2019冠狀病毒病疫苗授權列表:修订间差异
// Edit via Wikiplus |
无编辑摘要 |
||
| 第186行: | 第186行: | ||
}} |
}} |
||
</div> |
</div> |
||
==輝瑞-BNT== |
|||
輝瑞-BNT為一種[[信使核糖核酸]](mRNA)疫苗<ref name="CDC_Pfizer-BioNTech">{{cite web |title=辉瑞-生物科技COVID-19疫苗概述和安全性 |url=https://chinese.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html |publisher=美国卫生与公众服务部 |accessdate=2021-04-14}}</ref>,由德國[[BioNTech]]及美國[[輝瑞製藥]]合作開發及生產<ref>{{Cite web|title=What you need to know about BioNTech — the European company behind Pfizer's Covid-19 vaccine|url=https://www.cnbc.com/2020/11/11/biontech-the-european-company-behind-pfizers-covid-19-vaccine.html|accessdate=2021-04-04|date=2020-11-11|last=Browne|first=Ryan|work=CNBC|language=en}}</ref><ref name="nejm-2020-12-31">{{cite news |title=Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine |url=https://www.nejm.org/doi/full/10.1056/nejmoa2034577 |accessdate=2021-04-13 |work=The New England Journal of Medicine |date=2020-12-31 |archive-url=https://web.archive.org/web/20210101021944/https://www.nejm.org/doi/full/10.1056/nejmoa2034577 |archive-date=2021-01-01 |dead-url=no }}</ref>。 |
|||
*全面授權 |
|||
# 巴西<ref name="Brazil approves full vaccine">{{cite web|url=https://g1.globo.com/bemestar/vacina/noticia/2021/02/23/anvisa-concede-registro-definitivo-a-vacina-da-pfizer.ghtml|title=Vacina da Pfizer é a 1ª a obter registro definitivo no Brasil|publisher=G1|date=23 February 2021|access-date=23 February 2021|language=pt-br}}</ref> |
|||
# 紐西蘭<ref>{{cite web|date=3 February 2021|title=Robust assessment ahead of Medsafe approval of vaccine|url=https://www.health.govt.nz/news-media/media-releases/robust-assessment-ahead-medsafe-approval-vaccine|access-date=6 February 2021|work=Ministry of Health}}</ref> |
|||
# 沙烏地阿拉伯<ref>{{cite web|date=10 December 2020|title=Coronavirus: Saudi Arabia approves Pfizer COVID-19 vaccine for use|url=https://english.alarabiya.net/en/coronavirus/2020/12/10/Coronavirus-Saudi-Arabia-approves-Pfizer-COVID-19-vaccine-for-use|access-date=10 December 2020|website=Al Arabiya English}}</ref><ref>{{cite news|last1=Zimmer|first1=Carl|last2=Corum|first2=Jonathan|last3=Wee|first3=Sui-Lee|date=10 June 2020|title=Coronavirus Vaccine Tracker|work=[[The New York Times]]|url=https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html|access-date=12 December 2020|name-list-style=vanc}}</ref> |
|||
# 瑞士<ref name="Swiss authorization">{{cite press release|title=Swissmedic grants authorisation for the first COVID-19 vaccine in Switzerland|url=https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-81761.html|publisher=[[Swissmedic|Swiss Agency for Therapeutic Products (Swissmedic)]]|date=19 December 2020|access-date=19 December 2020}}</ref><ref>{{cite web|title=Comirnaty Product information|url=https://www.swissmedicinfo.ch/ShowText.aspx?textType=FI&lang=DE&authNr=68225|access-date=31 January 2021|language=DE|trans-quote=Due to incomplete clinical data at the time of the assessment of the authorization application, the Comirnaty medicinal product is authorized for a limited period (Art. 9a Medicinal Products Act).}}</ref> |
|||
*緊急授權使用 |
|||
<div style="margin-top: 0.3em; column-width: 20em;"> |
|||
</div> |
|||
== 衛星V == |
== 衛星V == |
||
衛星V是一款由[[俄罗斯]][[加马列亚流行病与微生物学国家研究中心]]开发的病毒載體疫苗<ref name="NCT04436471">{{cite web|date=17 June 2020|title=An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19|url=https://clinicaltrials.gov/ct2/show/NCT04436471|access-date=5 March 2021|website=ClinicalTrials.gov|publisher=United States National Library of Medicine|id=NCT04436471}}</ref>。 |
衛星V是一款由[[俄罗斯]][[加马列亚流行病与微生物学国家研究中心]]开发的病毒載體疫苗<ref name="NCT04436471">{{cite web|date=17 June 2020|title=An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19|url=https://clinicaltrials.gov/ct2/show/NCT04436471|access-date=5 March 2021|website=ClinicalTrials.gov|publisher=United States National Library of Medicine|id=NCT04436471}}</ref>。 |
||
2021年5月8日 (六) 16:33的版本
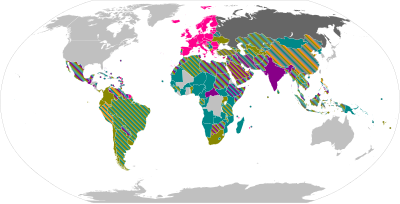
本列表列出各項2019冠状病毒病疫苗依據醫療產品法律規範所進行的授權,現有14款疫苗受到各地區政權頒發緊急使用授權,其中至少有6款疫苗受世界衛生組織PQ/EUL或嚴格監管機構通過緊急使用。
地圖總覽
|
|
Astra Zeneca
Astra Zeneca又名為Vaxzevria[1]、Covishield[2],是由英國牛津大學與阿斯利康製藥合作研發,以非複製型病毒載體為技術基礎,是一款針對2019冠狀病毒病的預防疫苗[3]。
- 全面授權
- 巴西[4]
- 緊急授權使用
- 阿富汗[5][6]
- 阿爾巴尼亞[7]
- 阿爾及利亞[8]
- 安道爾[9]
- 安哥拉[10]
- 阿根廷[11]
- 澳大利亞[12][13]
- 巴林[14]
- 孟加拉國[15][16]
- 不丹[17][18]
- 玻利維亞[19]
- 波斯尼亞和黑塞哥維那[20]
- 博茨瓦納[21]
- 汶萊[22]
- 柬埔寨[19]
- 加拿大[23][24]
- 維德角[25]
- 智利[26]
- 哥倫比亞[27]
- 剛果民主共和國[28]
- 哥斯大黎加[29]
- 吉布地[19]
- 多米尼加共和國[30]
- 東帝汶[31]
- 厄瓜多[32]
- 埃及[33]
- 薩爾瓦多[34]
- 史瓦帝尼[35]
- 衣索比亞[36][37][38]
- 斐濟[19]
- 甘比亞[39]
- 喬治亞[40]
- 迦納[41]
- 瓜地馬拉[42]
- 幾內亞比索 [43]
- 洪都拉斯[19]
- 匈牙利[44]
- 印度[45]
- 印尼[46]
- 伊朗[47]
- 伊拉克[48]
- 象牙海岸[49]
- 肯亞[50]
- 科索沃[51][52]
- 科威特[53]
- 黎巴嫩[54]
- 賴索托[55]
- 賴比瑞亞[56]
- 利比亞[57][58]
- 馬拉威[59][60]
- 馬來西亞[61]
- 馬爾地夫[62]
- 馬利[63]
- 模里西斯[64]
- 墨西哥[65]
- 摩多瓦[66]
- 蒙古[67]
- 摩洛哥[68]
