3.3 Friedlingstein, P., Jones, M., O'Sullivan, M., Andrew, R., Hauck, J., Peters, G., Peters, W., Pongratz, J., Sitch, S., Le Quéré, C. and 66 others (2019) "Global carbon budget 2019". Earth System Science Data, 11(4): 1783–1838. doi:10.5194/essd-11-1783-2019.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
- ^ 4.0 4.1 4.2 4.3 Prentice, I.C. The carbon cycle and atmospheric carbon dioxide. Houghton, J.T. (编). Climate change 2001: the scientific basis: contribution of Working Group I to the Third Assessment Report of the Intergouvernmental Panel on Climate Change. 2001. hdl:10067/381670151162165141.
- ^ 5.0 5.1 The NOAA Annual Greenhouse Gas Index (AGGI) - An Introduction. NOAA Global Monitoring Laboratory/Earth System Research Laboratories. [2020-10-30].
- ^ What is Ocean Acidification?. National Ocean Service, National Oceanic and Atmospheric Administration. [2020-10-30].
- ^ Report of the Ocean Acidification and Oxygen Working Group, SCOR Biological Observatories Workshop (PDF). scor-int.org/. International Council for Science's Scientific Committee on Ocean Research (SCOR). 2009-09-30. (原始内容存档 (PDF)于2011-07-15).
- ^ Heede, R. Tracing anthropogenic carbon dioxide and methane emissions to fossil fuel and cement producers, 1854–2010. Climatic Change. 2014, 122 (1–2): 229–241. Bibcode:2014ClCh..122..229H. doi:10.1007/s10584-013-0986-y
 .
.
- ^ Ritchie, Hannah; Roser, Max. CO₂ and Greenhouse Gas Emissions: CO₂ Emissions by Fuel. Our World in Data (Published online at OurWorldInData.org.). 2020 [2020-10-30].
- ^ Holmes, Richard (2008). "The Age Of Wonder", Pantheon Books. ISBN 978-0-375-42222-5.
- ^ Archer, David. The global carbon cycle. Princeton: Princeton University Press. 2010. ISBN 9781400837076.
- ^ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 Falkowski, P.; Scholes, R. J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Högberg, P.; Linder, S.; MacKenzie, F. T.; Moore, III, B.; Pedersen, T.; Rosenthal, Y.; Seitzinger, S.; Smetacek, V.; Steffen, W. The Global Carbon Cycle: A Test of Our Knowledge of Earth as a System. Science. 2000, 290 (5490): 291–296. Bibcode:2000Sci...290..291F. PMID 11030643. doi:10.1126/science.290.5490.291.
- ^ An Introduction to the Global Carbon Cycle (PDF). University of New Hampshire. 2009 [2016-02-06]. (原始内容存档 (PDF)于2016-10-08). 已忽略未知参数
|df=(帮助) - ^ A Year In The Life Of Earth's CO2 互联网档案馆的存檔,存档日期2021-12-26. NASA: Goddard Space Flight Center, 2014-11-17.
- ^ Forster, P.; Ramawamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; Nganga, J.; Prinn, R.; Raga, G.; Schulz, M.; Van Dorland, R. Changes in atmospheric constituents and in radiative forcing. Climate Change 2007: The Physical Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. 2007.
- ^ Many Planets, One Earth // Section 4: Carbon Cycling and Earth's Climate. Many Planets, One Earth. [2012-06-24]. (原始内容存档于2012-04-17). 已忽略未知参数
|df=(帮助) - ^ 17.0 17.1 O'Malley-James, Jack T.; Greaves, Jane S.; Raven, John A.; Cockell, Charles S. Swansong Biospheres: Refuges for life and novel microbial biospheres on terrestrial planets near the end of their habitable lifetimes. International Journal of Astrobiology. 2012, 12 (2): 99–112. Bibcode:2013IJAsB..12...99O. S2CID 73722450. arXiv:1210.5721
 . doi:10.1017/S147355041200047X.
. doi:10.1017/S147355041200047X.
- ^ Walker, James C. G.; Hays, P. B.; Kasting, J. F. A negative feedback mechanism for the long-term stabilization of Earth's surface temperature. Journal of Geophysical Research. 1981, 86 (C10): 9776. Bibcode:1981JGR....86.9776W. ISSN 0148-0227. doi:10.1029/JC086iC10p09776 (英语).
- ^ 19.0 19.1 Heath, Martin J.; Doyle, Laurance R. Circumstellar Habitable Zones to Ecodynamic Domains: A Preliminary Review and Suggested Future Directions. 2009-12-13. arXiv:0912.2482
 [astro-ph.EP].
[astro-ph.EP].
- ^ Crockford, Peter W.; Bar On, Yinon M.; Ward, Luce M.; Milo, Ron; Halevy, Itay. The geologic history of primary productivity. Current Biology. November 2023, 33 (21): 4741–4750.e5. doi:10.1016/j.cub.2023.09.040 (英语).
- ^ Lenton, Timothy M.; von Bloh, Werner. Biotic feedback extends the life span of the biosphere. Geophysical Research Letters. 2001-05-01, 28 (9): 1715–1718. Bibcode:2001GeoRL..28.1715L. doi:10.1029/2000GL012198
 (英语).
(英语).
- ^ Brownlee, Donald E. Planetary habitability on astronomical time scales. Schrijver, Carolus J.; Siscoe, George L. (编). Heliophysics: Evolving Solar Activity and the Climates of Space and Earth. Cambridge University Press. 2010: 94. ISBN 978-0-521-11294-9.
