舍曲林:修订间差异
小 (GR) File renamed: File:Zoloft Sells In Mainland China.png → File:Zoloft Sales in China.png File renaming criterion #3: To correct obvious errors in file names, including misspelled [[c::en:Noun#Prop... |
无编辑摘要 |
||
| 第23行: | 第23行: | ||
| protein_bound = 98.5% |
| protein_bound = 98.5% |
||
| metabolism = [[肝脏]](主要由[[CYP2B6]]酶进行N-去甲基化)<ref name="pmid15547048">{{cite journal | author = Obach RS, Cox LM, Tremaine LM | title = Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study | journal = Drug Metab. Dispos. | volume = 33 | issue = 2 | pages = 262–70 | year = 2005 | pmid = 15547048 | doi = 10.1124/dmd.104.002428 }}</ref> |
| metabolism = [[肝脏]](主要由[[CYP2B6]]酶进行N-去甲基化)<ref name="pmid15547048">{{cite journal | author = Obach RS, Cox LM, Tremaine LM | title = Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study | journal = Drug Metab. Dispos. | volume = 33 | issue = 2 | pages = 262–70 | year = 2005 | pmid = 15547048 | doi = 10.1124/dmd.104.002428 }}</ref> |
||
| elimination_half-life = ~23-26小时 (低活性的代谢产物 |
| elimination_half-life = ~23-26小时 (低活性的代谢产物去甲基舍曲林则为66小时)<ref name="FDALabel">Sertraline FDA Label. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4006b1_06_zoloft-label.pdf</ref><ref name = "GG">Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.</ref> |
||
| excretion = 尿液 |
| excretion = 尿液 |
||
| 第89行: | 第89行: | ||
===恐慌症=== |
===恐慌症=== |
||
使用舍曲林治疗恐慌症可以减少惊恐发作并提高生活质量。<ref name="pmid109451342">{{cite journal |author = Hirschfeld RM|title = Sertraline in the treatment of anxiety disorders|journal = Depress Anxiety|volume = 11|issue = 4|pages = 139–57|year = 2000|pmid = 10945134|doi = 10.1002/1520-6394(2000)11:4<139::AID-DA1>3.0.CO;2-C}}</ref> 四个双盲实验显示出舍曲林在治疗恐慌症时效果由于安慰剂,效果取决于剂量。舍曲林可以减少80%惊恐发作的几率(安慰剂为45%),并且可以减少广泛性焦虑,提升患者的生活质量。使用舍曲林的患者报告生活质量的提高程度优于安慰剂组。<ref name="pmid109451342"/><ref name="pmid16292466">{{cite journal | author = Clayton AH, Stewart RS, Fayyad R, Clary CM | title = Sex differences in clinical presentation and response in panic disorder: pooled data from sertraline treatment studies | journal = Arch Womens Ment Health | volume = 9 | issue = 3 | pages = 151–7 | year = 2006 | pmid = 16292466 | doi = 10.1007/s00737-005-0111-y | last2 = Stewart | last3 = Fayyad | last4 = Clary }}</ref> 性别不会影响舍曲林的效果。<ref name="pmid16292466"/> 如果粗略地将舍曲林与其他抗恐慌症药物(如[[氯米帕明]]、[[丙咪嗪]]、[[氯硝西泮]]、[[阿普唑仑]]、[[氟伏沙明]]、[[帕罗西汀]])进行单独比较,那么他们的效果是接近等价。<ref name="pmid109451342"/> |
|||
===社交恐惧症=== |
===社交恐惧症=== |
||
| 第105行: | 第106行: | ||
===自杀倾向=== |
===自杀倾向=== |
||
FDA要求包括舍曲林在内的所有抗抑郁药都要用黑 |