- 緬甸[69]
- 納米比亞[70]
- 諾魯[71]
- 尼泊爾[72]
- 尼加拉瓜[19]
- 奈及利亞[73]
- 北馬其頓[74]
- 阿曼[19]
- 巴基斯坦[75]
- 巴勒斯坦[19]
- 巴拿馬[19]
- 巴布亞新幾內亞[76][77]
- 祕魯 [78]
- 菲律賓[79]
- 盧安達[80]
- 薩摩亞[71]
- 聖多美普林西比[19]
- 沙烏地阿拉伯[81]
- 塞爾維亞[82]
- 塞席爾[83]
- 獅子山[84]
- 所羅門群島[19]
- 索馬利亞[85]
- 南非[86]
- 南韓[87][88]
- 南蘇丹[89]
- 斯里蘭卡[90]
- 蘇丹[91][92]
- 台灣[93]
- 塔吉克斯坦[94]
- 泰國[95]
- 多哥[96]
- 東加[97] [98][99]
- 吐瓦魯 [100]
- 烏干達[101]
- 烏克蘭[102]
- 英國[103][104][105]
- 烏拉圭[19]
- 烏茲別克斯坦[19]
- 越南[106]
- 葉門 [107]
- 尚比亞[108]
EMA夥伴國家 - 奧地利
- 比利時
- 保加利亞
- 克羅埃西亞
- 賽普勒斯
- 捷克
- 愛沙尼亞
- 芬蘭
- 法國
- 德國
- 希臘
- 匈牙利
- 冰島
- 愛爾蘭
- 義大利
- 拉脫維亞
- 列支敦士登
- 立陶宛
- 盧森堡
- 馬爾他
- 荷蘭
- 挪威
- 波蘭
- 葡萄牙
- 羅馬尼亞
- 斯洛伐克
- 斯洛維尼亞
- 西班牙
- 瑞典
CARPHA夥伴國家 - 安地卡及巴布達
- 巴哈馬
- 巴貝多
- 貝里斯
- 多明尼加
- 格瑞那達
- 蓋亞那
- 海地
- 牙買加
- 聖克里斯多福及尼維斯
- 聖露西亞
- 聖文森及格瑞那丁
- 蘇利南[109]
- 千里達及托巴哥
CARPHA 非獨立政體[110]
安奎拉
阿魯巴
百慕達
英屬維京群島
荷蘭加勒比區
開曼群島
庫拉索
蒙哲臘
荷屬聖馬丁
土克斯及開科斯群島
其他 非獨立政體
北賽普勒斯[111]
世界衛生組織[112][113][114]
輝瑞-BNT
輝瑞-BNT為一種信使核糖核酸(mRNA)疫苗[115],由德國BioNTech及美國輝瑞製藥合作開發及生產[116][117]。
- 全面授權
- 緊急授權使用
衛星V
衛星V是一款由俄罗斯加马列亚流行病与微生物学国家研究中心开发的病毒載體疫苗[124]。
- 全面授權
- 緊急授權使用
- 阿爾巴尼亞[7]
- 阿爾及利亞[128]
- 安哥拉[129]
- 安地卡及巴布達[130]
- 阿根廷[131]
- 亞美尼亞[132]
- 阿塞拜疆[133][134]
- 巴林[135]
- 孟加拉國[136]
- 白俄羅斯[137]
- 玻利維亞[138]
- 波士尼亞與赫塞哥維納[139]
- 喀麥隆[140]
- 剛果共和國[129]
- 吉布地[129]
- 埃及[33]
- 加彭[141]
- 迦納[142]
- 瓜地馬拉[143]
- 幾內亞[144]
- 蓋亞那[145]
- 宏都拉斯[146]
- 匈牙利[147][139][148]
- 印度[149]
- 伊朗[150]
- 伊拉克[151]
- 約旦[152]
- 哈薩克[153]
- 肯亞[154]
- 吉爾吉斯[155]
- 寮國[156]
- 黎巴嫩[157]
- 利比亞[57][58]
- 馬利[158]
- 模里西斯[159]
- 墨西哥[160]
- 蒙古[161]
- 摩爾多瓦[66]
- 蒙特內哥羅[162]
- 摩洛哥[163]
- 緬甸[164]
- 納米比亞[165]
- 尼泊爾[166]
- 尼加拉瓜[167]
- 北馬其頓[168]
- 巴基斯坦[169]
- 巴勒斯坦[170]
- 巴拿馬[171]
- 巴拉圭[172]
- 菲律賓[173]
- 俄羅斯[174]
- 聖文森及格瑞那丁[175]
- 聖馬利諾[176][177]
- 塞爾維亞[178]
- 塞席爾[179]
- 斯洛伐克[180]
- 斯里蘭卡[181]
- 敘利亞[182]
- 泰國[183]
- 土耳其[184]
- 突尼西亞[185]
- 阿拉伯聯合大公國[186]
- 烏拉圭[187]
- 委內瑞拉[188]
- 越南[189]
- 辛巴威[190]
莫德納
莫德納,藥品代號為mRNA-1273,是美國國家過敏和傳染病研究所、生物醫學高級研究與開發管理局和莫德纳合作開發[191][192]的2019冠状病毒病疫苗,為一種信使核糖核酸(mRNA)疫苗[193]。
- 全面授權
- 緊急授權使用
- 安多拉[9]
- 加拿大[197][24]
- 瓜地馬拉[42]
- 宏都拉斯[198]
- 以色列[199]
- 科威特[200]
- 馬歇爾群島
- 密克羅尼西亞
- 蒙古[67]
- 帛琉
- 巴勒斯坦[19]
- 菲律賓[201]
- 卡達[202]
- 盧安達[19]
- 聖文森及格瑞那丁[175]
- 沙烏地阿拉伯[81]
- 新加坡[203]
- 台灣[204]
- 泰國[205]
- 美國[206][207]
EMA夥伴國家[208] - 奧地利
- 比利時
- 保加利亞
- 克羅埃西亞
- 賽普勒斯
- 捷克
- 丹麥
- 愛沙尼亞
- 芬蘭
- 法國
- 德國
- 希臘
- 匈牙利
- 冰島
- 愛爾蘭
- 義大利
- 拉脫維亞
- 列支敦士登
- 立陶宛
- 盧森堡
- 馬爾他
- 荷蘭
- 挪威
- 波蘭
- 葡萄牙
- 羅馬尼亞
- 斯洛伐克
- 斯洛維尼亞
- 西班牙
- 瑞典
非獨立政體
世界衛生組織[209]
眾愛可維
眾愛可維,藥品代號為BBIBP-CorV,是一款由中國國藥集團北京生物製品研究所研發的一款滅活疫苗[210][211]。
- 全面授權
- 緊急授權使用
- 阿富汗[218]
- 阿爾及利亞[219]
- 安哥拉[220]
- 阿根廷[221]
- 孟加拉[222]
- 白俄羅斯[223]
- 玻利維亞[224]
- 汶萊[225]
- 柬埔寨[226]
- 喀麥隆[19]
- 剛果共和國[19]
- 多明尼加[227]
- 埃及[228]
- 赤道幾內亞[229]
- 衣索比亞[230]
- 加彭[231]
- 蓋亞那[232]
- 匈牙利[233][148]
- 印尼[234]
- 伊朗[235]
- 伊拉克[48]
- 約旦[236]
- 哈薩克[237]
- 吉爾吉斯[238]
- 寮國[156]
- 黎巴嫩[239]
- 馬爾地夫[240]
- 茅利塔尼亞[241]
- 摩爾多瓦[242]
- 蒙古[243]
- 蒙特內哥羅[244]
- 摩洛哥[245]
- 莫三比克[246]
- 納米比亞[247]
- 尼泊爾[248]
- 北馬其頓[249]
- 尼日[250]
- 巴勒斯坦[251]
- 巴基斯坦[252]
- 秘魯[253]
- 索馬利亞[254]
- 塞內加爾[255]
- 塞爾維亞[256]
- 獅子山[257]
- 斯里蘭卡[258]
- 蘇丹[259][260]
- 委內瑞拉[261]
- 辛巴威[262]
非獨立政體
世界衛生組織[263][264]
嬌生
嬌生是由位於荷兰莱顿的杨森疫苗[265]及其比利时母公司同時也是美国強生公司的子公司杨森制药[266]研发的2019冠状病毒病疫苗[267][268]。
- 全面授權
- 緊急授權使用
- 安道爾[9]
- 巴林[269][270]
- 巴西[271]
- 加拿大[272]
- 哥倫比亞[273]
- 馬歇爾群島
- 墨西哥[274]
- 密克羅尼西亞
- 帛琉
- 菲律賓[275]
- 聖文森及格瑞那丁[175]
- 南非[276]
- 南韓[277]
- 瑞士[278][279]
- 泰國[280]
- 美國[281][282]
- 尚比亞[108][283][284]
EMA夥伴國家[208] - 奧地利
- 比利時
- 保加利亞
- 克羅埃西亞
- 賽普勒斯
- 捷克
- 愛沙尼亞
- 芬蘭
- 法國
- 德國
- 希臘
- 匈牙利
- 冰島
- 愛爾蘭
- 義大利
- 拉脫維亞
- 列支敦士登
- 立陶宛
- 盧森堡
- 馬爾他
- 荷蘭
- 挪威
- 波蘭
- 葡萄牙
- 羅馬尼亞
- 斯洛伐克
- 斯洛維尼亞
- 西班牙
- 瑞典
非獨立政體
格陵蘭[285]
世界衛生組織[286]
CoronaVac
CoronaVac是由科興生物所研發的一款滅活疫苗[287][287][288][289]。
- 全面授權
- 中國[290]
- 緊急授權使用
Covaxin
Covaxin,藥物代號為BBV152,是一款由印度医学研究理事会與巴拉特生物技術公司共同研發的滅活疫苗[316]。
- 全面授權
- 緊急授權使用
克威莎
克威莎,藥物代號為Ad5-nCoV,是一款由康希诺生物與中國人民解放軍軍事科學院軍事醫學研究院生物工程研究所[326]所共同研發的病毒載體疫苗。
- 全面授權
- 中國[327]
- 緊急授權使用
EpiVacCorona
EpiVacCorona是一款由俄羅斯國家病毒學與生物技術研究中心所生產的肽疫苗
- 全面授權
- 緊急授權使用
RBD-Dimer
RBD-Dimer,又名為ZF2001,是一款由中國安徽智飞龙科马生物制药有限公司所生產的亞單位疫苗[338]。
- 全面授權
- 緊急授權使用
Sinopharm-WIBP
WIBP-CorV是由中國國藥集團武汉生物制品研究所研發的滅活疫苗。
- 全面授權
- 緊急授權使用
CoviVac
CoviVac 是一款由俄羅斯楚馬科夫研究中心所研發的滅活疫苗[343][344]。
- 全面授權
- 緊急授權使用
- 俄羅斯[345]
QazCovid-in
QazCovid-in,又名為QazVac,是一款由哈薩克教育和科学部生物安全问题研究所研發出的滅活疫苗[346]。
- 全面授權
- 緊急授權使用
- 哈薩克[346]
衛星 Light
衛星 Light 是一款病毒載體疫苗[347] ,由俄羅斯加馬利亞流行病學和微生物學研究所生產。
- 全面授權
- 緊急授權使用
- 俄羅斯[348]
参考资料
- ^ Vaxzevria (previously COVID-19 Vaccine AstraZeneca) EPAR. European Medicines Agency (EMA). [2021-04-01]. (原始内容存档于2021-04-21).
- ^ AstraZeneca / Covishield COVID-19 vaccine: What you should know. Health Canada. 2021-02-26 [2021-02-26]. (原始内容存档于2021-04-20).
- ^ COVID-19 Vaccine AstraZeneca. Therapeutic Goods Administration. 2021-02-16 [2020-02-16]. (原始内容存档于2021-03-18).
- ^ Brazil grants full approval to Oxford vaccine, orders Sputnik. Brasilia: France 24. Agence France-Presse. 12 March 2021 [13 March 2021]. (原始内容存档于2021-03-16).
