二碘化钕:修订间差异
删除的内容 添加的内容
小 Robot: 修正維基語法 2: 修正不正確的 HTML tag 如 </br> → <br /> |
无编辑摘要 |
||
| 第27行: | 第27行: | ||
== 制备 == |
== 制备 == |
||
二碘化钕可以由[[三碘化钕]]在[[真空]], 800 至900 °C 的温度下被金属钕还原而成。<ref name="brauer">{{ |
二碘化钕可以由[[三碘化钕]]在[[真空]], 800 至900 °C 的温度下被金属钕还原而成。<ref name="brauer">{{{cite book | title=Handbuch der präparativen anorganischen Chemie | publisher=Enke | publication-place=Stuttgart | year=1975 | isbn=3-432-02328-6 | oclc=310719485 | language=de | ref={{sfnref | Enke | 1975}} |page=1081}}</ref> |
||
:<math>\mathrm{ Nd + 2 \ NdI_3 \longrightarrow 3 \ NdI_2}</math> |
:<math>\mathrm{ Nd + 2 \ NdI_3 \longrightarrow 3 \ NdI_2}</math> |
||
| 第33行: | 第33行: | ||
:<math>\mathrm{Nd + HgI_2 \longrightarrow NdI_2 + Hg}</math> |
:<math>\mathrm{Nd + HgI_2 \longrightarrow NdI_2 + Hg}</math> |
||
它也可以由钕和碘直接反应而成。<ref name="Gschneidner">{{ |
它也可以由钕和碘直接反应而成。<ref name="Gschneidner">{{cite book | last=Gschneidner | first=Karl | title=Handbook on the physics and chemistry of rare earths | publisher=North Holland/Elsevier | publication-place=Amsterdam Boston | year=2010 | isbn=0-08-093257-6 | oclc=664572103 |page=247}}</ref> |
||
:<math>\mathrm{Nd + I_2 \longrightarrow NdI_2}</math> |
:<math>\mathrm{Nd + I_2 \longrightarrow NdI_2}</math> |
||
这种化合物是于 1961年由 John D. Corbett 首次合成的。<ref name="DOI10.1007/BF01317584"> |
这种化合物是于 1961年由 John D. Corbett 首次合成的。<ref name="DOI10.1007/BF01317584">{{cite journal | last=Jungmann | first=Angelika | last2=Claessen | first2=R. | last3=Zimmermann | first3=R. | last4=Meng | first4=Ge | last5=Steiner | first5=P. | last6=Hüfner | first6=S. | last7=Tratzky | first7=S. | last8=Stöwe | first8=K. | last9=Beck | first9=H. P. | title=Photoemission of LaI2 and CeI2 | journal=Zeitschrift für Physik B Condensed Matter | publisher=Springer Science and Business Media LLC | volume=97 | issue=1 | year=1995 | issn=0722-3277 | doi=10.1007/bf01317584 | pages=25–34}}</ref> |
||
== 性质== |
== 性质== |
||
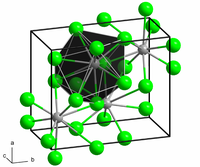
二碘化钕是一种深紫黑色固体,极易[[潮解]]。它的[[晶体结构]]为[[溴化锶]]结构。<ref name="brauer" />在高压下,它会变成[[二硅化钼]]结构,在常态下的其它镧系元素二碘化物中(例如[[二碘化镧]])中存在。<ref name="Ralf Alsfasser, Erwin Riedel">{{ |
二碘化钕是一种深紫黑色固体,极易[[潮解]]。它的[[晶体结构]]为[[溴化锶]]结构。<ref name="brauer" />在高压下,它会变成[[二硅化钼]]结构,在常态下的其它镧系元素二碘化物中(例如[[二碘化镧]])中存在。<ref name="Ralf Alsfasser, Erwin Riedel">{{cite book | last=Riedel | first=Erwin | title=Moderne anorganische Chemie : mit CD-ROM : [133 Tabellen | publisher=Gruyter | publication-place=Berlin | year=2007 | isbn=3-11-019060-5 | oclc=237200027 | language=de |page=188}}</ref>它和[[四氢呋喃]]或其它有机化合物反应,形成配合物。<ref name="DOI10.1002/1521-3757(20010903)">{{cite journal | last=Bochkarev | first=Mikhail N. | last2=Fedushkin | first2=Igor L. | last3=Dechert | first3=Sebastian | last4=Fagin | first4=Anatolii A. | last5=Schumann | first5=Herbert | title=[NdI2(thf)5], der erste kristallographisch charakterisierte Neodym(II)-Komplex | journal=Angewandte Chemie | publisher=Wiley | volume=113 | issue=17 | date=2001-09-03 | issn=0044-8249 | doi=10.1002/1521-3757(20010903)113:17<3268::aid-ange3268>3.0.co;2-k | pages=3268–3270 | language=de}}</ref><ref name="DOI10.1023/A:1026132017155">{{cite journal | last=Khoroshen'kov | first=G. V. | last2=Fagin | first2=A. A. | last3=Bochkarev | first3=M. N. | last4=Dechert | first4=S. | last5=Schumann | first5=H. | journal=Russian Chemical Bulletin | publisher=Springer Science and Business Media LLC | volume=52 | issue=8 | year=2003 | issn=1066-5285 | doi=10.1023/a:1026132017155 | pages=1715–1719}}</ref><ref name="DOI10.1016/j.poly.2005.08.050">{{cite journal | last=Fagin | first=Anatolii A. | last2=Balashova | first2=Tatyana V. | last3=Kusyaev | first3=Dmitrii M. | last4=Kulikova | first4=Tatyana I. | last5=Glukhova | first5=Tatyana A. | last6=Makarenko | first6=Natalya P. | last7=Kurskii | first7=Yurii A. | last8=Evans | first8=William J. | last9=Bochkarev | first9=Mikhail N. | title=Reactions of neodymium(II) iodide with organohalides | journal=Polyhedron | publisher=Elsevier BV | volume=25 | issue=5 | year=2006 | issn=0277-5387 | doi=10.1016/j.poly.2005.08.050 | pages=1105–1110}}</ref> |
||
二碘化钕可以还原热的氮气,形成[[氮碘化物]] (NdI<sub>2</sub>)<sub>3</sub>N,后者和THF反应生成 (Nd<sub>I</sub>)<sub>3</sub>N<sub>2</sub>。<ref>{{cite journal |last1=Fagin |first1=A. A. |last2=Salmova |first2=S. V. |last3=Bochkarev |first3=M. N. |title=Reduction of nitrogen with neodymium(II) and dysprosium(II) diiodides and selected properties of the resulting nitrides |journal=Russian Chemical Bulletin |date=January 2009 |volume=58 |issue=1 |pages=230–233 |doi=10.1007/s11172-009-0034-2}}</ref> |
|||
== 用处== |
== 用处== |
||
二碘化钕是一种[[还原剂]],也是有机化学<ref>{{ |
二碘化钕是一种[[还原剂]],也是有机化学<ref>{{cite book | last=Gschneidner | first=Karl | title=Handbook on the physics and chemistry of rare earths | publisher=North Holland/Elsevier | publication-place=Amsterdam Boston | year=2010 | isbn=0-08-093257-6 | oclc=664572103 |page=261}}</ref>的[[催化剂]]<ref name="Fundamental Chemistry">{{cite book | last=Nuyken | first=O | title=Neodymium based Ziegler catalysts : fundamental chemistry | publisher=Springer | publication-place=Berlin Great Britain | year=2006 | isbn=3-540-34809-3 | oclc=262692810 |page=13}}</ref>。 |
||
== 参考资料== |
== 参考资料== |
||
2022年4月4日 (一) 13:21的版本
| 二碘化钕 | |
|---|---|
| |
| 识别 | |
| CAS号 | 61393-36-0 |
| PubChem | 57351516 |
| SMILES |
|
| EC编号 | 622-142-8 |
| 性质 | |
| 化学式 | I2Nd |
| 摩尔质量 | 398.05 g·mol−1 |
| 外观 | 深紫黑色固体[1] |
| 密度 | 5.85 g·cm−3[2] |
| 熔点 | 562 °C[1] |
| 相关物质 | |
| 其他阴离子 | 二氟化钕 二氯化钕 二溴化钕 |
| 其他阳离子 | 二碘化钐 |
| 相关化学品 | 三碘化钕 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
制备
二碘化钕可以由三碘化钕在真空, 800 至900 °C 的温度下被金属钕还原而成。[1]
它也可以由钕和碘直接反应而成。[3]
这种化合物是于 1961年由 John D. Corbett 首次合成的。[4]
性质
二碘化钕是一种深紫黑色固体,极易潮解。它的晶体结构为溴化锶结构。[1]在高压下,它会变成二硅化钼结构,在常态下的其它镧系元素二碘化物中(例如二碘化镧)中存在。[5]它和四氢呋喃或其它有机化合物反应,形成配合物。[6][7][8]
二碘化钕可以还原热的氮气,形成氮碘化物 (NdI2)3N,后者和THF反应生成 (NdI)3N2。[9]
用处
二碘化钕是一种还原剂,也是有机化学[10]的催化剂[11]。
参考资料
- ^ 1.0 1.1 1.2 1.3 1.4 {Handbuch der präparativen anorganischen Chemie. Stuttgart: Enke. 1975: 1081. ISBN 3-432-02328-6. OCLC 310719485 (德语).