FDA要求包括舍曲林在内的所有抗抑郁药都要用黑框警告说明抗抑郁药会增加25岁以下服用者自杀的风险。此项警告的依据为两个独立的FDA专家组的统计分析,结果显示儿童和青少年产生自杀意向和行为的几率提高两倍,18-24岁的人群则提高1.5倍。<ref name=FDA>{{cite web|author = Levenson M, Holland C| title =Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)|publisher = FDA|accessdate = 11 July 2008|url = http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4272s1-04-FDA.ppt}}</ref><ref name =FDA2>{{cite web|url = http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf|title =Clinical review: relationship between antidepressant drugs and suicidality in adults| accessdate = 11 July 2008|author = Stone MB, Jones ML|date = 17 November 2006| format =PDF|work = Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC)|publisher = FDA| pages = 11–74}}</ref><ref name =FDA3>{{cite web|url = http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf|title = Statistical Evaluation of Suicidality in Adults Treated with Antidepressants|accessdate = 11 July 2008|author = Levenson M, Holland C|date = 17 November 2006| format =PDF|work =Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC) |publisher = FDA| pages = 75–140}}</ref> |
||
在临床试验中,很少表现出自杀意向。对于上述分析,FDA综合了11种抗抑郁药针对精神病的295次试验以取得具有显著统计学意义的结果。单独来讲,舍曲林减少成人产生自杀行为的具有统计学意义的几率是37%或50%,这取决于使用的统计方法。<ref name =FDA3/><ref name =FDA2/>The authors of the FDA analysis note that "given the large number of comparisons made in this review, chance is a very plausible explanation for this difference".<ref name =FDA2/> The more complete data submitted later by the sertraline manufacturer Pfizer indicated increased suicidal behavior.<ref>{{cite web |url=http://www.fda.gov/ohrms/dockets/dockets/06n0414/06N-0414-EC32-Attach-1.pdf |title=Memorandum from Pfizer Global Pharmaceuticals Re: DOCKET: 2006N-0414 –"Suicidality data from adult antidepressant trials" Background package for December 13 Advisory Committee|accessdate=11 July 2008 |author=Pfizer Inc. |date= 30 November 2006|format=PDF |work=FDA DOCKET 2006N-0414 |publisher=FDA}}</ref> Similarly, the analysis conducted by the UK [[Medicines and Healthcare products Regulatory Agency|MHRA]] found a 50% increase of odds of suicide-related events, not reaching statistical significance, in the patients on sertraline as compared to the ones on placebo.<ref>{{cite web|url=http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymessage/con019472.pdf |title=Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants|accessdate=11 July 2008 |date=December 2004 |format=PDF |publisher=MHRA}}</ref><ref name="pmid15718537">{{cite journal | author = Gunnell D, Saperia J, Ashby D | title = Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review | journal = BMJ | volume = 330 | issue = 7488 | page = 385 | year = 2005 | pmid = 15718537 | pmc = 549105 | doi = 10.1136/bmj.330.7488.385 | last2 = Saperia | last3 = Ashby }}</ref> |