- ^ 23.0 23.1 Janowiak, M.; Connelly, W.J.; Dante-Wood, K.; et al. Considering Forest and Grassland Carbon in Land Management. General Technical Report (United States Department of Agriculture, Forest Service). 2017: 1–68. doi:10.2737/WO-GTR-95
 .
.
- ^ Rice, Charles W. Storing carbon in soil: Why and how?. Geotimes. January 2002, 47 (1): 14–17 [2018-04-05]. (原始内容存档于2018-04-05). 已忽略未知参数
|df=(帮助) - ^ Yousaf, Balal; Liu, Guijian; Wang, Ruwei; Abbas, Qumber; Imtiaz, Muhammad; Liu, Ruijia. Investigating the biochar effects on C-mineralization and sequestration of carbon in soil compared with conventional amendments using the stable isotope (δ13C) approach. GCB Bioenergy. 2016, 9 (6): 1085–1099. doi:10.1111/gcbb.12401
 .
.
- ^ Lal, Rattan. Sequestration of atmospheric CO2 in global carbon pools. Energy and Environmental Science. 2008, 1: 86–100. doi:10.1039/b809492f.
- ^ Li, Mingxu; Peng, Changhui; Wang, Meng; Xue, Wei; Zhang, Kerou; Wang, Kefeng; Shi, Guohua; Zhu, Qiuan. The carbon flux of global rivers: A re-evaluation of amount and spatial patterns. Ecological Indicators. 2017, 80: 40–51. doi:10.1016/j.ecolind.2017.04.049.
- ^ Bond-Lamberty, Ben; Thomson, Allison. Temperature-associated increases in the global soil respiration record. Nature. 2010, 464 (7288): 579–582. Bibcode:2010Natur.464..579B. PMID 20336143. S2CID 4412623. doi:10.1038/nature08930.
- ^ Template:Cite web name=”World Agriculture”
- ^ Varney, Rebecca M.; Chadburn, Sarah E.; Friedlingstein, Pierre; Burke, Eleanor J.; Koven, Charles D.; Hugelius, Gustaf; Cox, Peter M. A spatial emergent constraint on the sensitivity of soil carbon turnover to global warming. Nature Communications. 2020-11-02, 11 (1): 5544. Bibcode:2020NatCo..11.5544V. ISSN 2041-1723. PMC 7608627
 . PMID 33139706. doi:10.1038/s41467-020-19208-8 (英语).
. PMID 33139706. doi:10.1038/s41467-020-19208-8 (英语).
- ^ Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics. Princeton University Press, Princeton, New Jersey, US. 2006.
- ^ Kleypas, J. A.; Buddemeier, R. W.; Archer, D.; Gattuso, J. P.; Langdon, C.; Opdyke, B. N. Geochemical Consequences of Increased Atmospheric Carbon Dioxide on Coral Reefs. Science. 1999, 284 (5411): 118–120. Bibcode:1999Sci...284..118K. PMID 10102806. doi:10.1126/science.284.5411.118.
- ^ Langdon, C.; Takahashi, T.; Sweeney, C.; Chipman, D.; Goddard, J.; Marubini, F.; Aceves, H.; Barnett, H.; Atkinson, M. J. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochemical Cycles. 2000, 14 (2): 639. Bibcode:2000GBioC..14..639L. S2CID 128987509. doi:10.1029/1999GB001195
 .
.
- ^ 34.0 34.1 The Slow Carbon Cycle. NASA. 2011-06-16 [2012-06-24]. (原始内容存档于2012-06-16). 已忽略未知参数
|df=(帮助) - ^ 35.0 35.1 The Carbon Cycle and Earth's Climate 互联网档案馆的存檔,存档日期2003-06-23. Information sheet for Columbia University Summer Session 2012 Earth and Environmental Sciences Introduction to Earth Sciences I
- ^ Berner, Robert A. A New Look at the Long-term Carbon Cycle (PDF). GSA Today. November 1999, 9 (11): 1–6. (原始内容存档 (PDF)于2019-02-13).
- ^ 37.0 37.1 Bush, Martin J. The Carbon Cycle. Climate Change and Renewable Energy. 2020: 109–141. ISBN 978-3-030-15423-3. S2CID 210305910. doi:10.1007/978-3-030-15424-0_3.
- ^ NASA Earth Observatory ( 2011-06-16). "The Fast Carbon Cycle". Archive.
 本文含有此來源中屬於公有领域的内容。
本文含有此來源中屬於公有领域的内容。
- ^ Rothman, D. H. Atmospheric carbon dioxide levels for the last 500 million years. Proceedings of the National Academy of Sciences. 2002, 99 (7): 4167–4171. Bibcode:2002PNAS...99.4167R. PMC 123620
 . PMID 11904360. doi:10.1073/pnas.022055499
. PMID 11904360. doi:10.1073/pnas.022055499  .
.
- ^ Carpinteri, Alberto; Niccolini, Gianni. Correlation between the Fluctuations in Worldwide Seismicity and Atmospheric Carbon Pollution. Sci. 2019, 1: 17. doi:10.3390/sci1010017
 .
.  Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived copy. [2020-07-05]. (原始内容存档于2017-10-16). 无效
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License Archived copy. [2020-07-05]. (原始内容存档于2017-10-16). 无效|url-status=bot: unknown(帮助). - ^ Rothman, Daniel. Earth's carbon cycle: A mathematical perspective. Bulletin of the American Mathematical Society. January 2015, 52 (1): 47–64. ISSN 0273-0979. doi:10.1090/S0273-0979-2014-01471-5. hdl:1721.1/97900
 (英语).
(英语).
- ^ NASA Earth Observatory (2011-06-16). "The Slow Carbon Cycle". Archive.