- ^ AMERICAN ELEMENTS: Neodymium Iodide NdI2, abgerufen am 2. Mai 2014
- ^ Gschneidner, Karl. Handbook on the physics and chemistry of rare earths. Amsterdam Boston: North Holland/Elsevier. 2010: 247. ISBN 0-08-093257-6. OCLC 664572103.
- ^ Jungmann, Angelika; Claessen, R.; Zimmermann, R.; Meng, Ge; Steiner, P.; Hüfner, S.; Tratzky, S.; Stöwe, K.; Beck, H. P. Photoemission of LaI2 and CeI2. Zeitschrift für Physik B Condensed Matter (Springer Science and Business Media LLC). 1995, 97 (1): 25–34. ISSN 0722-3277. doi:10.1007/bf01317584.
- ^ Riedel, Erwin. Moderne anorganische Chemie : mit CD-ROM : [133 Tabellen. Berlin: Gruyter. 2007: 188. ISBN 3-11-019060-5. OCLC 237200027 (德语).
- ^ Bochkarev, Mikhail N.; Fedushkin, Igor L.; Dechert, Sebastian; Fagin, Anatolii A.; Schumann, Herbert. [NdI2(thf)5], der erste kristallographisch charakterisierte Neodym(II)-Komplex. Angewandte Chemie (Wiley). 2001-09-03, 113 (17): 3268–3270. ISSN 0044-8249. doi:10.1002/1521-3757(20010903)113:17<3268::aid-ange3268>3.0.co;2-k (德语).
- ^ Khoroshen'kov, G. V.; Fagin, A. A.; Bochkarev, M. N.; Dechert, S.; Schumann, H. Russian Chemical Bulletin (Springer Science and Business Media LLC). 2003, 52 (8): 1715–1719. ISSN 1066-5285. doi:10.1023/a:1026132017155. 缺少或
|title=为空 (帮助) - ^ Fagin, Anatolii A.; Balashova, Tatyana V.; Kusyaev, Dmitrii M.; Kulikova, Tatyana I.; Glukhova, Tatyana A.; Makarenko, Natalya P.; Kurskii, Yurii A.; Evans, William J.; Bochkarev, Mikhail N. Reactions of neodymium(II) iodide with organohalides. Polyhedron (Elsevier BV). 2006, 25 (5): 1105–1110. ISSN 0277-5387. doi:10.1016/j.poly.2005.08.050.
- ^ Fagin, A. A.; Salmova, S. V.; Bochkarev, M. N. Reduction of nitrogen with neodymium(II) and dysprosium(II) diiodides and selected properties of the resulting nitrides. Russian Chemical Bulletin. January 2009, 58 (1): 230–233. doi:10.1007/s11172-009-0034-2.
- ^ Gschneidner, Karl. Handbook on the physics and chemistry of rare earths. Amsterdam Boston: North Holland/Elsevier. 2010: 261. ISBN 0-08-093257-6. OCLC 664572103.
- ^ Nuyken, O. Neodymium based Ziegler catalysts : fundamental chemistry. Berlin Great Britain: Springer. 2006: 13. ISBN 3-540-34809-3. OCLC 262692810.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||