|||
==引用== |
==引用== |
||
| 第113行: | 第112行: | ||
{{-}} |
{{-}} |
||
{{抗抑郁药}} |
|||
[[Category:抗抑郁药]] |
|||
[[Category:SSRI]] |
|||
2015年9月3日 (四) 18:32的版本
| 此條目目前正依照en:Sertraline上的内容进行翻译。 (2015年8月9日) |
 | |
 | |
| 臨床資料 | |
|---|---|
| 商品名 | 左洛复(Zoloft),彼迈乐, Lustral 等等[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697048 |
| 懷孕分級 |
|
| 给药途径 | 口服 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 44% |
| 血漿蛋白結合率 | 98.5% |
| 药物代谢 | 肝脏(主要由CYP2B6酶进行N-去甲基化)[4] |
| 生物半衰期 | ~23-26小时 (低活性的代谢产物去甲基舍曲林则为66小时)[2][3] |
| 排泄途徑 | 尿液 |
| 识别信息 | |
| |
| CAS号 | 79617-96-2 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化学信息 | |
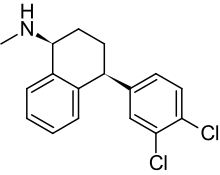
| 化学式 | C17H17Cl2N |
| 摩尔质量 | 306.229 g/mol |
| 3D模型(JSmol) | |
| |
| |
舍曲林(英語:Sertraline)(商品名:左洛复、彼迈乐等)是一种选择性5-羟色胺再吸收抑制剂(SSRI)类抗抑郁药,于1991年被瑞辉制药公司发明。舍曲林主要用于治疗成人重度抑郁症(MDD),也用来治疗儿童或成人的强迫症、恐慌症和社交恐惧症。在2013年,舍曲林是美国零售市场处方量最大的抗抑郁药与处方量第二大的精神类药物(在阿普唑仑之后),超过4100万的舍曲林被处方给患者。[5]
舍曲林与其他新型抗抑郁药的差别并不大,并且多只局限于副作用。与其他SSRI相比,舍曲林的耐受性与其他药物无明显差别,通常都会导致一些不良反应如腹泻、恶心、震颤、性功能障碍和体重增加。舍曲林导致腹泻的几率通常比其他SSRI要高。[6]
医疗用途
舍曲林适用于多种情况包括:重度抑郁症(MDD)、强迫症(OCD)、躯体变形障碍(BDD)、创伤后应激障碍(PTSD)、经前烦躁症(PMDD)、恐慌症和社交焦虑症。舍曲林也用来治疗早泄与血管性头痛,但是还没有足够充分的证据证明它的效果。[7][7]
抑郁症
一份2008年的报告指出只有51%的研究表明SSRI有积极效果。[8] 舍曲林的效果在统计学上与其他SSRI无明显差别,如帕罗西汀、西酞普兰、艾司西酞普兰、文拉法辛(属于SNRI)等。[9][10][11][12] 证据表明在治疗某些抑郁症亚型上,舍曲林比氟西汀(百优解)效果更明显。[13]
证据指出舍曲林对儿童抑郁症的治疗没有好处。[14]
对比其他抗抑郁药
一般认为三环类抗抑郁药(TCA)在治疗忧郁型抑郁症和住院病人上效果比SSRI更好,但并不一定对更加严重的抑郁症也有更好的效果。[16][17][18] 总的来说,舍曲林在治疗住院病人上并不优于安慰剂,在治疗重度抑郁症上与TCA类药物氯米帕明效果相当。[9] 尚未有针对在治疗忧郁型抑郁症上舍曲林与TCA的效果对比的研究。1998年有一种观点认为,由于舍曲林的药理特性,其在效果上会优于其他SSRI且在治疗忧郁型抑郁症上与TCA类药物效果相同。[19]
针对12种新型抗抑郁药的数据分析指出,舍曲林与艾司西酞普兰在治疗成人急性单极抑郁症上有着最好的效果和可接受性,而瑞波西汀的效果明显较差。[20]
具有比较性的临床试验表明,舍曲林治疗抑郁症的效果与吗氯贝胺、奈法唑酮、艾司西酞普兰、安非他酮、西酞普兰、氟伏沙明、帕罗西汀、米氮平相似。有质量较低的证据表明,舍曲林治疗抑郁症的效果优于氟西汀。[21][22][23][24][25]
针对老年患者
舍曲林在治疗老年患者(大于60岁)的效果上优于安慰剂,与其它SSRI类药物氟西汀和TCA类药物阿米替林、去甲替林、丙咪嗪效果相当。除恶心外,舍曲林的不良反应发生率低于这些TCA类药物。此外,针对大于70岁的患者,舍曲林的效果优于氟西汀和去甲替林。2003年的一项关于舍曲林与安慰剂的对比试验得出一个具有统计学上显著(也就是说不只是偶然发生)并且具有临床意义的结论,老年患者使用舍曲林后抑郁症状有适度改善,但是生活质量并没有得到提高。[26]2003年的一项关于舍曲林与安慰剂的对比试验得出一个具有统计学上显著(也就是说不只是偶然发生)并且具有临床意义的结论,老年患者使用舍曲林后抑郁症状有适度改善,但是生活质量并没有得到提高。[27]
强迫症
舍曲林对成人及儿童的强迫症治疗均有效果。[28]基于意向性治疗分析,舍曲林相比强迫症治疗金标准氯米帕明有着更好的效果。[29]普遍认为舍曲林用于治疗强迫症的剂量比治疗抑郁症的常用剂量高。[30]舍曲林治疗强迫症的起效时间比治疗抑郁症长。治疗的建议是,以最大推荐剂量的一半最为起始剂量并持续至少两个月,在此之后如果治疗效果不明显,可将剂量提高至最大。[31]
无论是儿童还是成人,单独应用行为认知疗法的效果优于舍曲林,但是最好的方法当然是结合药物治疗。[32][33]舍曲林可能对伴随抽动秽语综合征的强迫症有疗效。[34]
恐慌症
使用舍曲林治疗恐慌症可以减少惊恐发作并提高生活质量。[35] 四个双盲实验显示出舍曲林在治疗恐慌症时效果由于安慰剂,效果取决于剂量。舍曲林可以减少80%惊恐发作的几率(安慰剂为45%),并且可以减少广泛性焦虑,提升患者的生活质量。使用舍曲林的患者报告生活质量的提高程度优于安慰剂组。[35][36] 性别不会影响舍曲林的效果。[36] 如果粗略地将舍曲林与其他抗恐慌症药物(如氯米帕明、丙咪嗪、氯硝西泮、阿普唑仑、氟伏沙明、帕罗西汀)进行单独比较,那么他们的效果是接近等价。[35]