 本文含有此來源中屬於公有领域的内容。
本文含有此來源中屬於公有领域的内容。
- ^ 43.0 43.1 43.2 Ward, Nicholas D.; Bianchi, Thomas S.; Medeiros, Patricia M.; Seidel, Michael; Richey, Jeffrey E.; Keil, Richard G.; Sawakuchi, Henrique O. Where Carbon Goes when Water Flows: Carbon Cycling across the Aquatic Continuum. Frontiers in Marine Science. 2017, 4. doi:10.3389/fmars.2017.00007
 .
.  Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
- ^ Kerminen, Veli-Matti; Virkkula, Aki; Hillamo, Risto; Wexler, Anthony S.; Kulmala, Markku. Secondary organics and atmospheric cloud condensation nuclei production. Journal of Geophysical Research: Atmospheres. 2000, 105 (D7): 9255–9264. Bibcode:2000JGR...105.9255K. doi:10.1029/1999JD901203
 .
.
- ^ Riipinen, I.; Pierce, J. R.; Yli-Juuti, T.; Nieminen, T.; Häkkinen, S.; Ehn, M.; Junninen, H.; Lehtipalo, K.; Petäjä, T.; Slowik, J.; Chang, R.; Shantz, N. C.; Abbatt, J.; Leaitch, W. R.; Kerminen, V.-M.; Worsnop, D. R.; Pandis, S. N.; Donahue, N. M.; Kulmala, M. Organic condensation: A vital link connecting aerosol formation to cloud condensation nuclei (CCN) concentrations. Atmospheric Chemistry and Physics. 2011, 11 (8): 3865–3878. Bibcode:2011ACP....11.3865R. doi:10.5194/acp-11-3865-2011
 .
.
- ^ Waterloo, Maarten J.; Oliveira, Sylvia M.; Drucker, Debora P.; Nobre, Antonio D.; Cuartas, Luz A.; Hodnett, Martin G.; Langedijk, Ivar; Jans, Wilma W. P.; Tomasella, Javier; De Araújo, Alessandro C.; Pimentel, Tania P.; Múnera Estrada, Juan C. Export of organic carbon in run-off from an Amazonian rainforest blackwater catchment. Hydrological Processes. 2006, 20 (12): 2581–2597. Bibcode:2006HyPr...20.2581W. S2CID 129377411. doi:10.1002/hyp.6217.
- ^ Neu, Vania; Ward, Nicholas D.; Krusche, Alex V.; Neill, Christopher. Dissolved Organic and Inorganic Carbon Flow Paths in an Amazonian Transitional Forest. Frontiers in Marine Science. 2016, 3. S2CID 41290209. doi:10.3389/fmars.2016.00114
 .
.
- ^ Baldock, J.A.; Masiello, C.A.; Gélinas, Y.; Hedges, J.I. Cycling and composition of organic matter in terrestrial and marine ecosystems. Marine Chemistry. 2004, 92 (1–4): 39–64. Bibcode:2004MarCh..92...39B. doi:10.1016/j.marchem.2004.06.016.
- ^ Myers-Pigg, Allison N.; Griffin, Robert J.; Louchouarn, Patrick; Norwood, Matthew J.; Sterne, Amanda; Cevik, Basak Karakurt. Signatures of Biomass Burning Aerosols in the Plume of a Saltmarsh Wildfire in South Texas. Environmental Science & Technology. 2016, 50 (17): 9308–9314. Bibcode:2016EnST...50.9308M. PMID 27462728. doi:10.1021/acs.est.6b02132.
- ^ Field, C. B.; Behrenfeld, M. J.; Randerson, J. T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science. 1998, 281 (5374): 237–240. Bibcode:1998Sci...281..237F. PMID 9657713. doi:10.1126/science.281.5374.237.
- ^ Martens, Dean A.; Reedy, Thomas E.; Lewis, David T. Soil organic carbon content and composition of 130-year crop, pasture and forest land-use managements. Global Change Biology. 2004, 10 (1): 65–78. Bibcode:2004GCBio..10...65M. S2CID 5019983. doi:10.1046/j.1529-8817.2003.00722.x.
- ^ Bose, Samar K.; Francis, Raymond C.; Govender, Mark; Bush, Tamara; Spark, Andrew. Lignin content versus syringyl to guaiacyl ratio amongst poplars. Bioresource Technology. 2009, 100 (4): 1628–1633. PMID 18954979. doi:10.1016/j.biortech.2008.08.046.
- ^ Schlesinger, William H.; Andrews, Jeffrey A. Soil respiration and the global carbon cycle. Biogeochemistry. 2000, 48: 7–20. S2CID 94252768. doi:10.1023/A:1006247623877.
- ^ Schmidt, Michael W. I.; Torn, Margaret S.; Abiven, Samuel; Dittmar, Thorsten; Guggenberger, Georg; Janssens, Ivan A.; Kleber, Markus; Kögel-Knabner, Ingrid; Lehmann, Johannes; Manning, David A. C.; Nannipieri, Paolo; Rasse, Daniel P.; Weiner, Steve; Trumbore, Susan E. Persistence of soil organic matter as an ecosystem property. Nature. 2011, 478 (7367): 49–56. Bibcode:2011Natur.478...49S. PMID 21979045. S2CID 3461265. doi:10.1038/nature10386.
- ^ Lehmann, Johannes; Kleber, Markus. The contentious nature of soil organic matter. Nature. 2015, 528 (7580): 60–68. Bibcode:2015Natur.528...60L. PMID 26595271. S2CID 205246638. doi:10.1038/nature16069
 .
.
- ^ Qualls, Robert G.; Haines, Bruce L. Biodegradability of Dissolved Organic Matter in Forest Throughfall, Soil Solution, and Stream Water. Soil Science Society of America Journal. 1992, 56 (2): 578–586. Bibcode:1992SSASJ..56..578Q. doi:10.2136/sssaj1992.03615995005600020038x.