- ^ Sediqi, Abdul Qadir. First doses of COVID-19 vaccine arrive in Afghanistan from India. Reuters. 7 February 2021 [24 February 2021]. (原始内容存档于2021-03-05).
- ^ India donates 500,000 COVID vaccines to Afghanistan. Al Jazeera. [24 February 2021]. (原始内容存档于2021-03-08).
- ^ 7.0 7.1 7.2 Semini, Llazar. Albania starts mass COVID vaccinations before tourist season. ABC News. [2021-03-29]. (原始内容存档于2021-04-28).
- ^ Kreo. البلاد الوطني / وزارة الصناعات الصيدلانية ترخص باستعمال لقاح "أسترا زينيكا -أوكسفورد " المضاد لكورونا. elbilad.net. [2021-04-01]. (原始内容存档于2021-05-02) (阿拉伯语).
- ^ 9.0 9.1 9.2 Informació en relació amb la vacunació contra la COVID-19 (PDF). Govern d'Andorra. [14 March 2021]. (原始内容存档 (PDF)于2021-03-19) (加泰罗尼亚语).
- ^ AfricaNews. Angola begins Covid immunization fight with COVAX vaccines. Africanews. 2021-03-03 [2021-04-14]. (原始内容存档于2021-03-09).
|time=被忽略 (帮助) - ^ Argentine regulator approves AstraZeneca/Oxford COVID-19 vaccine. Reuters. 30 December 2020 [30 December 2020]. (原始内容存档于2021-02-04).
- ^ AstraZeneca coronavirus vaccine approved for use in Australia by TGA. ABC News. 16 February 2021 [16 February 2021]. (原始内容存档于2021-03-16).
- ^ TGA provisionally approves AstraZeneca's COVID-19 vaccine. Therapeutic Goods Administration (TGA). 16 February 2021 [16 February 2021]. (原始内容存档于2021-03-19).
- ^ Bahrain approves Oxford/AstraZeneca coronavirus vaccine produced in India. Saudigazette. 25 January 2021 [30 January 2021]. (原始内容存档于2021-02-01).
- ^ Oxford University-Astrazeneca vaccine: Bangladesh okays it for emergency use. The Daily Star. [6 January 2021]. (原始内容存档于2021-01-27).
- ^ Bangladesh approves Oxford-AstraZeneca COVID-19 vaccine. aa.com.tr. [6 January 2021]. (原始内容存档于2021-02-04).
- ^ Bhutan receives 400,000 doses of COVID-19 vaccine from India. Xinhuanet. [2021-04-01]. (原始内容存档于2021-04-01).
- ^ Bhutan begins biggest vaccination drive against COVID-19. CNA. [2021-04-01]. (原始内容存档于2021-05-02).
- ^ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 19.10 19.11 19.12 19.13 19.14 19.15 19.16 19.17 COVID Data Tracker. Centers for Disease Control and Prevention. 28 March 2020 [2021-05-08]. (原始内容存档于2021-01-25) (英语).
- ^ 引用错误:没有为名为
:3的参考文献提供内容 - ^ Botswana starts COVID-19 vaccine rollout. Xinhuanet. [2021-04-01]. (原始内容存档于2021-04-28).
- ^ 引用错误:没有为名为
auto的参考文献提供内容 - ^ Regulatory Decision Summary – AstraZeneca COVID-19 Vaccine. Health Canada. 26 February 2021 [26 February 2021]. (原始内容存档于2021-03-11).
- ^ 24.0 24.1 引用错误:没有为名为
hcapps的参考文献提供内容 - ^ COVID-19 vaccines sent by COVAX arrive in Cabo Verde. ReliefWeb. [2021-04-17]. (原始内容存档于2021-04-23).
- ^ Instituto de Salud Pública de Chile. [2021-04-01]. (原始内容存档于2021-05-01) (西班牙语).
- ^ Colombia approves emergency use of AstraZeneca coronavirus vaccine. Bogotá: Reuters. 23 February 2021 [22 March 2021]. (原始内容存档于2021-05-04).
- ^ More than 1.7 million COVID-19 vaccines arrive in the Democratic Republic of Congo. www.unicef.org. [2021-04-25]. (原始内容存档于2021-05-04) (英语).
- ^ Costa Rica approves use of AstraZeneca's COVID-19 vaccine. Q COSTA RICA. 9 April 2021 [2021-05-08]. (原始内容存档于2021-04-17).
- ^ La República Dominicana aprueba la vacuna de AstraZeneca contra la covid-19. Agencia EFE. 31 December 2020 [2021-05-08]. (原始内容存档于2021-01-24) (西班牙语).
- ^ First vaccines for East Timor arrive in Dili on Monday – LUSA. COVID-19 Timor-Leste Dashboard. April 1, 2021 [2021-05-08]. (原始内容存档于2021-04-02).
- ^ Ecuador approves use of AstraZeneca vaccine for COVID-19. Reuters. 24 January 2021 [30 January 2021]. (原始内容存档于2021-01-26).
- ^ 33.0 33.1 COVID-19: Egypt authorises Sputnik V, AstraZeneca virus jabs. Gulf News. [24 February 2021].
- ^ El Salvador greenlights AstraZeneca, Oxford University COVID-19 vaccine. Reuters. 30 December 2020 [2021-05-08]. (原始内容存档于2021-01-24).
- ^ Eswatini launches nationwide COVID-19 vaccination campaign. World Health Organization (WHO). [2021-04-01]. (原始内容存档于2021-05-04).
- ^ Ethiopia begins COVID-19 vaccine rollout. aa.com.tr. [19 March 2021]. (原始内容存档于2021-03-16).
- ^ Ethiopia introduces COVID-19 vaccine in a national launching ceremony. World Health Organization (WHO). [19 March 2021]. (原始内容存档于2021-03-13).
- ^ Ethiopia says it has secured 9 million doses of COVID-19 vaccines till April. Reuters. 9 February 2021 [19 March 2021]. (原始内容存档于2021-02-22).
- ^ The Arrival of COVAX vaccines Raises Hope in The Gambia. World Health Organization (WHO). [19 March 2021]. (原始内容存档于2021-03-11).
- ^ Georgia to receive first doses of COVID-19 vaccine in early March instead of February. Agenda.ge. [22 March 2021]. (原始内容存档于2021-05-01).
- ^ Welcome to Ghana Food And Drug Authority. fdaghana.gov.gh. [2021-04-01]. (原始内容存档于2021-05-02).
- ^ 42.0 42.1 引用错误:没有为名为
auto3的参考文献提供内容 - ^ 存档副本. [2021-05-08]. (原始内容存档于2021-05-04).
- ^ Hungary approves two more vaccines not authorised by EU regulator. euronews. 2021-03-22 [2021-04-01]. (原始内容存档于2021-05-06).
- ^ 45.0 45.1 Schmall, Emily; Yasir, Sameer. India Approves Oxford-AstraZeneca Covid-19 Vaccine and 1 Other. The New York Times. 3 January 2021 [3 January 2021]. (原始内容存档于2021-03-09).
- ^ BPOM Terbitkan Izin Penggunaan Darurat Vaksin Covid-19 AstraZeneca. Kompas.com. [10 February 2021]. (原始内容存档于2021-03-15).
- ^ Iran issues permit for emergency use for three other COVID-19 vaccines: Official. IRNA English. 2021-02-17 [2021-04-01]. (原始内容存档于2021-02-27).
- ^ 48.0 48.1 Iraq approves Sinopharm, AstraZeneca vaccines. Big News Network.com. [30 January 2021]. (原始内容存档于2021-01-31).
- ^ Covax: Ivory Coast and Ghana begin mass Covid vaccination rollouts. BBC News. 1 March 2021 [19 March 2021]. (原始内容存档于2021-03-14).
- ^ Kenya Receives 1M Vaccine Doses, Will Distribute to Health Workers First. Voice of America. [19 March 2021]. (原始内容存档于2021-03-09).
- ^ Kosovo receives first COVID-19 vaccines through Covax scheme. euronews. 2021-03-29 [2021-04-14]. (原始内容存档于2021-04-15).
- ^ Kosovo PM Becomes Nation's First Person To Receive COVID-19 Vaccine. RadioFreeEurope/RadioLiberty. [2021-04-14]. (原始内容存档于2021-05-08).
- ^ Godinho, Varun. Kuwait authorises emergency use of Oxford-AstraZeneca Covid-19 vaccine. Gulf Business. 2021-01-31 [2021-04-01]. (原始内容存档于2021-05-05).
- ^ Lebanon Received 33600 Doses of The AstraZeneca Vaccine as First Batch. Ministry of Public Health of Lebanon. [2021-05-08]. (原始内容存档于2021-04-21).
- ^ Lesotho receives 1st batch of COVID-19 vaccines. aa.com.tr. [19 March 2021]. (原始内容存档于2021-03-10).
- ^ 96,000 Doses of COVID-19 Vaccine Arrives in Liberia. WHO. [2021-04-11]. (原始内容存档于2021-04-11).
- ^ 57.0 57.1 Libya kicks off delayed COVID-19 vaccination drive. www.aljazeera.com. [2021-04-25]. (原始内容存档于2021-05-06) (英语).
- ^ 58.0 58.1 57,600 COVID-19 vaccine doses received today in Tripoli, Libya. ReliefWeb. [2021-04-25]. (原始内容存档于2021-04-28) (英语).
- ^ Malawi to receive COVID-19 vaccines in late February – Xinhua. Xinhuanet. [19 March 2021]. (原始内容存档于2021-02-13).
- ^ Malawi Sticks to AstraZeneca Despite Concerns Over Efficacy. Voice of America. [19 March 2021]. (原始内容存档于2021-02-08).
- ^ 61.0 61.1 Sipalan, Joseph; Donovan, Kirsten. Malaysia approves Sinovac, AstraZeneca COVID-19 vaccines for use. Reuters. 3 March 2021 [7 March 2021]. (原始内容存档于2021-03-19).
