重症肌無力:修订间差异
Karrie look(留言 | 贡献) |
Karrie look(留言 | 贡献) 无编辑摘要 |
||
| 第123行: | 第123行: | ||
===重肌無力症的病理學研究結果Pathological findings (4).(7)=== |
===重肌無力症的病理學研究結果Pathological findings (4).(7)=== |
||
Muscle biopsy is only performed if the diagnosis is in doubt and a muscular condition is suspected. [[Immunofluorescence]] shows [[IgG]] antibodies on the neuromuscular junction. (Note that it is not the antibody which causes myasthenia gravis that fluoresces, but rather a [[secondary antibody]] directed against it.) Muscle electron microscopy shows receptor infolding and loss of the tips of the folds, together with widening of the [[Chemical synapse|synaptic]] clefts. Both these techniques are currently used for research rather than diagnostically.<ref name="Losen">{{cite journal | author=Losen M, Stassen MH, Martínez-Martínez P, et al. | title=Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis | journal=Brain | year=2005 | volume=128 | pages=2327–37 | pmid=16150851 | doi=10.1093/brain/awh612 }}</ref> |
Muscle biopsy is only performed if the diagnosis is in doubt and a muscular condition is suspected. [[Immunofluorescence]] shows [[IgG]] antibodies on the neuromuscular junction. (Note that it is not the antibody which causes myasthenia gravis that fluoresces, but rather a [[secondary antibody]] directed against it.) Muscle electron microscopy shows receptor infolding and loss of the tips of the folds, together with widening of the [[Chemical synapse|synaptic]] clefts. Both these techniques are currently used for research rather than diagnostically.<ref name="Losen">{{cite journal | author=Losen M, Stassen MH, Martínez-Martínez P, et al. | title=Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis | journal=Brain | year=2005 | volume=128 | pages=2327–37 | pmid=16150851 | doi=10.1093/brain/awh612 }}</ref> |
||
==Treatment== |
|||
Treatment is by medication and/or surgery. Medication consists mainly of [[cholinesterase inhibitors]] to directly improve muscle function and [[immunosuppressant drug]]s to reduce the autoimmune process. [[Thymectomy]] is a surgical method to treat MG. For emergency treatment, [[plasmapheresis]] or [[IVIG]] can be used as a temporary measure to remove antibodies from the blood circulation. |
|||
===Medication=== |
|||
[[Image:Neostigmine.svg|thumb|Neostigmine, chemical structure.]] |
|||
* [[Acetylcholinesterase inhibitor]]s: [[neostigmine]] and [[pyridostigmine]] can improve muscle function by slowing the natural enzyme [[cholinesterase]] that degrades [[acetylcholine]] in the motor end plate; the neurotransmitter is therefore around longer to stimulate its receptor. Usually doctors will start with a low dose, eg 3x20mg pyridostigmine, and increase until the desired result is achieved. If taken 30 minutes before a meal, symptoms will be mild during eating. Side effects, like perspiration and diarrhea can be countered by adding [[atropine]]. Pyridostigmine is a short-lived drug with a half-life of about 4 hours. |
|||
[[Image:Azathioprine.svg|thumb|Azathioprine, chemical structure.]] |
|||
* [[Immunosuppressive drugs]]: [[prednisone]], [[cyclosporine]], [[mycophenolate mofetil]] and [[azathioprine]] may be used. It is common for patients to be treated with a combination of these drugs with a cholinesterase inhibitor. Treatments with some immunosuppressives take weeks to months before effects are noticed. Other immunomodulating substances, like drugs preventing acetylcholine receptor modulation by the immune system are currently being researched<ref name=Losen2008>{{cite journal |author=Losen M, Martínez-Martínez P, Phernambucq M, Schuurman J, Parren PW, DE Baets MH |title=Treatment of myasthenia gravis by preventing acetylcholine receptor modulation |journal=Ann N Y Acad Sci |volume=1132 |pages=174–9|year=2008 |pmid=18567867 |doi=10.1196/annals.1405.034}}</ref> |
|||
===Plasmapheresis and IVIG=== |
|||
If the myasthenia is serious (myasthenic crisis), [[plasmapheresis]] can be used to remove the putative antibody from the circulation. Also, [[Intravenous immunoglobulin]]s (IVIG) can be used to bind the circulating antibodies. Both of these treatments have relatively short-lived benefits, typically measured in weeks.<ref name=Juel2004>{{cite journal |author=Juel VC. |title=Myasthenia gravis: management of myasthenic crisis and perioperative care. |journal=Semin Neurol |volume=24 |issue=1 |pages=75–81 |year=2004|pmid=15229794 |doi=10.1055/s-2004-829595}}</ref> |