社交恐惧症
经前焦虑症
其他适应症
不良反应


对性功能的影响
自杀倾向
FDA要求包括舍曲林在内的所有抗抑郁药都要用黑框警告说明抗抑郁药会增加25岁以下服用者自杀的风险。此项警告的依据为两个独立的FDA专家组的统计分析,结果显示儿童和青少年产生自杀意向和行为的几率提高两倍,18-24岁的人群则提高1.5倍。[37][38][39]
引用
- ^ drugs.com drugs.com international Sertraline Page accessed May 11, 2015
- ^ Sertraline FDA Label. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4006b1_06_zoloft-label.pdf
- ^ Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.
- ^ Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab. Dispos. 2005, 33 (2): 262–70. PMID 15547048. doi:10.1124/dmd.104.002428.
- ^ John M. Grohol. Top 25 Psychiatric Medication Prescriptions for 2013. Psych Central. 2014 [3 April 2015].
- ^ Sanchez, C; Reines, E. H.; Montgomery, S. A. A comparative review of escitalopram, paroxetine, and sertraline: are they all alike?. International Clinical Psychopharmacology. 2014, 29 (4): 185–196. PMC 4047306
 . PMID 24424469. doi:10.1097/YIC.0000000000000023.
. PMID 24424469. doi:10.1097/YIC.0000000000000023.
- ^ 7.0 7.1 Sertraline hydrochloride. The American Society of Health-System Pharmacists. [3 April 2011].
- ^ Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R; Matthews; Linardatos; Tell; Rosenthal. Selective publication of antidepressant trials and its influence on apparent efficacy. N. Engl. J. Med. 2008, 358 (3): 252–60. PMID 18199864. doi:10.1056/NEJMsa065779.
- ^ 9.0 9.1 Lépine JP, Goger J, Blashko C, Probst C, Moles MF, Kosolowski J, Scharfetter B, Lane RM; Goger; Blashko; Probst; Moles; Kosolowski; Scharfetter; Lane. A double-blind study of the efficacy and safety of sertraline and clomipramine in outpatients with severe major depression. International Clinical Psychopharmacology. 2000, 15 (5): 263–71. PMID 10993128. doi:10.1097/00004850-200015050-00003.
- ^ Aberg-Wistedt A, Agren H, Ekselius L, Bengtsson F, Akerblad AC; Agren; Ekselius; Bengtsson; Akerblad. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. J Clin Psychopharmacol. 20 December 2000, 20 (6): 645–52. PMID 11106136. doi:10.1097/00004714-200012000-00010.
- ^ Ventura D, Armstrong EP, Skrepnek GH, Haim Erder M; Armstrong; Skrepnek; Haim Erder. Escitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trial. Current Medical Research and Opinion. 2007, 23 (2): 245–50. PMID 17288677. doi:10.1185/030079906X167273.
- ^ Matreja, P. S.; Badyal, D. K.; Khosla, P; Deswal, R. S. Effectiveness and acceptability of sertraline and citalopram in major depressive disorder: pragmatic randomized open-label comparison. Hum Psychopharmacol. 22 October 2007, 22 (7): 477–82. PMID 17647298. doi:10.1002/hup.864.