- ^ Maldives starts training healthcare workers on COVID-19 vaccine distribution – Xinhua. Xinhuanet. [19 February 2021]. (原始内容存档于2021-01-24).
- ^ AfricaNews. Mali receives first batch of COVID-19 vaccines. Africanews. 2021-03-06 [2021-04-01]. (原始内容存档于2021-05-03).
- ^ AfricaNews. Mauritius begins vaccinating frontline health workers against covid-19. Africanews. 26 January 2021 [24 February 2021]. (原始内容存档于2021-02-04).
- ^ Mexico approves AstraZeneca COVID-19 vaccine, minister says. Reuters. 5 January 2021 [14 January 2021]. (原始内容存档于2021-01-26).
- ^ 66.0 66.1 引用错误:没有为名为
:2的参考文献提供内容 - ^ 67.0 67.1 Mongolia approved three Covid-19 vaccines. News.MN. 2021-01-11 [2021-04-01]. (原始内容存档于2021-05-02).
- ^ Morocco approves AstraZeneca/Oxford COVID-19 vaccine – Minister. Reuters. 7 January 2021 [22 March 2021]. (原始内容存档于2021-05-01).
- ^ Myanmar launches nationwide COVID-19 vaccination program. Xinhua News. 27 January 2021 [2021-05-08]. (原始内容存档于2021-01-27).
- ^ 存档副本 (PDF). [2021-05-08]. (原始内容存档 (PDF)于2021-04-22).
- ^ 71.0 71.1 Samoa receives 24,000 doses of COVID-19 vaccines through the COVAX facility. [2021-05-08]. (原始内容存档于2021-05-01).
- ^ Nepal approves AstraZeneca COVID vaccine for emergency use – government statement. Reuters. 15 January 2021 [2021-05-08]. (原始内容存档于2021-01-21).
- ^ Wetin we sabi about NAFDAC approval of Oxford AstraZeneca vaccine use for Nigeria. BBC News Pidgin. [24 February 2021]. (原始内容存档于2021-02-18).
- ^ Сѐ за вакцините. Официјална веб-страница за вакцинација против КОВИД19. [2021-05-08]. (原始内容存档于2021-05-03) (mk-MK).
- ^ Pakistan approves AstraZeneca COVID-19 vaccine for emergency use. 16 January 2021 [2021-05-08]. (原始内容存档于2021-02-12).
- ^ PNG prime minister first to be vaccinated with Australian-supplied doses 'to show it's safe'. The Guardian. 2021-03-30 [2021-04-01]. (原始内容存档于2021-04-28).
- ^ Australia gives Covid-19 vaccine doses to hard-hit Papua New Guinea. France 24. 2021-03-17 [2021-04-01]. (原始内容存档于2021-05-06).
- ^ DIGEMID. www.digemid.minsa.gob.pe. [2021-05-08]. (原始内容存档于2021-05-07) (西班牙语).
- ^ Philippine regulator approves emergency use of AstraZeneca vaccine. Reuters. 28 January 2021 [28 January 2021]. (原始内容存档于2021-02-07).
- ^ Rwanda receives COVID-19 Vaccines through COVAX. World Health Organization (WHO). [19 March 2021]. (原始内容存档于2021-03-10).
- ^ 81.0 81.1 AstraZeneca and Moderna vaccines to be administered in Saudi Arabia. Gulf News. [19 January 2021]. (原始内容存档于2021-01-28).
- ^ Serbia receives first shipment of AstraZeneca COVID-19 vaccine. Reuters. 21 February 2021 [19 March 2021]. (原始内容存档于2021-03-15).
- ^ Bhattacherjee, Kallol. Coronavirus | Myanmar, Mauritius, Seychelles receive Covishield vaccine. The Hindu. 2021-01-22 [2021-04-01]. ISSN 0971-751X. (原始内容存档于2021-04-08).
- ^ COVID-19 vaccines shipped by COVAX arrive in Sierra Leone. World Health Organization (WHO). [19 March 2021]. (原始内容存档于2021-03-17).
- ^ Somalia Receives Its First Batch of COVID-19 Vaccines. Voice of America. [2021-04-01]. (原始内容存档于2021-05-05).
- ^ 86.0 86.1 引用错误:没有为名为
:1的参考文献提供内容 - ^ Kim, Han-joo. S. Korea approves AstraZeneca's COVID-19 vaccine for all adults. Yonhap News. 10 February 2021 [10 February 2021]. (原始内容存档于2021-02-13).
- ^ Maresca, Thomas. South Korea approves AstraZeneca COVID-19 vaccine. United Press International. 10 February 2021 [10 February 2021]. (原始内容存档于2021-03-12).
- ^ COVID-19 vaccination kicks-off in South Sudan. WHO. [2021-04-14]. (原始内容存档于2021-04-16).
- ^ Sri Lanka approves vaccine amid warnings of virus spread. Associated Press. 22 January 2021 [22 January 2021]. (原始内容存档于2021-01-29).
- ^ COVID-19 vaccination in Sudan. UNICEF. [19 March 2021]. (原始内容存档于2021-02-25).
- ^ Sudan receives first delivery of COVID-19 vaccines with over 800,000 doses. UNICEF. [19 March 2021]. (原始内容存档于2021-03-09).
- ^ Taiwan grants emergency authorisation for AstraZeneca COVID-19 vaccine. Reuters. 20 February 2021 [13 March 2021]. (原始内容存档于2021-02-22).
- ^ Tajikistan becomes first country in Central Asia to receive COVID-19 vaccine through COVAX Facility. unicef.org. [2021-04-14]. (原始内容存档于2021-04-13).
- ^ Thai Food and Drug registers COVID-19 vaccine developed by AstraZeneca. Pattaya Mail. 23 January 2021 [2021-05-08]. (原始内容存档于2021-02-11).
- ^ First, Togo. Covid-19: First vaccination campaign to soon begin. togofirst.com. [19 March 2021]. (原始内容存档于2021-02-16).
- ^ Over 500 people given first COVID-19 vaccine shot in Tonga – Xinhua. www.xinhuanet.com. [2021-04-25]. (原始内容存档于2021-04-23).
- ^ Tonga's Princess takes the lead on Covid-19 vaccination. RNZ. 2021-04-16 [2021-04-25]. (原始内容存档于2021-04-21) (New Zealand English).
- ^ Kingdom of Tonga receives 24,000 doses of COVID-19 vaccines through the COVAX facility. www.who.int. [2021-04-25]. (原始内容存档于2021-04-01) (英语).
- ^ 存档副本. [2021-05-08]. (原始内容存档于2021-05-01).
- ^ AfricaNews. Uganda starts COVID-19 vaccinations. Africanews. 10 March 2021 [19 March 2021]. (原始内容存档于2021-03-10).
- ^ COVID-19: AstraZeneca vaccine certified for use in Ukraine. covid.unian.info. [10 March 2021]. (原始内容存档于2021-03-13).
- ^ Information for Healthcare Professionals on COVID-19 Vaccine AstraZeneca. Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020 [4 January 2021]. (原始内容存档于2021-03-09).
- ^ Oxford University/AstraZeneca vaccine authorised by UK medicines regulator (新闻稿). Department of Health and Social Care. 30 December 2020 [30 December 2020]. (原始内容存档于2021-03-16).
- ^ Conditions of Authorisation for COVID-19 Vaccine AstraZeneca. Medicines and Healthcare products Regulatory Agency (MHRA). 30 December 2020 [4 January 2021]. (原始内容存档于2021-02-18).
- ^ Vietnam approves AstraZeneca COVID-19 vaccine, cuts short Communist Party congress. CNA. [5 February 2021]. (原始内容存档于2021-02-07).
- ^ 存档副本. [2021-05-08]. (原始内容存档于2021-05-01).
- ^ 108.0 108.1 Zambia sets out plans to vaccinate all people over 18 against COVID-19. Reuters. 2021-03-25 [2021-04-17]. (原始内容存档于2021-05-07).
- ^ Suriname begins coronavirus vaccination campaign with donated doses. Reuters. 23 February 2021 [28 February 2021]. (原始内容存档于2021-03-10).
- ^ 引用错误:没有为名为
carpha-oxford的参考文献提供内容 - ^ 111.0 111.1 Psyllides, George. Coronavirus: 10,000 vaccines handed over to north. cyprus-mail.com/. [2021-04-17]. (原始内容存档于2021-04-15).
- ^ 引用错误:没有为名为
WHO status的参考文献提供内容 - ^ Interim recommendations for use of the AZD1222 (ChAdOx1-S (recombinant)) vaccine against COVID-19 developed by Oxford University and AstraZeneca. World Health Organization (WHO). [27 February 2021]. (原始内容存档于2021-03-08).
- ^ AstraZeneca/Oxford Covid-19 Vaccine Gets Emergency Approval From WHO. Forbes. [16 February 2021]. (原始内容存档于2021-03-19).
- ^ 辉瑞-生物科技COVID-19疫苗概述和安全性. 美国卫生与公众服务部. [2021-04-14].
- ^ Browne, Ryan. What you need to know about BioNTech — the European company behind Pfizer's Covid-19 vaccine. CNBC. 2020-11-11 [2021-04-04] (英语).
- ^ Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. The New England Journal of Medicine. 2020-12-31 [2021-04-13]. (原始内容存档于2021-01-01).
- ^ Vacina da Pfizer é a 1ª a obter registro definitivo no Brasil. G1. 23 February 2021 [23 February 2021] (巴西葡萄牙语).
- ^ Robust assessment ahead of Medsafe approval of vaccine. Ministry of Health. 3 February 2021 [6 February 2021].
- ^ Coronavirus: Saudi Arabia approves Pfizer COVID-19 vaccine for use. Al Arabiya English. 10 December 2020 [10 December 2020].
