金属蛋白:修订间差异
外观
删除的内容 添加的内容
无编辑摘要 |
|||
| 第8行: | 第8行: | ||
==功能== |
==功能== |
||
据估计,所有蛋白质中有大约一半含有金属<ref>{{cite journal | last1 = Thomson | first1 = A. J. | last2 = Gray | first2 = H. B. | year = 1998 | title = Bioinorganic chemistry | url = | journal = Current Opinion in Chemical Biology | volume = 2 | issue = | pages = 155–158 | doi = 10.1016/S1367-5931(98)80056-2 }}</ref>。据另一个估计,大约四分之一到三分之一的所有蛋白质被提议要求有金属执行其功能<ref>{{cite journal |last1=Waldron |first1=K. J. |last2=Robinson |first2=N. J. |title=How do bacterial cells ensure that metalloproteins get the correct metal? |journal=Nat. Rev. Microbiol. |volume=7 |issue=1 |pages=25–35 |date=January 2009 |pmid=19079350 |doi=10.1038/nrmicro2057}}</ref>。因此,金属蛋白在[[细胞]]中具有许多不同的功能,例如蛋白质的储存和运输,[[酶]]和[[訊息傳遞 (生物)|信号转导]]蛋白。金属离子在感染性疾病中的作用已经被审查<ref>{{cite book|last1=Carver |first1=Peggy L. |editor1-last=Sigel |editor1-first=Astrid |editor2-last=Sigel |editor2-first=Helmut |editor3-last=Sigel |editor3-first=Roland K. O. |title=Interrelations between Essential Metal Ions and Human Diseases |series=Metal Ions in Life Sciences |volume=13 |year=2013 |publisher=Springer |pages=1–28 |chapter=Chapter 1. Metal Ions and Infectious Diseases. An Overview from the Clinic |doi=10.1007/978-94-007-7500-8_1}}</ref>。 |
|||
==配位化学原理== |
==配位化学原理== |
||
==存储与运输相关金属蛋白== |
==存储与运输相关金属蛋白== |
||
2016年11月10日 (四) 05:50的版本

金属蛋白(英語:Metalloprotein)是一类含有配位结合的金属离子作为辅因子的结合蛋白质[1][2]。所有蛋白质中有大量是属于这一类。
这是一类非常重要的蛋白质,在细胞中有多种不同的功能,包括作为酶、运输蛋白、贮存蛋白和信号转导蛋白等。其中的金属离子一般是与多肽链上氨基酸残基中的氮、氧或硫原子或与蛋白质相结合的大环配体相配位。由于金属离子的特殊氧化还原性质,金属酶常用作催化生氧化还原反应的催化剂。
功能
据估计,所有蛋白质中有大约一半含有金属[3]。据另一个估计,大约四分之一到三分之一的所有蛋白质被提议要求有金属执行其功能[4]。因此,金属蛋白在细胞中具有许多不同的功能,例如蛋白质的储存和运输,酶和信号转导蛋白。金属离子在感染性疾病中的作用已经被审查[5]。
配位化学原理
存储与运输相关金属蛋白
氧载体
细胞色素
红氧还蛋白

質體藍素
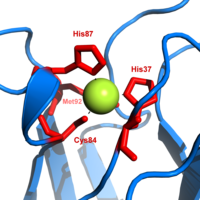
金属离子储存与转运
铁离子
铜离子
金属酶
碳酸酐酶
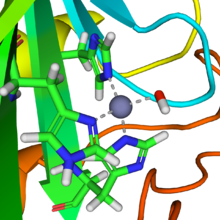
维生素B12依赖酶
固氮酶
超氧化物歧化酶

叶绿素结合蛋白
氢化酶

核酶、脱氧核酶
其它金属酶
There are two types of 一氧化碳脱氢酶: one contains copper and molybdenum, the other contains nickel and iron. Parallels and differences in catalytic strategies have been reviewed.[6] Some other metalloenzymes are given in the following table, according to the metal involved.
| Ion | Examples of enzymes containing this ion |
|---|---|
| Magnesium[7] | Glucose 6-phosphatase Hexokinase DNA polymerase |
| Vanadium | vanabins |
| Manganese[8] | Arginase |
| Iron[9] | Catalase Hydrogenase IRE-BP Aconitase |
| Cobalt[10] | Nitrile hydratase Methionyl aminopeptidase Methylmalonyl-CoA mutase Isobutyryl-CoA mutase |
| Nickel[11][12] | Urease Hydrogenase Methyl-coenzyme M reductase (MCR) |
| Copper[13] | Cytochrome oxidase Laccase Nitrous-oxide reductase Nitrite reductase |
| Zinc[14] | Alcohol dehydrogenase Carboxypeptidase Aminopeptidase Beta amyloid |
| Cadmium[15][16] | Metallothionein thiolate proteins |
| Molybdenum[17] | Nitrate reductase Sulfite oxidase Xanthine oxidase DMSO reductase |
| Tungsten[18] | Acetylene hydratase |
| various | Metallothionein Phosphatase |
信号转导相关金属蛋白
钙调素