|||
===Surgery=== |
|||
{{main|thymectomy}} |
|||
Thymectomy, the surgical removal of the [[thymus]], is essential in cases of [[thymoma]] in view of the potential neoplastic effects of the tumor. However, the procedure is more controversial in patients who do not show thymic abnormalities. Although some of these patients improve following thymectomy, some patients experience severe exacerbations and the highly controversial concept of "therapeutic thymectomy" for patients with thymus hyperplasia is disputed by many experts and efforts are underway to unequivocally answer this important question. |
|||
There are a number of surgical approaches to the removal of the thymus gland: transsternal (through the [[sternum]], or breast bone), transcervical (through a small neck incision), and transthoracic (through one or both sides of the chest). The transsternal approach is most common and uses the same length-wise incision through the sternum (breast bone)used for most open-heart surgery. The transcervical approach is a less invasive procedure that allows for removal of the entire thymus gland through a small neck incision. There has been no difference in success in symptom improvement between the transsternal approach and the minimally invasive transcervical approach.<ref name=Calhoun_1999>{{cite journal |author=Calhoun R, et al. |title=Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients. |journal=Annals of Surgery |volume=230 |issue=4 |pages=555–561 |year=1999 |pmid=10522725 |doi=10.1097/00000658-199910000-00011}}</ref> However for patients with a thymoma it is important that all the tissue is removed as thymic tissue can regrow. Thymomas can be malignant and are thought to be the onset of other diseases as well. For this reason, many surgeons will only recommend the full sternotomy approach to a thymectomy. |
|||
Thymoma is relatively rare in younger (<40) patients, but paradoxically especially younger patients with generalized MG without thymoma benefit from thymectomy. Of course, resection is also indicated for those with a thymoma, but it is less likely to improve the MG symptoms. |
|||
==Prognosis== |
|||
With treatment, patients have a normal life expectancy, except for those with a malignant [[thymoma]] (whose lesser life expectancy is on account of the thymoma itself and is otherwise unrelated to the myasthenia). Quality of life can vary depending on the severity and the cause. The drugs used to control MG either diminish in effectiveness over time ([[cholinesterase|cholinesterase inhibitors]]) or cause severe side effects of their own ([[Immunosuppressive drug|immunosuppressants]]). A small percentage (around 10%) of MG patients are found to have tumors in their [[thymus]] glands, in which case a [[thymectomy]] is a very effective treatment with long-term remission. However, most patients need treatment for the remainder of their lives, and their abilities vary greatly. It should be noted that MG is not usually a progressive disease. The symptoms may come and go, but the symptoms do not always get worse as the patient ages. For some, the symptoms decrease after a span of 3–5 years. |
|||
==Epidemiology流行病學 & MG in children== |
|||
Myasthenia gravis occurs in all ethnic groups and both genders. It most commonly affects women under 40 - and people from 50 to 70 years old of either sex, but it has been known to occur at any age. Younger patients rarely have thymoma. The prevalence in the [[United States]] is estimated at 20 cases per 100,000.<ref name="MGFA">{{cite web | title=What is Myasthenia Gravis (MG)? | url=http://www.myasthenia.org/amg_whatismg.cfm | publisher=Myasthenia Gravis Foundation of America}}</ref> Risk factors are the female gender with ages 20 – 40, familial myasthenia gravis, D-penicillamine ingestion (drug induced myasthenia), and having other autoimmune diseases. |
|||
Three types of myasthenic symptoms in children can be distinguished:<ref name="Baets"/> |
|||