- ^ Grøn, Christian; Tørsløv, Jens; Albrechtsen, Hans-Jørgen; Jensen, Hanne Møller. Biodegradability of dissolved organic carbon in groundwater from an unconfined aquifer. Science of the Total Environment. 1992,. 117-118: 241–251. Bibcode:1992ScTEn.117..241G. doi:10.1016/0048-9697(92)90091-6.
- ^ Pabich, Wendy J.; Valiela, Ivan; Hemond, Harold F. Relationship between DOC concentration and vadose zone thickness and depth below water table in groundwater of Cape Cod, U.S.A.. Biogeochemistry. 2001, 55 (3): 247–268. S2CID 140536437. doi:10.1023/A:1011842918260.
- ^ Linsley, Ray K. Solutions Manual to Accompany Hydrology for Engineers. 1975.
- ^ Horton, Robert E. The Rôle of infiltration in the hydrologic cycle. Transactions, American Geophysical Union. 1933, 14 (1): 446. Bibcode:1933TrAGU..14..446H. doi:10.1029/TR014i001p00446.
- ^ Richey, Jeffrey E.; Melack, John M.; Aufdenkampe, Anthony K.; Ballester, Victoria M.; Hess, Laura L. Outgassing from Amazonian rivers and wetlands as a large tropical source of atmospheric CO2. Nature. 2002, 416 (6881): 617–620. Bibcode:2002Natur.416..617R. PMID 11948346. S2CID 4345881. doi:10.1038/416617a.
- ^ Cole, J. J.; Prairie, Y. T.; Caraco, N. F.; McDowell, W. H.; Tranvik, L. J.; Striegl, R. G.; Duarte, C. M.; Kortelainen, P.; Downing, J. A.; Middelburg, J. J.; Melack, J. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems. 2007, 10: 172–185. S2CID 1728636. doi:10.1007/s10021-006-9013-8.
- ^ 63.0 63.1 Raymond, Peter A.; Hartmann, Jens; Lauerwald, Ronny; Sobek, Sebastian; McDonald, Cory; Hoover, Mark; Butman, David; Striegl, Robert; Mayorga, Emilio; Humborg, Christoph; Kortelainen, Pirkko; Dürr, Hans; Meybeck, Michel; Ciais, Philippe; Guth, Peter. Global carbon dioxide emissions from inland waters. Nature. 2013, 503 (7476): 355–359. Bibcode:2013Natur.503..355R. PMID 24256802. S2CID 4460910. doi:10.1038/nature12760.
- ^ Ward, Nicholas D.; Keil, Richard G.; Medeiros, Patricia M.; Brito, Daimio C.; Cunha, Alan C.; Dittmar, Thorsten; Yager, Patricia L.; Krusche, Alex V.; Richey, Jeffrey E. Degradation of terrestrially derived macromolecules in the Amazon River. Nature Geoscience. 2013, 6 (7): 530–533. Bibcode:2013NatGe...6..530W. doi:10.1038/ngeo1817.
- ^ Myers-Pigg, Allison N.; Louchouarn, Patrick; Amon, Rainer M. W.; Prokushkin, Anatoly; Pierce, Kayce; Rubtsov, Alexey. Labile pyrogenic dissolved organic carbon in major Siberian Arctic rivers: Implications for wildfire-stream metabolic linkages. Geophysical Research Letters. 2015, 42 (2): 377–385. Bibcode:2015GeoRL..42..377M. doi:10.1002/2014GL062762
 .
.
- ^ Tranvik, Lars J.; Downing, John A.; Cotner, James B.; Loiselle, Steven A.; Striegl, Robert G.; Ballatore, Thomas J.; Dillon, Peter; Finlay, Kerri; Fortino, Kenneth; Knoll, Lesley B.; Kortelainen, Pirkko L.; Kutser, Tiit; Larsen, Soren.; Laurion, Isabelle; Leech, Dina M.; McCallister, S. Leigh; McKnight, Diane M.; Melack, John M.; Overholt, Erin; Porter, Jason A.; Prairie, Yves; Renwick, William H.; Roland, Fabio; Sherman, Bradford S.; Schindler, David W.; Sobek, Sebastian; Tremblay, Alain; Vanni, Michael J.; Verschoor, Antonie M.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnology and Oceanography. 2009, 54 (6part2): 2298–2314. Bibcode:2009LimOc..54.2298T. doi:10.4319/lo.2009.54.6_part_2.2298
 .
.
- ^ Bastviken, David; Cole, Jonathan; Pace, Michael; Tranvik, Lars. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Global Biogeochemical Cycles. 2004, 18 (4): n/a. Bibcode:2004GBioC..18.4009B. doi:10.1029/2004GB002238
 .
.
- ^ Cooley, S. R.; Coles, V. J.; Subramaniam, A.; Yager, P. L. Seasonal variations in the Amazon plume-related atmospheric carbon sink. Global Biogeochemical Cycles. 2007, 21 (3): n/a. Bibcode:2007GBioC..21.3014C. doi:10.1029/2006GB002831
 .
.
- ^ Subramaniam, A.; Yager, P. L.; Carpenter, E. J.; Mahaffey, C.; Bjorkman, K.; Cooley, S.; Kustka, A. B.; Montoya, J. P.; Sanudo-Wilhelmy, S. A.; Shipe, R.; Capone, D. G. Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean. Proceedings of the National Academy of Sciences. 2008, 105 (30): 10460–10465. PMC 2480616
 . PMID 18647838. S2CID 8889134. doi:10.1073/pnas.0710279105
. PMID 18647838. S2CID 8889134. doi:10.1073/pnas.0710279105  .