- ^ Zimmer C, Corum J, Wee SL. Coronavirus Vaccine Tracker. The New York Times. 10 June 2020 [12 December 2020].
- ^ Comirnaty Product information. [31 January 2021] (德语). 已忽略未知参数
|trans-quote=(帮助) - ^ An Open Study of the Safety, Tolerability and Immunogenicity of the Drug "Gam-COVID-Vac" Vaccine Against COVID-19. ClinicalTrials.gov. United States National Library of Medicine. 17 June 2020 [5 March 2021]. NCT04436471.
- ^ Turkmenistan is the first in Central Asia to have registered 'Sputnik V' vaccine. Orient. 18 January 2021.
- ^ Session of the Cabinet of Ministers of Turkmenistan. Turkmenistan today. 22 January 2021.
- ^ Uzbekistan Certifies Russia's Sputnik Vaccine For Mass Use. Agence France-Presse (Barron's). 17 February 2021.
- ^ Covid19: National Pharmaceuticals Agency registers Sputnik V vaccine. Algeria Press service. 10 January 2021.
- ^ 129.0 129.1 129.2 Angola, Congo Republic and Djibouti approve Russia's Sputnik V vaccine. Reuters. 3 March 2021.
- ^ Antigua and Barbuda authorizes Sputnik V. sputnikvaccine.com (新闻稿). 26 March 2021.
- ^ Argentina has registered the Sputnik V vaccine based on Russian clinical trial data (新闻稿). Gamaleya Center. [1 January 2021].
- ^ Armenia approves Russia's Sputnik V coronavirus vaccine -Russia's RDIF. Reuters. 1 February 2021 [1 February 2021].
- ^ Azerbaijan approves Russia's Sputnik V vaccine for use against COVID-19 -Azeri health ministry. Reuters. 12 March 2021.
- ^ Russian Direct Investment Fund. rdif.ru. [2021-04-01].
- ^ Bahrain authorises Sputnik V COVID-19 vaccine for emergency use – Bahrain TV. Reuters. 10 February 2021 [19 February 2021].
- ^ Bangladesh approves Russia's Sputnik V Covid-19 vaccine for emergency use. Dhaka Tribune. 27 April 2021 [27 April 2021].
- ^ Belarus registers Sputnik V vaccine, in first outside Russia – RDIF. Reuters. 21 December 2020 [22 December 2020].
- ^ Ministerio de Salud de Bolivia – Bolivia y Rusia firman contrato para adquirir 5,2 millones de dosis de la vacuna Sputnik-V contra la COVID-19. minsalud.gob.bo. [1 January 2021].
- ^ 139.0 139.1 Sputnik V vaccine registered in Bosnia and Herzegovina's Republika Srpska. TASS. 5 February 2021 [8 February 2021].
- ^ Cameroon approves Russia's Sputnik V coronavirus vaccine for use – RDIF. Reuters. 19 March 2021.
- ^ Sputnik V authorised in Gabon (新闻稿). Gamaleya Center. [17 February 2021].
- ^ Ghana approves Russia's Sputnik V vaccine for emergency use – RDIF. Reuters. 20 February 2021.
- ^ Guatemala authorizes Sputnik V. PharmiWeb.com. [2021-04-01].
- ^ Guinea Begins Administering Russia's Sputnik V Covid-19 Vaccine. Africa news. 31 December 2020.
- ^ Russia's Sputnik V vaccine expands its reach in Latin America. CNN. 3 March 2021.
- ^ Honduras approves use of Sputnik V vaccine against COVID-19. Xinhuanet. 25 February 2021.
- ^ Hungarian drug regulator approves Sputnik V vaccine: website. The Moscow Times. 7 February 2021.
- ^ 148.0 148.1 148.2 Spike J. Hungary approves 2 more vaccines from outside EU amid spike. The Washington Post. 22 March 2021 [22 March 2021].
- ^ India approves Russia's 'Sputnik V' COVID-19 vaccine: Economic Times. Reuters. 12 April 2021.
- ^ Iran approves Russian coronavirus vaccine Sputnik V. Reuters. 26 January 2021.
- ^ Sputnik V authorized in Iraq (新闻稿). PharmiWeb.com. 4 March 2021.
- ^ Jordan approves Russia's Sputnik V vaccine for use against COVID-19 (新闻稿). Reuters. 10 March 2021.
- ^ Kazakhstan begins mass vaccination by Russian Sputnik V. 1 February 2021 [19 February 2021].
- ^ Morocco, Kenya approve Russian coronavirus vaccine for use – RDIF. 10 March 2021 [12 March 2021].
- ^ Sputnik V registered in Kyrgyzstan. Gamaleya Center (新闻稿). 23 February 2021.
- ^ 156.0 156.1 Laos declares Covid-19 vaccinations safe, more to be inoculated next week. The Star. Malaysia. [19 February 2021].
- ^ Lebanon authorises emergency use of Russia's Sputnik V vaccine. Reuters. 5 February 2021.
- ^ Mali approves Russia's Sputnik V vaccine – Russian sovereign wealth fund. Reuters. 30 March 2021.
- ^ Mauritius approves Russia's Sputnik V coronavirus vaccine – Russian RDIF fund. Reuters. 22 March 2021.
- ^ Mexico, Germany warm to Russia's Sputnik V virus vaccine. The Jakarta Post. 3 February 2021.
- ^ Mongolia Approves Russia's Sputnik V Coronavirus Vaccine – RDIF. Urdu Point. 9 February 2021.
- ^ Montenegro and St. Vincent approve Russia's Sputnik V vaccine – RDIF. Reuters. 12 February 2021.
- ^ Morocco orders one million doses of Russia's Sputnik V vaccine. Yabiladi. 11 March 2021.
- ^ Myanmar registers Russia's Sputnik V COVID-19 vaccine. Tass. [19 February 2021].
- ^ Russian Direct Investment Fund. rdif.ru. [2021-04-01].
- ^ Staff, Reuters. Nepal approves Russia's Sputnik V COVID-19 vaccine. Reuters. 2021-04-20 [2021-04-23] (英语).
- ^ Nicaragua approves Russian COVID-19 vaccine. wsoctv. 3 February 2021.
- ^ NRussia's Sputnik V COVID 19 vaccine registered in North Macedonia. TASS. 7 March 2021.
- ^ Govt okays Russian vaccine for 'emergency use'. Dawn. 24 January 2021.
- ^ Palestine has become the first country in the Middle East to register Sputnik V vaccine. RFID. 11 January 2021.
- ^ Panama approves use of Russian vaccine. Newsroom Panama. 1 April 2021 [2 April 2021].
- ^ Paraguay approves Russia's Sputnik V vaccine: RDIF. Reuters. 15 January 2021 [15 January 2021].
- ^ Russia's Sputnik V approved for emergency use in PH. CNN Philippines. 19 March 2021 [19 March 2021].
- ^ Burki TK. The Russian vaccine for COVID-19. The Lancet. Respiratory Medicine //www.ncbi.nlm.nih.gov/pmc/articles/PMC7837053
|PMC=缺少标题 (帮助). November 2020, 8 (11): e85–e86. PMC 7837053 . PMID 32896274. doi:10.1016/S2213-2600(20)30402-1
. PMID 32896274. doi:10.1016/S2213-2600(20)30402-1  .
.
- ^ 175.0 175.1 175.2 Public Health (Emergency Authorisation of COVID-19 Vaccine) Rules, 2021 (PDF). Government of Saint Vincent and the Grenadines. 11 February 2021 [12 February 2021].
- ^ San Marino buys the Sputnik vaccine: "First doses already in the next few days". Unioneonline. 20 February 2021.
- ^ San Marino reopens thanks to Sputnik covid vaccine from Russia. Wanted in Rome. 12 April 2021.
- ^ Agencija odobrila uvoz ruske vakcine Sputnjik V u Srbiju. N1. 31 December 2020 (sr-RS).
- ^ Sputnik V vaccine approved in Seychelles. TASS. 19 March 2021.
- ^ Sputnik V approved for use in Slovakia. rdif.ru. [1 March 2021].
- ^ Sri Lanka approves Russia's Sputnik V vaccine. The Hindu. 4 March 2021.
- ^ Syria authorizes use of Sputnik-V. Roya. 22 February 2021.
- ^ Thai Prime Minister reassures public after most severe COVID-19 wave (新闻稿). Reuters. [26 April 2021].
- ^ Merve Aydogan. Turkey OKs emergency use of Sputnik-V vaccine. Anadolu Agency. 2021-04-30.
- ^ Sputnik V vaccine authorized in Tunisia (新闻稿). Gamaleya Center. [30 January 2021].
- ^ UAE approves Russia's Sputnik vaccine for emergency use. Khaleej Times. 21 January 2021 [21 January 2021].
- ^ Uruguay to start covid 19 Vaccination in April 2021. India Blooms.
- ^ Venezuela firma contrato para la adquisición de la vacuna rusa Sputnik V. Reuters. 29 December 2020 (西班牙语).
- ^ Vietnam approves Russia Covid-19 vaccine for emergency use. VnExpress. [2 April 2021].
- ^ 190.0 190.1 Covid-19: Zimbabwe authorises Sputnik V, Sinovac vaccines for emergency use. news24.com. 9 March 2021.
- ^ A Study to Evaluate Efficacy, Safety, and Immunogenicity of mRNA-1273 Vaccine in Adults Aged 18 Years and Older to Prevent COVID-19. ClinicalTrials.gov. United States National Library of Medicine. 14 July 2020 [27 July 2020]. NCT04470427. (原始内容存档于2020-10-11).
|url-status=和|dead-url=只需其一 (帮助) - ^ Palca J. COVID-19 vaccine candidate heads to widespread testing in U.S.. NPR. 27 July 2020 [27 July 2020]. (原始内容存档于2020-10-11).
|url-status=和|dead-url=只需其一 (帮助) - ^ Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19). ClinicalTrials.gov. United States National Library of Medicine. 16 March 2020 [5 March 2021]. NCT04283461. (原始内容存档于2020-02-27).