# Neonatal: In 12% of the pregnancies with a mother with MG, she passes the antibodies to the infant through the [[placenta]] causing neonatal myasthenia gravis. The symptoms will start in the first two days and disappear within a few weeks after birth. With the mother it is not uncommon for the symptoms to even improve during pregnancy, but they might worsen after labor. |
|||
# Congenital: Children of a healthy mother can, very rarely, develop myasthenic symptoms beginning at birth. This is called [[congenital myasthenic syndrome]] or CMS. Other than Myasthenia gravis, CMS is not caused by an autoimmune process, but due to synaptic malformation, which in turn is caused by genetic [[mutations]]. Thus, CMS is a [[hereditary disease]]. More than 11 different mutations have been identified and the inheritance pattern is typically [[recessive gene|autosomal recessive]]. |
|||
# Juvenile myasthenia gravis: myasthenia occurring in childhood but after the peripartum period. |
|||
The congenital myasthenias cause muscle weakness and fatigability similar to those of MG. The symptoms of CMS usually begin within the first two years of life, although in a few forms patients can develop their first symptoms as late as the seventh decade of life. A diagnosis of CMS is suggested by the following: |
|||
* Onset of symptoms in infancy or childhood. |
|||
* Weakness which increases as muscles tire. |
|||
* A decremental EMG response, on low frequency, of the compound muscle action potential (CMAP). |
|||
* No anti-AChR or MuSK antibodies. |
|||
* No response to immunosuppressant therapy. |
|||
* Family history of symptoms which resemble CMS. |
|||
The symptoms of CMS can vary from mild to severe. It is also common for patients with the same form, even members of the same family, to be affected to differing degrees. In most forms of CMS weakness does not progress, and in some forms, the symptoms may diminish as the patient gets older. Only rarely do symptoms of CMS become worse with time. |
|||
==In pregnancy== |
|||
In the long term, [[pregnancy]] does not affect myasthenia gravis. Up to 10% of infants with parents affected by the condition are born with transient (periodic) [[neonatal]] myasthenia (TNM) which generally produces feeding and [[Respiratory system|respiratory]] difficulties.<ref name="OTM3">{{cite book |title=Oxford Texbook of Medicine — Fourth Edition — Volume 3 |last=Warrell |first=David A |coauthors=Timothy M Cox, ''et al.'' |year=2003 |publisher=Oxford |isbn=0-19852787-X| pages=1170}}</ref> TNM usually presents as poor [[sucking]] and generalized [[hypotonia]] (low muscle tone). Other reported symptoms include a weak cry, facial [[diplegia]] (paralysis of one part of the body) or [[paresis]] (impaired or lack of movement) and mild respiratory distress. A child with TNM typically responds very well to [[acetylcholinesterase inhibitor]]s. The mothers themselves suffer from exasperated myasthenia in a third of cases and for those who it does worsen, it usually occurs in the [[first trimester]] of pregnancy. Signs and symptoms in pregnant mothers tend to improve during the [[second trimester|second]] and [[third trimester]]. Complete [[remission]] can occur in some mothers.<ref name="tellez">{{cite journal | author = Téllez-Zenteno JF, Hernández-Ronquillo L, Salinas V, Estanol B, da Silva O | title = Myasthenia gravis and pregnancy: clinical implications and neonatal outcome | journal = BMC Musculoskeletal Disorders | volume = 5 | issue = | pages = 42 | year = 2004 | pmid = 15546494 | pmc = 534111 | doi = 10.1186/1471-2474-5-42 | url = http://www.biomedcentral.com/1471-2474/5/42| accessdate = 2008-07-10}}</ref> [[Immunosuppressive therapy]] should be maintained throughout pregnancy as this reduces the chance of neonatal muscle weakness, as well as controlling the mother's myasthenia.<ref name="OTM3"/> |
|||
Very rarely, an infant can be born with [[arthrogryposis multiplex congenita]], secondary to profound intrauterine weakness. This is due to maternal [[Antibody|antibodies]] that target an infant's [[acetylcholine]] receptors. In some cases, the mother remains [[asymptomatic]].<ref name="OTM3"/> |
|||
==參考資料== |
|||
===名人=== |
|||
*[[Augustus Pablo]], [[reggae]] musician. Died May 18, 1999 due to a collapsed lung and had suffered from the disease for some time. |
|||