肌钙蛋白
转录因子
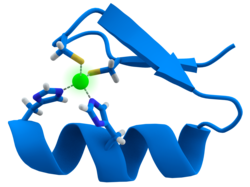
例子
参考文献
- ^ Banci, Lucia. Sigel, Astrid; Sigel, Helmut; Sigel, Roland K. O. , 编. Metallomics and the Cell. Springer. 2013. ISBN 978-94-007-5561-1. ISSN 1868-0402.
- ^ Shriver, D. F.; Atkins, P. W. Chapter 19, Bioinorganic chemistry. Inorganic chemistry 3rd. Oxford University Press. 1999. ISBN 0-19-850330-X.
- ^ Thomson, A. J.; Gray, H. B. Bioinorganic chemistry. Current Opinion in Chemical Biology. 1998, 2: 155–158. doi:10.1016/S1367-5931(98)80056-2.
- ^ Waldron, K. J.; Robinson, N. J. How do bacterial cells ensure that metalloproteins get the correct metal?. Nat. Rev. Microbiol. January 2009, 7 (1): 25–35. PMID 19079350. doi:10.1038/nrmicro2057.
- ^ Carver, Peggy L. Chapter 1. Metal Ions and Infectious Diseases. An Overview from the Clinic. Sigel, Astrid; Sigel, Helmut; Sigel, Roland K. O. (编). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences 13. Springer. 2013: 1–28. doi:10.1007/978-94-007-7500-8_1.
- ^ Jeoung, Jae-Hun; Fesseler, Jochen; Goetzl, Sebastian; Dobbek, Holger. Chapter 3. Carbon Monoxide. Toxic Gas and Fuel for Anaerobes and Aerobes: Carbon Monoxide Dehydrogenases. Peter M.H. Kroneck and Martha E. Sosa Torres (编). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences 14. Springer. 2014: 37–69. doi:10.1007/978-94-017-9269-1_3.
- ^ Romani, Andrea M.P. Chapter 4 Magnesium Homeostasis in Mammalian Cells. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-1_4. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Roth, Jerome; Ponzoni, Silvia; Aschner, Michael. Chapter 6 Manganese Homeostasis and Transport. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-1_6. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Dlouhy, Adrienne C.; Outten, Caryn E. Chapter 8 The Iron Metallome in Eukaryotic Organisms. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-1_8. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Cracan, Valentin; Banerjee, Ruma. Chapter 10 Cobalt and Corrinoid Transport and Biochemistry. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-10_10. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Astrid Sigel, Helmut Sigel and Roland K.O. Sigel (编). Nickel and Its Surprising Impact in Nature. Metal Ions in Life Sciences 2. Wiley. 2008. ISBN 978-0-470-01671-8.
- ^ Sydor, Andrew M.; Zambie, Deborah B. Chapter 11 Nickel Metallomics: General Themes Guiding Nickel Homeostasis. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-10_11. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Vest, Katherine E.; Hashemi, Hayaa F.; Cobine, Paul A. Chapter 13 The Copper Metallome in Eukaryotic Cells. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-10_12. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Maret, Wolfgang. Chapter 14 Zinc and the Zinc Proteome. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-10_14. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ Peackock, Anna F.A.; Pecoraro, Vincent. Chapter 10. Natural and artificial proteins containing cadmium. Astrid Sigel, Helmut Sigel and Roland K. O. Sigel (编). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences 11. Springer. 2013: 303–337. doi:10.1007/978-94-007-5179-8_10.
- ^ Freisinger, Elsa F.A.; Vasac, Milan. Chapter 11. Cadmium in Metallothioneins. Astrid Sigel, Helmut Sigel and Roland K. O. Sigel (编). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences 11. Springer. 2013: 339–372. doi:10.1007/978-94-007-5179-8_11.
- ^ Mendel, Ralf R. Chapter 15 Metabolism of Molybdenum. Banci, Lucia (Ed.) (编). Metallomics and the Cell. Metal Ions in Life Sciences 12. Springer. 2013. ISBN 978-94-007-5560-4. doi:10.1007/978-94-007-5561-10_15. electronic-book ISBN 978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ^ ten Brink, Felix. Chapter 2. Living on acetylene. A Primordial Energy Source. Peter M.H. Kroneck and Martha E. Sosa Torres (编). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences 14. Springer. 2014: 15–35. doi:10.1007/978-94-017-9269-1_2.
| |||||||||||||||||||||||||||||||
|