.
- ^ 70.0 70.1 Cai, Wei-Jun. Estuarine and Coastal Ocean Carbon Paradox: CO2Sinks or Sites of Terrestrial Carbon Incineration?. Annual Review of Marine Science. 2011, 3: 123–145. Bibcode:2011ARMS....3..123C. PMID 21329201. doi:10.1146/annurev-marine-120709-142723.
- ^ Livingston, R. J. Ecological Processes in Coastal and Marine Systems. Springer. 2012-12-06. ISBN 9781461591467.
- ^ Dittmar, Thorsten; Lara, Rubén José; Kattner, Gerhard. River or mangrove? Tracing major organic matter sources in tropical Brazilian coastal waters. Marine Chemistry. 2001, 73 (3–4): 253–271. Bibcode:2001MarCh..73..253D. doi:10.1016/s0304-4203(00)00110-9.
- ^ Moore, W.S.; Beck, M.; Riedel, T.; Rutgers Van Der Loeff, M.; Dellwig, O.; Shaw, T.J.; Schnetger, B.; Brumsack, H.-J. Radium-based pore water fluxes of silica, alkalinity, manganese, DOC, and uranium: A decade of studies in the German Wadden Sea. Geochimica et Cosmochimica Acta. 2011, 75 (21): 6535–6555. Bibcode:2011GeCoA..75.6535M. doi:10.1016/j.gca.2011.08.037.
- ^ Wehrli, Bernhard. Conduits of the carbon cycle. Nature. 2013, 503 (7476): 346–347. PMID 24256800. S2CID 205079291. doi:10.1038/503346a
 .
.
- ^ Moran, Mary Ann; Kujawinski, Elizabeth B.; Stubbins, Aron; Fatland, Rob; Aluwihare, Lihini I.; Buchan, Alison; Crump, Byron C.; Dorrestein, Pieter C.; Dyhrman, Sonya T.; Hess, Nancy J.; Howe, Bill; Longnecker, Krista; Medeiros, Patricia M.; Niggemann, Jutta; Obernosterer, Ingrid; Repeta, Daniel J.; Waldbauer, Jacob R. Deciphering ocean carbon in a changing world. Proceedings of the National Academy of Sciences. 2016, 113 (12): 3143–3151. Bibcode:2016PNAS..113.3143M. PMC 4812754
 . PMID 26951682. S2CID 10255391. doi:10.1073/pnas.1514645113
. PMID 26951682. S2CID 10255391. doi:10.1073/pnas.1514645113  .
.
- ^ 76.0 76.1 76.2 Gao, Yang; Jia, Junjie; Lu, Yao; Sun, Kun; Wang, Jing; Wang, Shuoyue. Carbon transportation, transformation, and sedimentation processes at the land-river-estuary continuum. Fundamental Research (Elsevier BV). 2022. ISSN 2667-3258. S2CID 251168582. doi:10.1016/j.fmre.2022.07.007
 .
.  Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ^ Schlünz, B.; Schneider, R. R. Transport of terrestrial organic carbon to the oceans by rivers: re-estimating flux- and burial rates. International Journal of Earth Sciences (Springer Science and Business Media LLC). 2000-03-22, 88 (4): 599–606. Bibcode:2000IJEaS..88..599S. ISSN 1437-3254. S2CID 128411658. doi:10.1007/s005310050290.
- ^ Blair, Neal E.; Leithold, Elana L.; Aller, Robert C. From bedrock to burial: The evolution of particulate organic carbon across coupled watershed-continental margin systems. Marine Chemistry. 2004, 92 (1–4): 141–156. Bibcode:2004MarCh..92..141B. doi:10.1016/j.marchem.2004.06.023.
- ^ Bouchez, Julien; Beyssac, Olivier; Galy, Valier; Gaillardet, Jérôme; France-Lanord, Christian; Maurice, Laurence; Moreira-Turcq, Patricia. Oxidation of petrogenic organic carbon in the Amazon floodplain as a source of atmospheric CO2. Geology (Geological Society of America). 2010, 38 (3): 255–258. Bibcode:2010Geo....38..255B. ISSN 1943-2682. S2CID 53512466. doi:10.1130/g30608.1.
- ^ Regnier, Pierre; Friedlingstein, Pierre; Ciais, Philippe; Mackenzie, Fred T.; et al. Anthropogenic perturbation of the carbon fluxes from land to ocean (PDF). Nature Geoscience (Springer Science and Business Media LLC). 2013-06-09, 6 (8): 597–607. Bibcode:2013NatGe...6..597R. ISSN 1752-0894. S2CID 53418968. doi:10.1038/ngeo1830.
- ^ 81.0 81.1 Bauer, James E.; Cai, Wei-Jun; Raymond, Peter A.; Bianchi, Thomas S.; Hopkinson, Charles S.; Regnier, Pierre A. G. The changing carbon cycle of the coastal ocean. Nature (Springer Science and Business Media LLC). 2013-12-04, 504 (7478): 61–70. Bibcode:2013Natur.504...61B. ISSN 0028-0836. PMID 24305149. S2CID 4399374. doi:10.1038/nature12857.
- ^ Cai, Wei-Jun. Estuarine and Coastal Ocean Carbon Paradox: CO2 Sinks or Sites of Terrestrial Carbon Incineration?. Annual Review of Marine Science (Annual Reviews). 2011-01-15, 3 (1): 123–145. Bibcode:2011ARMS....3..123C. ISSN 1941-1405. PMID 21329201. doi:10.1146/annurev-marine-120709-142723.