*[[Suzanne Rogers]], [[Emmy]] award winning daytime television actress; plays [[Maggie Horton]] on ''[[Days of our Lives]]''. Diagnosed in 1984, but currently in remission; her condition was dramatized on the series as her character was shown to be suffering from it as well. |
|||
*[[John Spencer]], World professional snooker champion 1969, 1971 and 1977. Double vision, associated with the disease, effectively ended his career in the mid 1980s. |
|||
*[[Vijay Tendulkar]], A renowned Indian playwright; died May 19, 2008 due to complications arising out of Myasthenia gravis. |
|||
*[[Aristotle Onassis]]. |
|||
*[[Brandon Cox]]- Starting Auburn QB from 2005-2007. Finished with a record of 29-9. |
|||
*[[Madame Web]], a fictional character from the [[Spider-Man]] comics and other media. |
|||
*[[Amitabh Bachchan]], Bollywood superstar, Star of Millennium (voted on BBC). |
|||
*[[Christopher Robin Milne]], 1920-1996, of Winnie-the-Pooh fame and son of author A.A. Milne. |
|||
* [[Mary Broadfoot Walker]], British physician who first discovered the effectiveness of physostigmine in the treatment of myasthenia gravis. |
|||
===外部連結=== |
|||
* [http://www.myasthenia.org The Myasthenia Gravis Foundation of America] |
|||
* [http://www.mgauk.org The Myasthenia Gravis Association (MGA) in the United Kingdom & the Republic of Ireland] |
|||
* [http://www.mgcc-ccmg.org The Myasthenia Gravis Coalition of Canada] |
|||
{{Diseases of myoneural junction and muscle}} |
|||
{{Autoimmune diseases}} |
|||
[[Category:Autoimmune diseases]] |
|||
[[Category:Neurology]] |
|||
[[bg:Миастения гравис]] |
|||
[[ca:Miastènia greu]] |
|||
[[cs:Myasthenia gravis]] |
|||
[[de:Myasthenia gravis]] |
|||
[[es:Miastenia gravis]] |
|||
[[fa:میاستنی گراویس]] |
|||
[[fr:Myasthénie]] |
|||
[[it:Miastenia gravis]] |
|||
[[lt:Sunkioji miastenija]] |
|||
[[hu:Myasthenia gravis]] |
|||
[[nl:Myasthenia gravis]] |
|||
[[ja:重症筋無力症]] |
|||
[[no:Myasthenia gravis]] |
|||
[[pl:Miastenia]] |
|||
[[pt:Miastenia grave]] |
|||
[[ru:Миастения]] |
|||
[[sq:Miastenia gravis]] |
|||
[[sk:Ťažká myasténia]] |
|||
[[sv:Myasthenia gravis]] |
|||
[[zea:Myasthenia Gravis]] |
|||
[[zh:重症肌無力]] |
|||
2009年6月21日 (日) 12:28的版本
重肌無力症,中國內地稱為「重症肌無力」,醫學學名為Myasthenia Gravis,簡稱M.G.。
此乃神經肌的疾病引致肌肉顫動、軟弱及容易疲勞。這是一種自我免疫系統的紊亂,它會阻礙抗體的循環,阻塞乙醯膽素感受體 (acetylcholine receptors)在突觸後神經肌的接合點(post-synaptic neuromuscular junction)。此症會禁止神經傳送素乙醯膽素(neurotransmitter acetylcholine)而引致的刺激效果。
重肌無力症在醫學上會使用膽素酯脢抗化劑(cholinesterase inhibitors)或抑制免疫力的藥劑(immuno-suppressants)及有些個案會使用thymectomy藥劑。而每1,000,000個個案中,有200–400個個案能減少自我免疫系統的紊亂。
重肌無力症的分類
重肌無力症廣泛被接受的分類是以美國臨床分類為基礎:
- 第一類:出現眼肌軟弱或疲勞情況,眼皮可能下垂(ptosis),並沒其他證據顯示身體其他地方出現肌肉軟弱或疲勞。
- 第二類:眼肌軟弱或疲勞情況較嚴重,其他肌肉軟弱或疲勞情況溫和。
- 第2A類:主要在肢體或軸向肌肉。
- 第2B類:主要在球莖(bulbar)及/或 呼吸肌肉。
- 第三類:眼肌軟弱或疲勞情況嚴重及其他肌肉出現軟弱情況。
- 第3A類:主要在肢體或軸向肌肉。
- 第3B類:主要在球莖(bulbar)及/或 呼吸肌肉。
- 第四類:眼肌軟弱或疲勞情況嚴重及其他肌肉軟弱情況嚴重。
- 第4A類:主要在肢體或軸向肌肉。
- 第4B類:主要在球莖(bulbar)及/或 呼吸肌肉。(需要以喉管進食但不需要插喉)
- 第五類:需要插喉以維持呼吸。
重肌無力症的徵狀
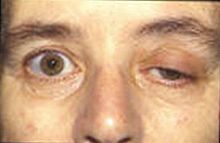
重肌無力症的徵狀是容易疲勞。肌肉會在活動期間逐漸變得軟弱,經過一段時間的休息便能恢復。肌肉能夠控制眼睛及眼皮的運動,及面部表情。 咀嚼及吞嚥、清楚的發音/談話技巧都是特別容易受影響。控制呼吸的肌肉、脖子和肢體運動都會受到影響。定期的身體檢查能保持身體於正常的極限內。
人類的免疫系統的紊亂起源可以是突然的。紊亂的症狀通常是斷斷續續的。如果症狀是微妙或易變的話,會引致延遲診斷。
在許多個案中,首先令人注意到的症狀是眼部肌肉的軟弱。在許多個案中,眼皮肌肉容易疲勞和軟弱就是首個令人注目的徵狀。另外,吞嚥困難和言語不清也許是第一個徵兆。在不同的病人中肌肉無力的程度有著很大的差異,範圍能從局部的形式縮窄至眼部的肌肉(視覺的重肌無力症),到一個嚴重或廣泛到許多肌肉 - 有時包括控制呼吸的那些肌肉都會受到影響。那些症狀,在類型和嚴重性的變化,可能包括不對稱的眼皮下垂(ptosis (eyelid)) (一隻或雙眼的眼皮下垂)、複視(雙眼看到重影)。由於肌肉無力控制眼球移動,頸部、手臂、手指、手部、腿部的關係,會引致呼吸短暫而急促、斷續的講話、吞嚥困難、在面部表情上只有一個變化、說話經常出現鼻音不穩定或步履不穩的步態。
在重肌無力症危機裡,痲痺的情況會發生於呼吸肌肉,需要呼吸機協助(assisted ventilation)維持生命。如病人是的呼吸肌肉已經軟弱,傳染、發燒、藥物或治療過程的不良反應,或緊張/有壓力的情緒都也許會引發危機。即使心臟肌肉(cardiac muscle)受到不同的刺激,重肌無力症都不會對它有影響。
重肌無力症的病理 Pathophysiology
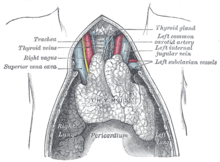
重肌無力症是一個自我免疫的channelopathy:它的特點是抗體被指揮對抗自身的蛋白質。
當各種相似的疾病與其他疾病交叉感染的媒體,這是沒有已知的起因(病原體)令人患上重肌無力症。有一個微細起源的傾向:特別的HLA類型(Human leukocyte antigen)似乎為MG預先處理 (DR1的B8及DR3為特定的視覺上的重肌無力症)。有75%患者的胸腺有異常;其餘25%有腫瘤(thymoma)(良性或惡性),及經常會找到其他異常。此症通常會在切除胸腺後保持穩定。

在重肌無力症,抗體通常會直接共同對抗菸鹼酸的乙醯膽素感受體 (Nicotinic acetylcholine receptor)(nAChR),那些馬達終板的感受體刺激神經傳送素乙醯膽素去刺激肌肉的收縮。Some forms of the antibody impair the ability of acetylcholine to bind to receptors. Others lead to the destruction of receptors, either by complement fixation or by inducing the muscle cell to eliminate the receptors through endocytosis.