- ^ Flament MF, Lane RM, Zhu R, Ying Z; Lane; Zhu; Ying. Predictors of an acute antidepressant response to fluoxetine and sertraline. Int Clin Psychopharmacol. 1999, 14 (5): 259–75. PMID 10529069. doi:10.1097/00004850-199914050-00001.
- ^ Cohen D. Should the use of selective serotonin reuptake inhibitors in child and adolescent depression be banned?. Psychotherapy and psychosomatics. 2007, 76 (1): 5–14. PMID 17170559. doi:10.1159/000096360.
- ^ Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, Baldwin R, Bentham P, Fox C, Holmes C, Katona C, Lawton C, Lindesay J, Livingston G, McCrae N, Moniz-Cook E, Murray J, Nurock S, Orrell M, O'Brien J, Poppe M, Thomas A, Walwyn R, Wilson K, Burns A; Hellier; Romeo; Dewey; Knapp; Ballard; Baldwin; Bentham; Fox; Holmes; Katona; Lawton; Lindesay; Livingston; McCrae; Moniz-Cook; Murray; Nurock; Orrell; O'Brien; Poppe; Thomas; Walwyn; Wilson; Burns. Study of the use of antidepressants for depression in dementia: the HTA-SADD trial – a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess. 2007, 17 (7): 1–116. PMID 23438937. doi:10.3310/hta17070.
- ^ Parker G, Roy K, Wilhelm K, Mitchell P; Roy; Wilhelm; Mitchell. Assessing the comparative effectiveness of antidepressant therapies: a prospective clinical practice study. J Clin Psychiatry. 2001, 62 (2): 117–25. PMID 11247097. doi:10.4088/JCP.v62n0209.
- ^ Anderson IM. SSRIS versus tricyclic antidepressants in depressed inpatients: a meta-analysis of efficacy and tolerability. Depress Anxiety. 1998, 7 (S1): 11–7. PMID 9597346. doi:10.1002/(SICI)1520-6394(1998)7:1+<11::AID-DA4>3.0.CO;2-I.
- ^ Hirschfeld RM. Efficacy of SSRIs and newer antidepressants in severe depression: comparison with TCAs. J Clin Psychiatry. 1999, 60 (5): 326–35. PMID 10362442. doi:10.4088/JCP.v60n0511.
- ^ Amsterdam JD. Selective serotonin reuptake inhibitor efficacy in severe and melancholic depression. J. Psychopharmacol. (Oxford). 1998, 12 (3 Suppl B): S99–111. PMID 9808081. doi:10.1177/0269881198012003061 (不活跃 2015-05-19).
- ^ Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C; Furukawa; Salanti; Geddes; Higgins; Churchill; Watanabe; Nakagawa; Omori; McGuire; Tansella; Barbui. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. The Lancet. 2009, 373 (9665): 746–758. PMID 19185342. doi:10.1016/S0140-6736(09)60046-5. 简明摘要 – The Washington Post (29 January 2009).
- ^ Papakostas GI, Fava M; Fava. A metaanalysis of clinical trials comparing moclobemide with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. Canadian Journal of Psychiatry. 2006, 51 (12): 783–90. PMID 17168253.
- ^ Feiger A, Kiev A, Shrivastava RK, Wisselink PG, Wilcox CS; Kiev; Shrivastava; Wisselink; Wilcox. Nefazodone versus sertraline in outpatients with major depression: focus on efficacy, tolerability, and effects on sexual function and satisfaction. The Journal of Clinical Psychiatry. 57. 1996,. Suppl 2: 53–62. PMID 8626364.
- ^ Kavoussi RJ, Segraves RT, Hughes AR, Ascher JA, Johnston JA; Segraves; Hughes; Ascher; Johnston. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. The Journal of Clinical Psychiatry. 1997, 58 (12): 532–7. PMID 9448656. doi:10.4088/JCP.v58n1204.
- ^ For the review, see:Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS; Gartlehner; Lohr; Gaynes; Carey. Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann. Intern. Med. 2005, 143 (6): 415–26. PMID 16172440. doi:10.7326/0003-4819-143-6-200509200-00006.