- ^ Galy, Valier; Peucker-Ehrenbrink, Bernhard; Eglinton, Timothy. Global carbon export from the terrestrial biosphere controlled by erosion. Nature (Springer Science and Business Media LLC). 2015, 521 (7551): 204–207. Bibcode:2015Natur.521..204G. ISSN 0028-0836. PMID 25971513. S2CID 205243485. doi:10.1038/nature14400.
- ^ Sigman DM & GH Haug. 2006. The biological pump in the past. In: Treatise on Geochemistry; vol. 6, (ed.). Pergamon Press, pp. 491–528
- ^ Sanders, Richard; Henson, Stephanie A.; Koski, Marja; de la Rocha, Christina L.; Painter, Stuart C.; Poulton, Alex J.; Riley, Jennifer; Salihoglu, Baris; Visser, Andre; Yool, Andrew; Bellerby, Richard; Martin, Adrian P. The Biological Carbon Pump in the North Atlantic. Progress in Oceanography. 2014, 129: 200–218. Bibcode:2014PrOce.129..200S. doi:10.1016/j.pocean.2014.05.005.
- ^ Boyd, Philip W. Toward quantifying the response of the oceans' biological pump to climate change. Frontiers in Marine Science. 2015, 2. S2CID 16787695. doi:10.3389/fmars.2015.00077
 .
.
- ^ Basu, Samarpita; MacKey, Katherine. Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate. Sustainability. 2018, 10 (3): 869. doi:10.3390/su10030869
 .
.
- ^ Steinberg, Deborah; Goldthwait, Sarah; Hansell, Dennis. Zooplankton vertical migration and the active transport of dissolved organic and inorganic nitrogen in the Sargasso Sea. Deep-Sea Research Part I. 2002, 49 (8): 1445–1461. Bibcode:2002DSRI...49.1445S. CiteSeerX 10.1.1.391.7622
 . ISSN 0967-0637. doi:10.1016/S0967-0637(02)00037-7.
. ISSN 0967-0637. doi:10.1016/S0967-0637(02)00037-7.
- ^ 89.0 89.1 Ducklow, H.W., Steinberg, D.K. and Buesseler, K.O. (2001) "Upper Ocean Carbon Export and the Biological Pump". Oceanography, 14(4): 50–58. doi:10.5670/oceanog.2001.06.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
- ^ De La Rocha C.L. (2006) "The Biological Pump". In: Treatise on Geochemistry; vol. 6, Pergamon Press, pp. 83–111.
- ^ Wong, Kevin; Mason, Emily; Brune, Sascha; East, Madison; Edmonds, Marie; Zahirovic, Sabin. Deep Carbon Cycling over the Past 200 Million Years: A Review of Fluxes in Different Tectonic Settings. Frontiers in Earth Science. 2019, 7: 263. Bibcode:2019FrEaS...7..263W. S2CID 204027259. doi:10.3389/feart.2019.00263
 .
.
- ^ The Deep Carbon Cycle and our Habitable Planet | Deep Carbon Observatory. deepcarbon.net. [2019-02-19]. (原始内容存档于2020-07-27).
- ^ Wilson, Mark. Where do Carbon Atoms Reside within Earth's Mantle?. Physics Today. 2003, 56 (10): 21–22. Bibcode:2003PhT....56j..21W. doi:10.1063/1.1628990.
- ^ Dasgupta, Rajdeep. From Magma Ocean to Crustal Recycling: Earth's Deep Carbon Cycle. 2011-12-10 [2019-03-09]. (原始内容存档于2016-04-24).
- ^ Carbon cycle reaches Earth's lower mantle: Evidence of carbon cycle found in 'superdeep' diamonds From Brazil. ScienceDaily. [2019-02-06].
- ^ Stagno, V.; Frost, D. J.; McCammon, C. A.; Mohseni, H.; Fei, Y. The oxygen fugacity at which graphite or diamond forms from carbonate-bearing melts in eclogitic rocks. Contributions to Mineralogy and Petrology. 2015-02-05, 169 (2): 16. Bibcode:2015CoMP..169...16S. ISSN 1432-0967. S2CID 129243867. doi:10.1007/s00410-015-1111-1.
- ^ 97.0 97.1 Fiquet, Guillaume; Guyot, François; Perrillat, Jean-Philippe; Auzende, Anne-Line; Antonangeli, Daniele; Corgne, Alexandre; Gloter, Alexandre; Boulard, Eglantine. New host for carbon in the deep Earth. Proceedings of the National Academy of Sciences. 2011-03-29, 108 (13): 5184–5187. Bibcode:2011PNAS..108.5184B. ISSN 0027-8424. PMC 3069163
 . PMID 21402927. doi:10.1073/pnas.1016934108
. PMID 21402927. doi:10.1073/pnas.1016934108  .
.
- ^ Dorfman, Susannah M.; Badro, James; Nabiei, Farhang; Prakapenka, Vitali B.; Cantoni, Marco; Gillet, Philippe. Carbonate stability in the reduced lower mantle (PDF). Earth and Planetary Science Letters. 2018-05-01, 489: 84–91. Bibcode:2018E&PSL.489...84D. ISSN 0012-821X. OSTI 1426861. S2CID 134119145. doi:10.1016/j.epsl.2018.02.035. (原始内容存档 (PDF)于2021-07-18).
- ^ Kelley, Katherine A.; Cottrell, Elizabeth. Redox Heterogeneity in Mid-Ocean Ridge Basalts as a Function of Mantle Source. Science. 2013-06-14, 340 (6138): 1314–1317. Bibcode:2013Sci...340.1314C. ISSN 0036-8075. PMID 23641060. S2CID 39125834. doi:10.1126/science.1233299
 .
.