The antibodies are produced by plasma cells, derived from B cells. B-cells convert into plasma cells by T-helper cell stimulation. In order to carry out this activation T-helpers must first be activated themselves, which is done by binding of the T-cell receptor (TCR) to the acetylcholine receptor antigenic peptide fragment (epitope) resting within the major histocompatibility complex of an antigen presenting cells. Since the thymus plays an important role in the development of T-cells and the selection of the TCR myasthenia gravis is closely associated with thymoma. The exact mechanisms are however not convincingly clarified although resection of the thymus (thymectomy) in MG patients without a thymus neoplasm often have positive results.
In normal muscle contraction, cumulative activation of the nAChR leads to influx of sodium ions which in turn causes depolarization of muscle cell and subsequent opening of voltage gated sodium channels. This ion influx then travels down the cell membrane via T-tubules and, via calcium channel complexes leads to the release of calcium from the sarcoplasmic reticulum. Only when the levels of calcium inside the muscle cell are high enough will it contract. Decreased numbers of functioning nAChRs therefore impairs muscular contraction by limiting depolarization. In fact, MG causes the motor neuron action potential to muscular twitch ratio to vary from the non-pathological one to one ratio.
It has recently been realized that a second category of gravis is due to auto-antibodies against the MuSK protein (muscle specific kinase), a tyrosine kinase receptor which is required for the formation of the neuromuscular junction. Antibodies against MuSK inhibit the signaling of MuSK normally induced by its nerve-derived ligand, agrin. The result is a decrease in patency of the neuromuscular junction, and the consequent symptoms of MG.
People treated with penicillamine can develop MG symptoms. Their antibody titer is usually similar to that of MG, but both the symptoms and the titer disappear when drug administration is discontinued.
MG is more common in families with other autoimmune diseases. A familial predisposition is found in 5% of the cases. This is associated with certain genetic variations such as an increased frequency of HLA-B8 and DR3. People with MG suffer from co-existing autoimmune diseases at a higher frequency than members of the general population. Of particular mention is co-existing thyroid disease where episodes of hypothyroidism may precipitate a severe exacerbation.
The acetylcholine receptor is clustered and anchored by the Rapsyn protein, research in which might eventually lead to new treatment options.[1]
重肌無力症的診斷(4)(4.1-7)
重肌無力症是很難診斷的,因為症狀是難以捉摸,和較難分別出是正常變形與其他神經紊亂。[2]
一個全面的身體檢查能顯露出病人容易疲累的症狀,當經過休息之後能改善疲累的情況,及重複和延長測試會令疲累的情況惡化。使用冰到弱的肌肉部分能有代表性地改善那部分的肌肉強度。以下所提及額外的測試會經常執行。此外,在療程中的良好反應都會被考慮為病理學中自動免疫的標記。
臨床檢查4.1
很多肌肉都能測試出肌肉疲累。[3]一個徹底的檢查包括:
- 病人的眼睛向上望和向側看30秒:眼皮下垂(ptosis)及複視(diplopia)。
- 橫臥時望向腳60秒。
- 持續把手臂伸直60秒。
- 膝彎約10次。
- 腳尖跟腳跟步行30步。
- 仰臥起坐5次,需要完全躺下及坐起來。
- "窺視標誌(Peek sign)":完成眼瞼邊緣最初的並列後,病人很快(30秒內)開始分開和眼球的鞏膜開始表現出來。[2]
血液測試4.2
如診斷是有懷疑的,血清學 (serology)會用於血液測試來鑑別某抗體:
- 有一個測試是為抗體阻擋乙醯膽素感受體(acetylcholine receptor)[2]的。這個是合理的敏感性測試。這測試的合理敏感性是 80–96%,但重肌無力症限制了眼球肌肉 (視覺的重肌無力症), 這個測試或許是消極至個案的50%。
- 病人體內沒有抗體的比例以乙醯膽素感受體的抗體抑制MuSK蛋白質。[4]
- 在特殊的情況(減少反映促進增加、現行共同的自律的特色、懷疑腫瘍或癌的出現,特別是肺部,反覆的EMG測試能增加或促進其存在性)。進行肌肉委縮症候群(Lambert-Eaton syndrome)測試,並從中找到其他的抗體。
Edrophonium測試4.4

Edrophonium測試是很少用以確認重肌無力症的存在。當其他調查不能確定的診斷時,情況會限制了它的應用情況。 這個測試要求edrophonium 氯化物 (Tensilon, Reversol)於靜脈內intravenous administration or neostigmine (Prostigmin), drugs that block the breakdown of acetylcholine by cholinesterase and temporarily increases the levels of acetylcholine at the neuromuscular junction. In people with myasthenia gravis involving the eye muscles, edrophonium chloride will briefly relieve weakness.[5]
Imaging 4.5

A chest X-ray is frequently performed; it may point towards alternative diagnoses (e.g. Lambert-Eaton due to a lung tumor) and comorbidity. It may also identify widening of the mediastinum suggestive of thymoma, but computed tomography (CT) or magnetic resonance imaging (MRI) are more sensitive ways to identify thymomas, and are generally done for this reason.[6]