- ^ Cipriani, A; La Ferla, T; Furukawa, TA; Signoretti, A; Nakagawa, A; Churchill, R; McGuire, H; Barbui, C. Sertraline versus other antidepressive agents for depression. The Cochrane database of systematic reviews. 14 April 2010, (4): CD006117. PMC 4163971
 . PMID 20393946. doi:10.1002/14651858.CD006117.pub4.
. PMID 20393946. doi:10.1002/14651858.CD006117.pub4.
- ^ Muijsers RB, Plosker GL, Noble S; Plosker; Noble. Sertraline: a review of its use in the management of major depressive disorder in elderly patients. Drugs & Aging. 2002, 19 (5): 377–92. PMID 12093324. doi:10.2165/00002512-200219050-00006.
- ^ Schneider LS, Nelson JC, Clary CM, Newhouse P, Krishnan KR, Shiovitz T, Weihs K; Nelson; Clary; Newhouse; Krishnan; Shiovitz; Weihs; Sertraline Elderly Depression Study Group. An 8-week multicenter, parallel-group, double-blind, placebo-controlled study of sertraline in elderly outpatients with major depression. Am J Psychiatry. 2003, 160 (7): 1277–85. PMID 12832242. doi:10.1176/appi.ajp.160.7.1277.
- ^ Geller DA, Biederman J, Stewart SE, Mullin B, Martin A, Spencer T, Faraone SV; Biederman; Stewart; Mullin; Martin; Spencer; Faraone. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. The American Journal of Psychiatry. 2003, 160 (11): 1919–28. PMID 14594734. doi:10.1176/appi.ajp.160.11.1919.
- ^ Flament MF, Bisserbe JC; Bisserbe. Pharmacologic treatment of obsessive-compulsive disorder: comparative studies. The Journal of Clinical Psychiatry. 58. 1997,. Suppl 12: 18–22. PMID 9393392.
- ^ Math SB, Janardhan Reddy YC. Issues In The Pharmacological Treatment of Obsessive-Compulsive Disorder: First-Line Treatment Options for OCD. medscape.com. 19 July 2007 [28 July 2009].
- ^ Blier P, Habib R, Flament MF; Habib; Flament. Pharmacotherapies in the management of obsessive-compulsive disorder (PDF). Can J Psychiatry. 2006, 51 (7): 417–30. PMID 16838823.
- ^ Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004, 292 (16): 1969–76. PMID 15507582. doi:10.1001/jama.292.16.1969.
- ^ Sousa MB, Isolan LR, Oliveira RR, Manfro GG, Cordioli AV; Isolan; Oliveira; Manfro; Cordioli. A randomized clinical trial of cognitive-behavioral group therapy and sertraline in the treatment of obsessive-compulsive disorder. The Journal of Clinical Psychiatry. 2006, 67 (7): 1133–9. PMID 16889458. doi:10.4088/JCP.v67n0717.
- ^ Jiménez-Jiménez FJ, García-Ruiz PJ; García-Ruiz. Pharmacological options for the treatment of Tourette's disorder. Drugs. 2001, 61 (15): 2207–20. PMID 11772131. doi:10.2165/00003495-200161150-00005.
- ^ 35.0 35.1 35.2 Hirschfeld RM. Sertraline in the treatment of anxiety disorders. Depress Anxiety. 2000, 11 (4): 139–57. PMID 10945134. doi:10.1002/1520-6394(2000)11:4<139::AID-DA1>3.0.CO;2-C.
- ^ 36.0 36.1 Clayton AH, Stewart RS, Fayyad R, Clary CM; Stewart; Fayyad; Clary. Sex differences in clinical presentation and response in panic disorder: pooled data from sertraline treatment studies. Arch Womens Ment Health. 2006, 9 (3): 151–7. PMID 16292466. doi:10.1007/s00737-005-0111-y.
- ^ Levenson M, Holland C. Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006). FDA. [11 July 2008].
- ^ Stone MB, Jones ML. Clinical review: relationship between antidepressant drugs and suicidality in adults (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA: 11–74. 17 November 2006 [11 July 2008].
- ^ Levenson M, Holland C. Statistical Evaluation of Suicidality in Adults Treated with Antidepressants (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA: 75–140. 17 November 2006 [11 July 2008].
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