- ^ Kono, Yoshio; Sanloup, Chrystèle. Magmas Under Pressure | ScienceDirect. Elsevier Science. 2018-04-10 [2019-02-07]. ISBN 9780128113011.
- ^ Mao, Wendy L.; Liu, Zhenxian; Galli, Giulia; Pan, Ding; Boulard, Eglantine. Tetrahedrally coordinated carbonates in Earth's lower mantle. Nature Communications. 2015-02-18, 6: 6311. Bibcode:2015NatCo...6.6311B. ISSN 2041-1723. PMID 25692448. S2CID 205335268. arXiv:1503.03538
 . doi:10.1038/ncomms7311.
. doi:10.1038/ncomms7311.
- ^ Carmody, Laura; Genge, Matthew; Jones, Adrian P. Carbonate Melts and Carbonatites. Reviews in Mineralogy and Geochemistry. 2013-01-01, 75 (1): 289–322. Bibcode:2013RvMG...75..289J. ISSN 1529-6466. S2CID 49365059. doi:10.2138/rmg.2013.75.10.
- ^ Dasgupta, Rajdeep; Hirschmann, Marc M. The deep carbon cycle and melting in Earth's interior. Earth and Planetary Science Letters. 2010-09-15, 298 (1): 1–13. Bibcode:2010E&PSL.298....1D. ISSN 0012-821X. doi:10.1016/j.epsl.2010.06.039.
- ^ Frost, Daniel J.; McCammon, Catherine A. The Redox State of Earth's Mantle. Annual Review of Earth and Planetary Sciences. 2008, 36: 389–420. Bibcode:2008AREPS..36..389F. doi:10.1146/annurev.earth.36.031207.124322.
- ^ Does Earth's Core Host a Deep Carbon Reservoir? | Deep Carbon Observatory. deepcarbon.net. [2019-03-09]. (原始内容存档于27 July 2020).
- ^ Li, Jie; Chow, Paul; Xiao, Yuming; Alp, E. Ercan; Bi, Wenli; Zhao, Jiyong; Hu, Michael Y.; Liu, Jiachao; Zhang, Dongzhou. Hidden carbon in Earth's inner core revealed by shear softening in dense Fe7C3. Proceedings of the National Academy of Sciences. 2014-12-16, 111 (50): 17755–17758. Bibcode:2014PNAS..11117755C. ISSN 0027-8424. PMC 4273394
 . PMID 25453077. doi:10.1073/pnas.1411154111
. PMID 25453077. doi:10.1073/pnas.1411154111  .
.
- ^ Li, Jie; Chow, Paul; Xiao, Yuming; Alp, E. Ercan; Bi, Wenli; Zhao, Jiyong; Hu, Michael Y.; Liu, Jiachao; Zhang, Dongzhou. Hidden carbon in Earth's inner core revealed by shear softening in dense Fe7C3. Proceedings of the National Academy of Sciences. 2014-12-16, 111 (50): 17755–17758. Bibcode:2014PNAS..11117755C. ISSN 0027-8424. PMC 4273394
 . PMID 25453077. doi:10.1073/pnas.1411154111
. PMID 25453077. doi:10.1073/pnas.1411154111  .
.
- ^ Overview of greenhouse gases. U.S. Environmental Protection Agency. 2015-12-23 [2020-11-02].
- ^ 109.0 109.1 The known unknowns of plastic pollution. The Economist. 2018-03-03 [2018-06-17].
- ^ Overview of greenhouse gases. U.S. Environmental Protection Agency. 2015-12-23 [2020-11-02].
- ^ 111.0 111.1 111.2 Lade, Steven J.; Donges, Jonathan F.; Fetzer, Ingo; Anderies, John M.; Beer, Christian; Cornell, Sarah E.; Gasser, Thomas; Norberg, Jon; Richardson, Katherine; Rockström, Johan; Steffen, Will. Analytically tractable climate–carbon cycle feedbacks under 21st century anthropogenic forcing. Earth System Dynamics. 2018, 9 (2): 507–523. Bibcode:2018ESD.....9..507L. doi:10.5194/esd-9-507-2018
 .
.  Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License 互联网档案馆的存檔,存档日期2017-10-16..
- ^ Takahashi, Taro; Sutherland, Stewart C.; Sweeney, Colm; Poisson, Alain; Metzl, Nicolas; Tilbrook, Bronte; Bates, Nicolas; Wanninkhof, Rik; Feely, Richard A.; Sabine, Christopher; Olafsson, Jon; Nojiri, Yukihiro. Global sea–air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Research Part II: Topical Studies in Oceanography. 2002, 49 (9–10): 1601–1622. Bibcode:2002DSRII..49.1601T. doi:10.1016/S0967-0645(02)00003-6.
- ^ Orr, James C.; Fabry, Victoria J.; Aumont, Olivier; Bopp, Laurent; Doney, Scott C.; Feely, Richard A.; Gnanadesikan, Anand; Gruber, Nicolas; Ishida, Akio; Joos, Fortunat; Key, Robert M.; Lindsay, Keith; Maier-Reimer, Ernst; Matear, Richard; Monfray, Patrick; Mouchet, Anne; Najjar, Raymond G.; Plattner, Gian-Kasper; Rodgers, Keith B.; Sabine, Christopher L.; Sarmiento, Jorge L.; Schlitzer, Reiner; Slater, Richard D.; Totterdell, Ian J.; Weirig, Marie-France; Yamanaka, Yasuhiro; Yool, Andrew. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms (PDF). Nature. 2005, 437 (7059): 681–686. Bibcode:2005Natur.437..681O. PMID 16193043. S2CID 4306199. doi:10.1038/nature04095. (原始内容存档 (PDF)于2019-04-26).