肺功能測試4.6
Spirometry (lung function testing) may be performed to assess respiratory function if there are concerns about a patient's ability to breathe adequately. The forced vital capacity may be monitored at intervals in order not to miss a gradual worsening of muscular weakness. Severe myasthenia may cause respiratory failure due to exhaustion of the respiratory muscles.[7]
重肌無力症的神經生理學 Neurophysiology (4.3)
Muscle fibers of patients with MG are easily fatigued, and thus do not respond as well as muscles in healthy individuals to repeated stimulation. By repeatedly stimulating a muscle with electrical impulses, the fatiguability of the muscle can be measured. This is called the repetitive nerve stimulation test. In single fiber electromyography, which is considered to be the most sensitive (although not the most specific) test for MG,[2] a thin needle electrode is inserted into a muscle to record the electric potentials of individual muscle fibers. By finding two muscle fibers belonging to the same motor unit and measuring the temporal variability in their firing patterns (i.e. their 'jitter'), the diagnosis can be made.
重肌無力症的病理學研究結果Pathological findings (4).(7)
Muscle biopsy is only performed if the diagnosis is in doubt and a muscular condition is suspected. Immunofluorescence shows IgG antibodies on the neuromuscular junction. (Note that it is not the antibody which causes myasthenia gravis that fluoresces, but rather a secondary antibody directed against it.) Muscle electron microscopy shows receptor infolding and loss of the tips of the folds, together with widening of the synaptic clefts. Both these techniques are currently used for research rather than diagnostically.[1]
Treatment
Treatment is by medication and/or surgery. Medication consists mainly of cholinesterase inhibitors to directly improve muscle function and immunosuppressant drugs to reduce the autoimmune process. Thymectomy is a surgical method to treat MG. For emergency treatment, plasmapheresis or IVIG can be used as a temporary measure to remove antibodies from the blood circulation.
Medication

- Acetylcholinesterase inhibitors: neostigmine and pyridostigmine can improve muscle function by slowing the natural enzyme cholinesterase that degrades acetylcholine in the motor end plate; the neurotransmitter is therefore around longer to stimulate its receptor. Usually doctors will start with a low dose, eg 3x20mg pyridostigmine, and increase until the desired result is achieved. If taken 30 minutes before a meal, symptoms will be mild during eating. Side effects, like perspiration and diarrhea can be countered by adding atropine. Pyridostigmine is a short-lived drug with a half-life of about 4 hours.

- Immunosuppressive drugs: prednisone, cyclosporine, mycophenolate mofetil and azathioprine may be used. It is common for patients to be treated with a combination of these drugs with a cholinesterase inhibitor. Treatments with some immunosuppressives take weeks to months before effects are noticed. Other immunomodulating substances, like drugs preventing acetylcholine receptor modulation by the immune system are currently being researched[8]
Plasmapheresis and IVIG
If the myasthenia is serious (myasthenic crisis), plasmapheresis can be used to remove the putative antibody from the circulation. Also, Intravenous immunoglobulins (IVIG) can be used to bind the circulating antibodies. Both of these treatments have relatively short-lived benefits, typically measured in weeks.[9]
Surgery
Thymectomy, the surgical removal of the thymus, is essential in cases of thymoma in view of the potential neoplastic effects of the tumor. However, the procedure is more controversial in patients who do not show thymic abnormalities. Although some of these patients improve following thymectomy, some patients experience severe exacerbations and the highly controversial concept of "therapeutic thymectomy" for patients with thymus hyperplasia is disputed by many experts and efforts are underway to unequivocally answer this important question.
