托法替尼
外观
 | |
| 临床资料 | |
|---|---|
| 商品名 | 捷抑炎/Xeljanz, Jaquinus, Tofacinix, Others |
| 其他名称 | CP-690550 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613025 |
| 核准状况 | |
| 怀孕分级 | |
| 给药途径 | 口服给药 |
| 药物类别 | JAK激酶抑制剂 |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | 74% |
| 血浆蛋白结合率 | 40% |
| 药物代谢 | 肝脏(透过CYP3A4和CYP2C19) |
| 生物半衰期 | 3小时 |
| 排泄途径 | Urine |
| 识别信息 | |
| |
| CAS号 | 477600-75-2 |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB配体ID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.928 |
| 化学信息 | |
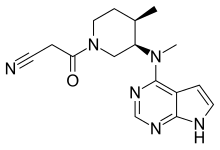
| 化学式 | C16H20N6O |
| 摩尔质量 | 312.38 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
托法替尼(英语:Tofacitinib),商品名捷抑炎(英语:Xeljanz),用于治疗类风湿性关节炎、干癣性关节炎、僵直性脊椎炎、多关节型幼年特发性关节炎和溃疡性结肠炎的药物[9] [10]。它是一种JAK激酶抑制剂, [9] 由美国国家卫生院和辉瑞公司合作发现和研发。
常见副作用包括腹泻、头痛和高血压[11]。严重的副作用可能包括感染、癌症、血管性水肿和肺栓塞[11]。高剂量使用时可能会增加死亡风险[11]。孕期使用可能对胎儿有害[12]。2019 年,欧洲药品管理局安全委员会审查后,建议医师对于肺栓塞高风险族群,暂时不要使用每日两次 10 毫克剂量[13]。美国食品药品监督管理局也发布了有关血栓风险的警告[14] [15] [16]。 另一个重要副作用是严重的细菌、分枝杆菌、真菌和病毒感染。在托法替尼的 III 期临床试验中,有 3 例在试验中得到肺结核 ,他们在研究进行前的结核病筛查均为阴性[17]。
托法替尼分别于2012年及2017年在美国及欧洲获得医疗使用许可[18] [19]。缓释药剂是在2016年2月在美国拿到医疗使用许可[20]。已有学名药存在[21]。
参考文献
[编辑]- ^ Tofacitinib Use During Pregnancy. Drugs.com. 15 April 2020 [23 October 2020].
- ^ Xeljanz/Xeljanz XR (Pfizer Australia Pty Ltd). Therapeutic Goods Administration (TGA). 16 February 2023 [10 April 2023].
- ^ Prescription medicines: registration of new chemical entities in Australia, 2015. Therapeutic Goods Administration (TGA). 21 June 2022 [10 April 2023].
- ^ Product monograph brand safety updates. 加拿大卫生部. 6 June 2024 [8 June 2024].
- ^ 10 mg film-coated tablets - Summary of Product Characteristics (SmPC). (emc). 13 October 2020 [3 November 2020].
- ^ Xeljanz 11 mg prolonged release tablets - Summary of Product Characteristics (SmPC). (emc). [3 November 2020].
- ^ Xeljanz- tofacitinib tablet, film coated Xeljanz XR- tofacitinib tablet, film coated, extended release Xeljanz- tofacitinib solution. DailyMed. 2 October 2020 [3 November 2020].
- ^ Xeljanz EPAR. 欧洲药品管理局 (EMA). 17 September 2018 [3 November 2020]. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ 9.0 9.1 Xeljanz- tofacitinib tablet, film coated Xeljanz XR- tofacitinib tablet, film coated, extended release Xeljanz- tofacitinib solution. DailyMed. 2 October 2020 [3 November 2020].
- ^ Tofacitinib Citrate. The American Society of Health-System Pharmacists. [1 June 2018].
- ^ 11.0 11.1 11.2 Tofacitinib Monograph for Professionals. Drugs.com. [5 October 2021]. (原始内容存档于28 September 2021) (英语).
- ^ Tofacitinib Use During Pregnancy | Drugs.com. Drugs.com. [5 October 2021]. (原始内容存档于29 November 2020).
- ^ Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 13-16 May 2019, May 17, 2019. 欧洲药品管理局. 17 May 2019 [17 May 2019].
- ^ Xeljanz, Xeljanz XR (tofacitinib): Drug Safety Communication - Due to an Increased Risk of Blood Clots and Death with Higher Dose. U.S. 美国食品药品监督管理局 (FDA). 26 July 2019 [10 August 2019]. (原始内容存档于15 December 2019).
- ^ FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) (Podcast). 5 August 2019 [15 December 2019].
- ^ FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). U.S. Food and Drug Administration. 15 December 2019 [15 December 2019]. (原始内容存档于15 December 2019).
- ^ O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Annals of the Rheumatic Diseases. April 2013, 72 (Suppl 2): ii111–ii115. PMC 3616338
 . PMID 23532440. doi:10.1136/annrheumdis-2012-202576.
. PMID 23532440. doi:10.1136/annrheumdis-2012-202576.
- ^ Tofacitinib Monograph for Professionals. Drugs.com. [5 October 2021]. (原始内容存档于28 September 2021) (英语).
- ^ Xeljanz EPAR. European Medicines Agency (EMA). [3 November 2020]. (原始内容存档于28 October 2020). Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Drug Approval Package: Xeljanz (tofacitinib) Extended Release (XR) Tablets NDA #208246. U.S. 美国食品药品监督管理局 (FDA). 26 June 2017 [30 June 2023].
- ^ First Generic Drug Approvals 2023. U.S. 美国食品药品监督管理局 (FDA). 30 May 2023 [30 June 2023]. (原始内容存档于30 June 2023).