- ^ Swissmedic grants authorisation for the COVID-19 vaccine from Moderna (新闻稿). Swiss Agency for Therapeutic Products (Swissmedic). 12 January 2020 [12 January 2020]. (原始内容存档于2021-02-11).
- ^ Information for Healthcare Professionals on COVID-19 Vaccine Moderna. Medicines and Healthcare products Regulatory Agency (MHRA). 1 April 2021 [6 April 2021]. (原始内容存档于2021-02-18).
- ^ Summary of the Public Assessment Report for COVID-19 Vaccine Moderna. Medicines and Healthcare products Regulatory Agency (MHRA). 19 February 2021 [6 April 2021]. (原始内容存档于2021-04-22).
- ^ Regulatory Decision Summary – Moderna COVID-19 Vaccine. Health Canada, Government of Canada. 23 December 2020 [23 December 2020]. (原始内容存档于2021-01-15).
- ^ Honduras a la zaga de la vacunación en Centroamérica. Proceso Digital. 10 April 2021 (西班牙语).
- ^ Israeli Ministry of Health Authorizes COVID-19 Vaccine Moderna for Use in Israel. modernatx.com. 4 January 2021 [4 January 2021]. (原始内容存档于2021-02-17).
- ^ COVID-19: Kuwait unlikely to import Sputnik V. gulfnews.com. [21 April 2021]. (原始内容存档于2021-04-24) (英语).
- ^ Galvez, Daphne. Moderna COVID-19 vaccine gets emergency use authorization. Philippine Daily Inquirer. 5 May 2021 [2021-05-08]. (原始内容存档于2021-05-06).
- ^ Qatar approves emergency use of Moderna's COVID-19 vaccine. [31 March 2021]. (原始内容存档于2021-05-04).
- ^ Singapore becomes first in Asia to approve Moderna's COVID-19 vaccine. Reuters. 3 February 2021 [3 February 2021]. (原始内容存档于2021-02-07).
- ^ 莫德納疫苗獲緊急授權 自費AZ疫苗供應不間斷. Central News Agency. 5 May 2021 [2021-05-08]. (原始内容存档于2021-05-07).
- ^ Thailand reduces quarantine, paperwork for vaccinated. AP NEWS. 1 April 2021 [2021-05-08]. (原始内容存档于2021-04-24).
- ^ FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine (新闻稿). U.S. Food and Drug Administration (FDA). [18 December 2020]. (原始内容存档于2021-03-17).
- ^ Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Moderna COVID-19 Vaccine – United States, December 2020 (PDF). MMWR. Morbidity and Mortality Weekly Report. December 2020, 69 (5152): 1653–56 [2021-05-08]. PMID 33382675. S2CID 229945697. doi:10.15585/mmwr.mm695152e1
 . (原始内容存档 (PDF)于2021-02-09).
. (原始内容存档 (PDF)于2021-02-09).
- ^ WHO lists anti-COVID Moderna vaccine for emergency use. Agence France-Presse (Philippine Daily Inquirer). 1 May 2021 [2021-05-08]. (原始内容存档于2021-05-01).
- ^ Chen W, Al Kaabi N. A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells). Chinese Clinical Trial Registry. 18 July 2020 [15 August 2020]. (原始内容存档于2020-10-11).
- ^ Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. The Lancet. Infectious Diseases. October 2020, 21 (1): 39–51. PMC 7561304
 . PMID 33069281. doi:10.1016/s1473-3099(20)30831-8.
. PMID 33069281. doi:10.1016/s1473-3099(20)30831-8.
- ^ Bahrain approves China's Sinopharm coronavirus vaccine. Arabian Business Industries. 13 December 2020 [2021-05-08]. (原始内容存档于2021-01-21).
- ^ Kuo L. China approves Sinopharm coronavirus vaccine, the country's first for general use. The Washington Post. [2021-05-08]. (原始内容存档于2021-02-09).
- ^ President Ramkalawan and First Lady receives second dose SinoPharm Vaccine. statehouse.gov.sc. [5 February 2021]. (原始内容存档于2021-02-01).
- ^ Wee SL. Chinese Covid-19 Vaccine Gets Key Push, but Doubts Swirl. The New York Times. 9 December 2020 [12 December 2020]. (原始内容存档于2021-03-17).
- ^ Coronavirus: UAE authorises emergency use of vaccine for frontline workers. The National. [24 November 2020]. (原始内容存档于2020-01-02).
- ^ Macau receives first batch of COVID-19 vaccines. IAG. 7 February 2021 [24 February 2021]. (原始内容存档于2021-03-12).
- ^ China 'to provide 400,000 COVID vaccine doses' to Afghanistan. Al Jazeera. [22 March 2021]. (原始内容存档于2021-04-26).
- ^ Presse, AFP-Agence France. Algeria Receives 200,000 Coronavirus Jabs From China. Barrons. [22 March 2021].
- ^ Angola recebeu doação chinesa de 200 mil doses de vacinas Sinopharm. Notícias ao Minuto. 2021-03-25 [2021-04-14]. (原始内容存档于2021-04-16) (葡萄牙语).
- ^ Biannchi, Walter. Argentina approves Sinopharm COVID-19 vaccine for emergency use. Reuters. 21 February 2021 [23 February 2021]. (原始内容存档于2021-02-22).
- ^ Bangladesh approves emergency use of China's Sinopharm vaccine amid supply squeeze. bdnews24.com. 29 April 2021 [29 April 2021]. (原始内容存档于2021-05-01).
- ^ Belarus begins COVID-19 vaccinations with Chinese shots. Belta. 15 March 2021 [16 March 2021]. (原始内容存档于2021-03-18).
- ^ Bolivia begins inoculation with Sinopharm jabs. The Star. Malaysia. [28 February 2021]. (原始内容存档于2021-03-19).
- ^ Vaccine donation from China arrives. The Star. Malaysia. [22 March 2021]. (原始内容存档于2021-03-25).
- ^ Rinith T. Health Ministry grants Emergency Use Authorization to China's Sinopharm vaccine. Khmer Times. 4 February 2021 [4 February 2021].
- ^ Dominica: Melissa Skerrit receives the Sinopharm COVID-19 vaccine. WIC News. 4 March 2021 [7 March 2021]. (原始内容存档于2021-03-10).
- ^ Egypt licenses China's Sinopharm COVID-19 vaccine for emergency use: health minister – Xinhua. Xinhuanet. [2021-05-08]. (原始内容存档于2021-01-05).
- ^ StackPath. dailynewsegypt.com. [22 March 2021].
- ^ Ethiopia to get 300,000 doses of Sinopharm COVID-19 shot, health minister says. Reuters. 2021-03-29 [2021-04-14]. (原始内容存档于2021-05-06).
- ^ Gabon receives 100,000 doses of Sinopharm's vaccine from China. Gabon 24. 12 March 2021 [15 March 2021]. (原始内容存档于2021-03-19).
- ^ Sinopharm vaccine rollout starts this weekend. Stabroek News. 6 March 2021 [7 March 2021]. (原始内容存档于2021-03-10).
- ^ Hungary signs deal for Chinese Sinopharm's COVID-19 vaccine, first in EU. National Post.
- ^ Indonesia approves Sinopharm COVID-19 vaccine for emergency use. Kontan. 30 April 2021 [30 April 2021]. (原始内容存档于2021-05-01).
- ^ Iran Launches Phase Two of Mass Inoculation Campaign. Financial Tribune. 22 February 2021 [23 February 2021]. (原始内容存档于2021-03-07).
- ^ First batch of Chinese Sinopharm vaccine arrives in Jordan. Roya News. [9 January 2021]. (原始内容存档于2021-02-04).
- ^ Satubaldina, Assel. Three Vaccines to Become Available to Kazakh Citizens. The Astana Times. 2021-04-30 [2021-05-01]. (原始内容存档于2021-05-03) (英语).
|url-status=和|dead-url=只需其一 (帮助) - ^ Kharizov, Ruslan. 150,000 doses of Sinopharm coronavirus vaccine delivered to Kyrgyzstan. 24.kg. 19 March 2021 [22 March 2021]. (原始内容存档于2021-03-25).
- ^ Crean, Rosabel. China donates 50,000 doses of Sinopharm vaccine to Lebanon. The Daily Star. [2 March 2021]. (原始内容存档于2021-03-18).
- ^ Rasheed, Aishath Hanaan Hussain. MFDA approves Pfizer, Sinopharm Covid-19 vaccines for emergency use. raajje.mv. [16 March 2021]. (原始内容存档于2021-03-18).
- ^ Mauritania begins COVID-19 vaccination campaign. aa.com.tr. [2021-03-29]. (原始内容存档于2021-04-28).
- ^ Cristina. O mie de studenți și medici-rezidenți din cadrul USMF vor fi imunizați anti-COVID cu vaccinul BBIBP-CorV, produs de către Sinopharm Beijing Institute of Biological Products. Ziarul de Gardă. 19 March 2021 [19 March 2021]. (原始内容存档于2021-04-29) (ro-RO).
- ^ Deputy PM and City Governor get the first dose of Sinopharm vaccine. Montsame. [15 March 2021]. (原始内容存档于2021-03-10).
- ^ Montenegro receives 30,000 doses of China's COVID-19 vaccine. seenews.com. [22 March 2021]. (原始内容存档于2021-03-10).
- ^ Covid-19: Morocco authorizes use of the Sinopharm vaccine. en.yabiladi.com. [2021-05-08]. (原始内容存档于2021-01-30).
- ^ Mozambique expects to vaccinate 16 million against coronavirus by 2022. Reuters. 6 March 2021 [7 March 2021]. (原始内容存档于2021-03-10).