There are a number of surgical approaches to the removal of the thymus gland: transsternal (through the sternum, or breast bone), transcervical (through a small neck incision), and transthoracic (through one or both sides of the chest). The transsternal approach is most common and uses the same length-wise incision through the sternum (breast bone)used for most open-heart surgery. The transcervical approach is a less invasive procedure that allows for removal of the entire thymus gland through a small neck incision. There has been no difference in success in symptom improvement between the transsternal approach and the minimally invasive transcervical approach.[10] However for patients with a thymoma it is important that all the tissue is removed as thymic tissue can regrow. Thymomas can be malignant and are thought to be the onset of other diseases as well. For this reason, many surgeons will only recommend the full sternotomy approach to a thymectomy.
Thymoma is relatively rare in younger (<40) patients, but paradoxically especially younger patients with generalized MG without thymoma benefit from thymectomy. Of course, resection is also indicated for those with a thymoma, but it is less likely to improve the MG symptoms.
Prognosis
With treatment, patients have a normal life expectancy, except for those with a malignant thymoma (whose lesser life expectancy is on account of the thymoma itself and is otherwise unrelated to the myasthenia). Quality of life can vary depending on the severity and the cause. The drugs used to control MG either diminish in effectiveness over time (cholinesterase inhibitors) or cause severe side effects of their own (immunosuppressants). A small percentage (around 10%) of MG patients are found to have tumors in their thymus glands, in which case a thymectomy is a very effective treatment with long-term remission. However, most patients need treatment for the remainder of their lives, and their abilities vary greatly. It should be noted that MG is not usually a progressive disease. The symptoms may come and go, but the symptoms do not always get worse as the patient ages. For some, the symptoms decrease after a span of 3–5 years.
Epidemiology流行病學 & MG in children
Myasthenia gravis occurs in all ethnic groups and both genders. It most commonly affects women under 40 - and people from 50 to 70 years old of either sex, but it has been known to occur at any age. Younger patients rarely have thymoma. The prevalence in the United States is estimated at 20 cases per 100,000.[11] Risk factors are the female gender with ages 20 – 40, familial myasthenia gravis, D-penicillamine ingestion (drug induced myasthenia), and having other autoimmune diseases.
Three types of myasthenic symptoms in children can be distinguished:[3]
- Neonatal: In 12% of the pregnancies with a mother with MG, she passes the antibodies to the infant through the placenta causing neonatal myasthenia gravis. The symptoms will start in the first two days and disappear within a few weeks after birth. With the mother it is not uncommon for the symptoms to even improve during pregnancy, but they might worsen after labor.
- Congenital: Children of a healthy mother can, very rarely, develop myasthenic symptoms beginning at birth. This is called congenital myasthenic syndrome or CMS. Other than Myasthenia gravis, CMS is not caused by an autoimmune process, but due to synaptic malformation, which in turn is caused by genetic mutations. Thus, CMS is a hereditary disease. More than 11 different mutations have been identified and the inheritance pattern is typically autosomal recessive.
- Juvenile myasthenia gravis: myasthenia occurring in childhood but after the peripartum period.
The congenital myasthenias cause muscle weakness and fatigability similar to those of MG. The symptoms of CMS usually begin within the first two years of life, although in a few forms patients can develop their first symptoms as late as the seventh decade of life. A diagnosis of CMS is suggested by the following:
- Onset of symptoms in infancy or childhood.
- Weakness which increases as muscles tire.
- A decremental EMG response, on low frequency, of the compound muscle action potential (CMAP).
- No anti-AChR or MuSK antibodies.
- No response to immunosuppressant therapy.
- Family history of symptoms which resemble CMS.
The symptoms of CMS can vary from mild to severe. It is also common for patients with the same form, even members of the same family, to be affected to differing degrees. In most forms of CMS weakness does not progress, and in some forms, the symptoms may diminish as the patient gets older. Only rarely do symptoms of CMS become worse with time.
In pregnancy
In the long term, pregnancy does not affect myasthenia gravis. Up to 10% of infants with parents affected by the condition are born with transient (periodic) neonatal myasthenia (TNM) which generally produces feeding and respiratory difficulties.[12] TNM usually presents as poor sucking and generalized hypotonia (low muscle tone). Other reported symptoms include a weak cry, facial diplegia (paralysis of one part of the body) or paresis (impaired or lack of movement) and mild respiratory distress. A child with TNM typically responds very well to acetylcholinesterase inhibitors. The mothers themselves suffer from exasperated myasthenia in a third of cases and for those who it does worsen, it usually occurs in the first trimester of pregnancy. Signs and symptoms in pregnant mothers tend to improve during the second and third trimester. Complete remission can occur in some mothers.[13] Immunosuppressive therapy should be maintained throughout pregnancy as this reduces the chance of neonatal muscle weakness, as well as controlling the mother's myasthenia.[12]
Very rarely, an infant can be born with arthrogryposis multiplex congenita, secondary to profound intrauterine weakness. This is due to maternal antibodies that target an infant's acetylcholine receptors. In some cases, the mother remains asymptomatic.[12]
參考資料
名人
- Augustus Pablo, reggae musician. Died May 18, 1999 due to a collapsed lung and had suffered from the disease for some time.