- ^ Le Quéré, Corinne; Andrew, Robbie M.; Canadell, Josep G.; Sitch, Stephen; Korsbakken, Jan Ivar; Peters, Glen P.; Manning, Andrew C.; Boden, Thomas A.; Tans, Pieter P.; Houghton, Richard A.; Keeling, Ralph F.; Alin, Simone; Andrews, Oliver D.; Anthoni, Peter; Barbero, Leticia; Bopp, Laurent; Chevallier, Frédéric; Chini, Louise P.; Ciais, Philippe; Currie, Kim; Delire, Christine; Doney, Scott C.; Friedlingstein, Pierre; Gkritzalis, Thanos; Harris, Ian; Hauck, Judith; Haverd, Vanessa; Hoppema, Mario; Klein Goldewijk, Kees; et al. Global Carbon Budget 2016. Earth System Science Data. 2016, 8 (2): 605–649. Bibcode:2016ESSD....8..605L. doi:10.5194/essd-8-605-2016
 .
.
- ^ Intergovernmental Panel On Climate Change (编). Carbon and Other Biogeochemical Cycles. Climate Change 2013 - the Physical Science Basis. Cambridge University Press. 2014: 465–570. ISBN 9781107415324. doi:10.1017/CBO9781107415324.015. hdl:11858/00-001M-0000-0023-E34E-5.
- ^ Joos, F.; Roth, R.; Fuglestvedt, J. S.; Peters, G. P.; Enting, I. G.; von Bloh, W.; Brovkin, V.; Burke, E. J.; Eby, M.; Edwards, N. R.; Friedrich, T.; Frölicher, T. L.; Halloran, P. R.; Holden, P. B.; Jones, C.; Kleinen, T.; MacKenzie, F. T.; Matsumoto, K.; Meinshausen, M.; Plattner, G.-K.; Reisinger, A.; Segschneider, J.; Shaffer, G.; Steinacher, M.; Strassmann, K.; Tanaka, K.; Timmermann, A.; Weaver, A. J. Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis. Atmospheric Chemistry and Physics. 2013, 13 (5): 2793–2825. Bibcode:2013ACP....13.2793J. doi:10.5194/acp-13-2793-2013
 .
.
- ^ Hausfather, Zeke; Betts, Richard. Analysis: How 'carbon-cycle feedbacks' could make global warming worse. Carbon Brief. 2020-04-14 [2022-01-04]. (原始内容存档于2020-04-16) (英语).
- ^ IPCC (2007) 7.4.5 Minerals 互联网档案馆的存檔,存档日期2016-05-25. in Climate Change 2007: Working Group III: Mitigation of Climate Change,
- ^ Buis, Alan; Ramsayer, Kate; Rasmussen, Carol. A Breathing Planet, Off Balance. NASA. 2015-11-12 [2015-11-13]. (原始内容存档于2015-11-14). 已忽略未知参数
|df=(帮助) - ^ Audio (66:01) - NASA News Conference - Carbon & Climate Telecon. NASA. 2015-11-12 [2015-11-12]. (原始内容存档于2015-11-17). 已忽略未知参数
|df=(帮助) - ^ St. Fleur, Nicholas. Atmospheric Greenhouse Gas Levels Hit Record, Report Says. The New York Times. 2015-11-10 [2015-11-11]. (原始内容存档于2015-11-11). 已忽略未知参数
|df=(帮助) - ^ Ritter, Karl. UK: In 1st, global temps average could be 1 degree C higher. AP News. 2015-11-09 [2015-11-11]. (原始内容存档于2015-11-17). 已忽略未知参数
|df=(帮助) - ^ 123.0 123.1 Morse, John W.; Morse, John W. Autor; Morse, John W.; MacKenzie, F. T.; MacKenzie, Fred T. Chapter 9 the Current Carbon Cycle and Human Impact. Geochemistry of Sedimentary Carbonates. Developments in Sedimentology 48. 1990: 447–510. ISBN 9780444873910. doi:10.1016/S0070-4571(08)70338-8.
- ^ Figure 8.SM.4 (PDF). Intergovernmental Panel on Climate Change Fifth Assessment Report. : 8SM-16. (原始内容存档 (PDF)于2019-03-13).
- ^ Archer, David. Atmospheric lifetime of fossil fuel carbon dioxide. Annual Review of Earth and Planetary Sciences. 2009, 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:10.1146/annurev.earth.031208.100206. hdl:2268/12933.
- ^ Joos, F.; Roth, R.; Fuglestvedt, J.D.; et al. Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis. Atmospheric Chemistry and Physics. 2013, 13 (5): 2793–2825. doi:10.5194/acpd-12-19799-2012
 . hdl:20.500.11850/58316
. hdl:20.500.11850/58316  .
.
- ^ Butler, J.; Montzka, S. The NOAA Annual Greenhouse Gas Index (AGGI). NOAA Global Monitoring Laboratory/Earth System Research Laboratories. 2020.
- ^ Sciance, Fred. The Transition from HFC- 134a to a Low -GWP Refrigerant in Mobile Air Conditioners HFO -1234yf (PDF). General Motors Public Policy Center. 2013-10-29 [2018-08-01]. (原始内容存档 (PDF)于2015-10-15).
外部連結
- Carbon Cycle Science Program – an interagency partnership.
- NOAA's Carbon Cycle Greenhouse Gases Group
- Global Carbon Project – initiative of the Earth System Science Partnership
- UNEP – The present carbon cycle – Climate Change 互联网档案馆的存檔,存档日期2008-09-15. carbon levels and flows
- NASA's Orbiting Carbon Observatory 互联网档案馆的存檔,存档日期2018-09-09.
| ||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||