- ^ Namibian, The. Khomas, Erongo first to get vaccinated. The Namibian. [17 March 2021]. (原始内容存档于2021-04-29).
- ^ Poudel, Arjun. China's Shinopharm vaccine gets emergency use authorisation in Nepal. Kathmandu Post. [19 February 2021]. (原始内容存档于2021-02-17).
- ^ North Macedonia looks to Chinese vaccine to revive program. ABC News. [2021-05-01]. (原始内容存档于2021-05-02) (英语).
|url-status=和|dead-url=只需其一 (帮助) - ^ China donates 400,000 doses of Sinopharm COVID-19 vaccine to Niger. CNA. [2021-04-01]. (原始内容存档于2021-03-23).
- ^ What we know about China's Sinopharm COVID vaccine. The Financial Express. [2021-05-01]. (原始内容存档于2021-05-02) (英语).
|url-status=和|dead-url=只需其一 (帮助) - ^ Shahzad A. Pakistan approves Chinese Sinopharm COVID-19 vaccine for emergency use. Reuters. 19 January 2021 [2021-05-08]. (原始内容存档于2021-01-29).
- ^ Peru grants 'exceptional' approval for Sinopharm COVID-19 vaccine – government sources. Reuters. 27 January 2021 [2021-05-08]. (原始内容存档于2021-02-02).
- ^ Somalia rolls out Sinopharm vaccines to boost fight against COVID-19. News Ghana. 2021-04-15 [2021-04-18]. (原始内容存档于2021-04-18).
|url-status=和|dead-url=只需其一 (帮助) - ^ Asala, Kizzi. Senegal Kicks Off COVID-19 Vaccination Campaign with China's Sinopharm. Africanews. [23 February 2021]. (原始内容存档于2021-02-18).
- ^ Serbia Becomes First European Nation To Use China's Sinopharm Vaccine. RadioFreeEurope/RadioLiberty. [2021-05-08]. (原始内容存档于2021-03-09).
- ^ Thomas, Abdul Rashid. Sierra Leone's President Bio leads the way in taking COVID-19 Vaccine. Sierra Leone Telegraph. 15 March 2021 [16 March 2021]. (原始内容存档于2021-03-16).
- ^ NMRA approves sinopharm vaccine for emergency use. Colombo Gazette. 2021-03-19 [2021-03-29]. (原始内容存档于2021-03-19).
|url-status=和|dead-url=只需其一 (帮助) - ^ China To Provide Sudan With 250,000 Doses Of Sinopharm Vaccine On Friday – Ambassador. UrduPoint. [2021-04-14]. (原始内容存档于2021-04-17).
- ^ StackPath. dailynewsegypt.com. [2021-04-14]. (原始内容存档于2021-03-29).
- ^ Sequera, Vivian. Venezuela approves use of China's Sinopharm coronavirus vaccine. Reuters. 1 March 2021 [2 March 2021]. (原始内容存档于2021-03-03).
- ^ Mutsaka, Farai. Zimbabwe starts administering China's Sinopharm vaccines. Toronto Star. 18 February 2021 [20 February 2021]. (原始内容存档于2021-02-18).
- ^ WHO lists additional COVID-19 vaccine for emergency use and issues interim policy recommendations (新闻稿). World Health Organization (WHO). 7 May 2021 [7 May 2021]. (原始内容存档于2021-05-08).
- ^ Taylor, Adam. WHO grants emergency use authorization for Chinese-made Sinopharm coronavirus vaccine. The Washington Post. 7 May 2021 [7 May 2021]. (原始内容存档于2021-05-07).
- ^ Leiden developed Covid-19 vaccine submitted to EMA for approval. 2021-02-16.
- ^ Clinical trial COVID-19 vaccine candidate underway. Janssen Belgium. [2021-03-13]. (原始内容存档于2021-01-15).
- ^ EMA recommends Johnson & Johnson Covid vaccine for approval; Developed in Leiden. NL Times.
- ^ Beth Israel is working with Johnson & Johnson on a coronavirus vaccine. The Boston Globe. 2020-03-12 [2021-05-08]. (原始内容存档于2021-04-11).
- ^ Bahrain first to approve Johnson & Johnson COVID-19 vaccine for emergency use. Reuters. 25 February 2021 [25 February 2021]. (原始内容存档于2021-03-10).
- ^ Bahrain becomes 1st nation to grant J&J shot emergency use. ABC News. 25 February 2021 [25 February 2021]. (原始内容存档于2021-03-10).
- ^ Brazil health regulator approves emergency use of Johnson & Johnson COVID-19 vaccine. Rio de Janeiro: Reuters. 2021-03-31 [2021-04-01].
- ^ Johnson & Johnson COVID-19 vaccine becomes 4th to receive Health Canada approval. CBC News. [5 March 2021]. (原始内容存档于2021-03-19).
- ^ Acosta, Luis Jaime. Colombia grants emergency use for J&J coronavirus vaccine. Bogotá: Reuters. 25 March 2021 [25 March 2021].
- ^ El Comité de Moléculas Nuevas de COFEPRIS informa sobre los resultados de votación para la vacuna COVID-19 (AD26.COV2-S [Recombinante]). www.gob.mx/cofepris. [2021-05-08]. (原始内容存档于2021-05-07) (西班牙语).
- ^ 275.0 275.1 Covaxin, Janssen approved for emergency use in PH. CNN Philippines. 19 April 2021 [2021-05-08]. (原始内容存档于2021-04-20).
- ^ Coronavirus: South Africa rolls out vaccination programme. BBC News. 17 February 2021 [19 February 2021]. (原始内容存档于2021-03-03).
- ^ Lardieri, Alexa. South Korea Approves Johnson & Johnson Vaccine. U.S. News & World Report. 2021-04-07 [2021-04-08]. (原始内容存档于2021-04-13).
- ^ Johnson & Johnson Covid vaccine approved for use in Switzerland. SWI swissinfo.ch. Keystone-SDA/Reuters/sb. [2021-04-01]. (原始内容存档于2021-04-03).
- ^ Swissmedic regulator approves Johnson & Johnson COVID-19 vaccine. Reuters. 22 March 2021 [3 April 2021]. (原始内容存档于2021-03-24).
- ^ Thailand approves Johnson & Johnson's COVID-19 vaccine. Reuters. 2021-03-25 [2021-04-01]. (原始内容存档于2021-04-01).
- ^ FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine (新闻稿). U.S. Food and Drug Administration (FDA). 27 February 2021 [27 February 2021]. (原始内容存档于2021-03-18).
- ^ 存档副本. [2021-05-08]. (原始内容存档于2021-03-10).
- ^ 引用错误:没有为名为
:6的参考文献提供内容 - ^ 引用错误:没有为名为
:8的参考文献提供内容 - ^ Zimmer, Carl; Corum, Jonathan; Wee, Sui-Lee. Coronavirus Vaccine Tracker. The New York Times. [2021-05-08]. (原始内容存档于2020-12-24).
- ^ WHO approves J&J's COVID-19 vaccine for emergency listing. Channel NewsAsia. 13 March 2021 [13 March 2021]. (原始内容存档于2021-03-18).
- ^ 287.0 287.1 Safety and Immunogenicity Study of Inactivated Vaccine for Prevention of SARS-CoV-2 Infection (COVID-19) (Renqiu). ClinicalTrials.gov. United States National Library of Medicine. 12 May 2020 [14 July 2020]. NCT04383574. (原始内容存档于2020-10-11).
- ^ Clinical Trial of Efficacy and Safety of Sinovac's Adsorbed COVID-19 (Inactivated) Vaccine in Healthcare Professionals (PROFISCOV). ClinicalTrials.gov. United States National Library of Medicine. 2 July 2020 [3 August 2020]. NCT04456595. (原始内容存档于2020-10-11).
- ^ PT. Bio Farma. A Phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-COV-2 inactivated vaccine in healthy adults aged 18–59 years in Indonesia. Registri Penyakit Indonesia. 10 August 2020 [15 August 2020]. (原始内容存档于2020-10-11).
- ^ 290.0 290.1 290.2 Liu R. China approves Sinovac Biotech COVID-19 vaccine for general public use. Reuters. 6 February 2021 [7 February 2021]. (原始内容存档于2021-03-03).
- ^ Aliyev J. Azerbaijan kicks off COVID-19 vaccination. Anadolu Agency. [7 February 2021]. (原始内容存档于2021-02-06).
- ^ Ayosso, Akpédjé. Deux ministres vaccinés contre Covid-19. 24haubenin.info. 2021-03-29 [2021-04-07]. (原始内容存档于2021-03-29) (法语).
- ^ Bolívia autoriza uso de vacinas Sputnik V e CoronaVac contra covid-19. noticias.uol.com.br. [6 January 2021]. (原始内容存档于2021-03-04) (巴西葡萄牙语).
- ^ Agency, Anadolu. Turkey sends Chinese COVID-19 vaccines to Bosnia-Herzegovina. Daily Sabah. 2021-03-28 [2021-04-14]. (原始内容存档于2021-04-17).
- ^ McGeever J, Fonseca P. Brazil clears emergency use of Sinovac, AstraZeneca vaccines, shots begin. Reuters. 17 January 2021 [17 January 2021]. (原始内容存档于2021-01-30).
- ^ Chanritheara, Torn. Cambodia Approves AstraZeneca and Sinovac Vaccines for COVID-19 Emergency Use. Cambodianess. [12 February 2021]. (原始内容存档于2021-02-19).
- ^ Chile aprueba el uso de emergencia de la vacuna china de Sinovac contra covid-19. France 24. 20 January 2021 [30 January 2021]. (原始内容存档于2021-02-05).
- ^ Aliyev J. Colombia approves emergency use of CoronaVac vaccine. Anadolu Agency. [7 February 2021]. (原始内容存档于2021-02-17).