- Suzanne Rogers, Emmy award winning daytime television actress; plays Maggie Horton on Days of our Lives. Diagnosed in 1984, but currently in remission; her condition was dramatized on the series as her character was shown to be suffering from it as well.
- John Spencer, World professional snooker champion 1969, 1971 and 1977. Double vision, associated with the disease, effectively ended his career in the mid 1980s.
- Vijay Tendulkar, A renowned Indian playwright; died May 19, 2008 due to complications arising out of Myasthenia gravis.
- Aristotle Onassis.
- Brandon Cox- Starting Auburn QB from 2005-2007. Finished with a record of 29-9.
- Madame Web, a fictional character from the Spider-Man comics and other media.
- Amitabh Bachchan, Bollywood superstar, Star of Millennium (voted on BBC).
- Christopher Robin Milne, 1920-1996, of Winnie-the-Pooh fame and son of author A.A. Milne.
- Mary Broadfoot Walker, British physician who first discovered the effectiveness of physostigmine in the treatment of myasthenia gravis.
外部連結
- The Myasthenia Gravis Foundation of America
- The Myasthenia Gravis Association (MGA) in the United Kingdom & the Republic of Ireland
- The Myasthenia Gravis Coalition of Canada
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- ^ 1.0 1.1 Losen M, Stassen MH, Martínez-Martínez P; et al. Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain. 2005, 128: 2327–37. PMID 16150851. doi:10.1093/brain/awh612.
- ^ 2.0 2.1 2.2 2.3 Scherer K, Bedlack RS, Simel DL. Does this patient have myasthenia gravis?. JAMA. 2005, 293: 1906–14. PMID 15840866. doi:10.1001/jama.293.15.1906.
- ^ 3.0 3.1 Baets, M.H.; H.J.G.H. Oosterhuis. Myasthenia gravis. DRD Press. 1993: 158. ISBN 3805547366.
- ^ Leite MI, Jacob S, Viegas S; et al. IgG1 antibodies to acetylcholine receptors in 'seronegative' myasthenia gravis. Brain. 2008, 131 (Pt 7): 1940–52. PMC 2442426
 . PMID 18515870. doi:10.1093/brain/awn092. 已忽略未知参数
. PMID 18515870. doi:10.1093/brain/awn092. 已忽略未知参数|month=(建议使用|date=) (帮助) - ^ Seybold ME. The office Tensilon test for ocular myasthenia gravis. Arch Neurol. 1986, 43: 842–3. PMID 3729766.
- ^ de Kraker M, Kluin J, Renken N, Maat AP, Bogers AJ. CT and myasthenia gravis: correlation between mediastinal imaging and histopathological findings. Interact Cardiovasc Thorac Surg. 2005, 4: 267–71. PMID 17670406. doi:10.1510/icvts.2004.097246.
- ^ Thieben MJ, Blacker DJ, Liu PY, Harper CM Jr, Wijdicks EF. Pulmonary function tests and blood gases in worsening myasthenia gravis. Muscle Nerve. 2005, 32: 664–667. PMID 16025526. doi:10.1002/mus.20403.
- ^ Losen M, Martínez-Martínez P, Phernambucq M, Schuurman J, Parren PW, DE Baets MH. Treatment of myasthenia gravis by preventing acetylcholine receptor modulation. Ann N Y Acad Sci. 2008, 1132: 174–9. PMID 18567867. doi:10.1196/annals.1405.034.
- ^ Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care.. Semin Neurol. 2004, 24 (1): 75–81. PMID 15229794. doi:10.1055/s-2004-829595.
- ^ Calhoun R; et al. Results of transcervical thymectomy for myasthenia gravis in 100 consecutive patients.. Annals of Surgery. 1999, 230 (4): 555–561. PMID 10522725. doi:10.1097/00000658-199910000-00011.
- ^ What is Myasthenia Gravis (MG)?. Myasthenia Gravis Foundation of America.
- ^ 12.0 12.1 12.2 Warrell, David A; Timothy M Cox, et al.. Oxford Texbook of Medicine — Fourth Edition — Volume 3. Oxford. 2003: 1170. ISBN 0-19852787-X.
- ^ Téllez-Zenteno JF, Hernández-Ronquillo L, Salinas V, Estanol B, da Silva O. Myasthenia gravis and pregnancy: clinical implications and neonatal outcome. BMC Musculoskeletal Disorders. 2004, 5: 42 [2008-07-10]. PMC 534111
 . PMID 15546494. doi:10.1186/1471-2474-5-42.
. PMID 15546494. doi:10.1186/1471-2474-5-42.