- ^ Anticovid vaccines run out as Dominican Republic awaits arrival of more doses. DominicanToday. [10 March 2021]. (原始内容存档于2021-03-11).
- ^ Ecuador signs agreement with Sinovac for 2 million COVID-19 vaccine: minister. National Post. [26 February 2021].
- ^ Egypt approves Sinovac COVID-19 vaccine for emergency use. gulfnews.com. [2021-04-27]. (原始内容存档于2021-05-06) (英语).
- ^ Llegan a El Salvador un millón de dosis de la vacuna china CoronaVac contra el covid-19 de la farmacéutica Sinovac. Noticias de El Salvador – La Prensa Gráfica | Informate con la verdad. [2021-03-29]. (原始内容存档于2021-04-21) (欧洲西班牙语).
- ^ China to donate Sinovac Vaccine to Fiji. Fiji Broadcasting Corporation. [22 March 2021]. (原始内容存档于2021-03-18).
- ^ Soeriaatmadja W. Indonesia grants emergency use approval to Sinovac's vaccine, local trials show 65% efficacy. The Straits Times. 11 January 2021 [11 January 2021]. (原始内容存档于2021-01-30).
- ^ BPOM Grants Emergency Use Authorization for Sinovac Vaccine. Tempo. 11 January 2021 [11 January 2021]. (原始内容存档于2021-01-13).
- ^ 306.0 306.1 Barrera, Adriana. Mexico approves China's CanSino and Sinovac COVID-19 vaccines. Reuters. 11 February 2021 [11 February 2021]. (原始内容存档于2021-03-02).
- ^ DRAP allows emergency authorisation to fifth Covid-19 vaccine. The News International. [2021-04-10]. (原始内容存档于2021-04-10).
- ^ Panama approves emergency use of Chinese vaccine against COVID-19. Xinhuanet. 9 April 2021 [9 April 2021]. (原始内容存档于2021-04-10).
- ^ CoronaVac, vacuna de alta eficacia. Ministerio de Salud Publica Y Bienestar Social. [2021-05-08]. (原始内容存档于2021-03-15).
- ^ Philippines approves Sinovac's COVID-19 vaccine for emergency use. Reuters. 22 February 2021 [2021-05-08]. (原始内容存档于2021-03-19).
- ^ Thepgumpanat, Panarat. Thailand allows emergency use of Sinovac's COVID-19 vaccine. Reuters. 22 February 2021 [23 February 2021]. (原始内容存档于2021-03-16).
- ^ Tunisia approva vaccino cinese Sinovac. Agenzia Nazionale Stampa Associata (in Italian). 5 March 2021 [7 March 2021]. (原始内容存档于2021-03-19) (意大利语).
- ^ Turkey to begin COVID-19 vaccine jabs by this weekend. Anadolu. 11 January 2021 [11 January 2021]. (原始内容存档于2021-02-02).
- ^ Zinets, Natalia. Ukraine approves China's Sinovac COVID-19 vaccine. Reuters. 9 March 2021 [10 March 2021]. (原始内容存档于2021-03-16).
- ^ Use of Sinovac vaccine authorised. Government of Hong Kong. 18 February 2021 [19 February 2021]. (原始内容存档于2021-03-19).
- ^ Whole-Virion Inactivated SARS-CoV-2 Vaccine (BBV152) for COVID-19 in Healthy Volunteers. ClinicalTrials.gov. [2021-05-08]. NCT04471519. (原始内容存档于2021-04-30).
- ^ 317.0 317.1 317.2 317.3 317.4 COVAXIN® – India's First Indigenous COVID-19 Vaccine. Bharat Biotech. [6 April 2021]. (原始内容存档于2021-05-06).
- ^ 318.0 318.1 318.2 318.3 318.4 Covid-19: Bharat Biotech ramps up Covaxin capacity to 700 million doses per annum | India News. The Times of India. [2021-05-08]. (原始内容存档于2021-04-21).
- ^ Iran issues permit for emergency use for three other COVID-19 vaccines: Official. IRNA English. 17 February 2021 [2021-05-08]. (原始内容存档于2021-02-27).
- ^ COVID-19: COVAXIN receives approval of National COVID-19 Vaccination Committee – beSafeMoris. [2021-04-09]. (原始内容存档于2021-03-21).
- ^ México autoriza Covaxin, la vacuna india contra covid-19. Excelsior. 6 April 2021.
- ^ Sharma, Gopal. Nepal becomes third country to give emergency nod to Indian vaccine COVAXIN. Reuters. 19 March 2021 [19 March 2021]. (原始内容存档于2021-04-25).
- ^ India provides 100,000 doses of Covaxin vaccine to Paraguay. Hindustan Times. 30 March 2021 [2021-05-08]. (原始内容存档于2021-05-04).
- ^ @MaritoAbdo. Acaban de llegar 100.000 dosis de vacunas contra el Covid-19, provenientes de la India. La prioridad es avanzar hacia la inmunización de todo el personal de blanco y luego la población de riesgo. Gracias a la República de la India por el apoyo. (推文). 30 March 2021 –通过Twitter.
- ^ Manral, Karan. Zimbabwe approves Covaxin, first in Africa to okay India-made Covid-19 vaccine. Hindustan Times. 4 March 2021 [6 March 2021]. (原始内容存档于2021-03-05).
- ^ Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. August 2020, 396 (10249): 479–88. ISSN 0140-6736. PMID 32702299. doi:10.1016/s0140-6736(20)31605-6. (原始内容存档于2021-04-30) 使用
|archiveurl=需要含有|url=(帮助). 简明摘要. - ^ China approves two more domestic COVID-19 vaccines for public use. Reuters. 25 February 2021 [16 March 2021]. (原始内容存档于2021-02-28).
- ^ ISP Approves Emergency Use And Importation Of Cansino Vaccine To Fight COVID-19. Institute of Public Health of Chile. [2021-04-08]. (原始内容存档于2021-04-18).
- ^ China's CanSino Biologics COVID-19 vaccine receives emergency use approval in Hungary. MSN. 22 March 2021 [22 March 2021]. (原始内容存档于2021-04-16).
- ^ Malaysia's Solution Group to supply 3.5 million doses of CanSino vaccine to government. Reuters. 4 February 2021 [22 March 2021]. (原始内容存档于2021-03-02).
- ^ Shahzad, Asif. Pakistan approves Chinese CanSinoBIO COVID vaccine for emergency use. Reuters. 12 February 2021 [12 February 2021]. (原始内容存档于2021-02-16).
- ^ Study of the Safety, Reactogenicity and Immunogenicity of "EpiVacCorona" Vaccine for the Prevention of COVID-19 (EpiVacCorona). ClinicalTrials.gov. United States National Library of Medicine. 22 September 2020 [16 November 2020]. NCT04368988. (原始内容存档于2021-04-30).
- ^ Turkmenistan registers vaccines for the prevention of infectious diseases. Turkmenistan Today. 29 January 2021 [2021-05-08]. (原始内容存档于2021-03-13).
- ^ Russian vaccine "EpiVacCorona" was registered in Turkmenistan. EN24. 29 January 2021 [2021-05-08]. (原始内容存档于2021-03-19).
- ^ Belarus receives Russia's EpiVacCorona vaccine. Belta. 2021-02-03 [2021-04-05]. (原始内容存档于2021-02-13).
- ^ О регистрации вакцины ФБУН ГНЦ ВБ "Вектор" Роспотребнадзора "ЭпиВакКорона". Rospotrebnadzor. 14 October 2020 [2021-05-08]. (原始内容存档于2020-11-01) (俄语).
- ^ Russia's EpiVacCorona vaccine post-registration trials started. The Pharma Letter. 18 November 2020 [2021-05-08]. (原始内容存档于2021-03-02).
- ^ COVID-19 vaccine development pipeline (Refresh URL to update). Vaccine Centre, London School of Hygiene and Tropical Medicine. 1 March 2021 [10 March 2021]. (原始内容存档于2020-05-18).
- ^ Liu, Roxanne. China IMCAS's COVID-19 vaccine obtained emergency use approval in China. Reuters. 15 March 2021 [15 March 2021]. (原始内容存档于2021-03-18).
- ^ Mamatkulov, Mukhammadsharif. Uzbekistan approves Chinese-developed COVID-19 vaccine. Reuters. 1 March 2021 [2 March 2021]. (原始内容存档于2021-03-10).
- ^ Uzbekistan certifies Chinese vaccine to fight against COVID-19. China Daily. 2 March 2021 [8 March 2021]. (原始内容存档于2021-03-03).
- ^ Wee, Sui-Lee. China approves two more Covid-19 vaccines.. The New York Times. 2021-02-25 [2021-04-01]. ISSN 0362-4331. (原始内容存档于2021-05-03).
- ^ Ryumin, Alexander. Russia registers its third COVID-19 vaccine CoviVac. Moscow: TASS. 20 February 2021 [6 March 2021]. (原始内容存档于2021-02-26).
- ^ Briefing with Deputy Prime Minister Tatyana Golikova, Health Minister Mikhail Murashko and Head of Rospotrebnadzor Anna Popova. Government of Russia. 18 January 2021 [20 February 2021].
- ^ Russia approves its third COVID-19 vaccine, CoviVac. Reuters. 20 February 2021 [2021-05-08]. (原始内容存档于2021-02-20).
- ^ 346.0 346.1 A new vaccine on the scene: Kazakhstan begins rollout of homegrown QazVac. Fortune. 26 April 2021 [2021-05-08]. (原始内容存档于2021-05-04) (英语).
- ^ Ryumin, Alexander. Russia registers its third COVID-19 vaccine CoviVac. Moscow: TASS. 20 February 2021 [6 March 2021]. (原始内容存档于2021-02-26).
- ^ Russia Approves Single-Dose Sputnik Light Covid Vaccine For Use. NDTV Coronavirus. 6 May 2021 [2021-05-08]. (原始内容存档于2021-05-06).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||