利洛司酮
外觀
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | ZK-98734、ZK-734 |
| 識別資訊 | |
| |
| CAS號 | 97747-88-1 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| 化學資訊 | |
| 化學式 | C29H37NO3 |
| 摩爾質量 | 447.62 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
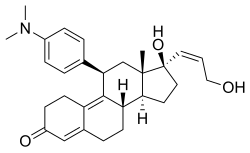
利洛司酮(INN:liropristone;開發代號:ZK-98734、ZK-734)是一種合成類甾體抗孕激素,具有額外的抗糖皮質激素活性。該藥物由先靈公司開發,於1985年獲得專利。[1][2][3][4]它被描述為一種墮胎藥和子宮內膜避孕藥。[1][4][5]該藥物與米非司酮的不同之處僅在於其C17α側鏈的結構,據說相比之下,其抗糖皮質激素活性大大降低。[6]
參考資料
[編輯]- ^ 1.0 1.1 Elks J. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014: 733–. ISBN 978-1-4757-2085-3.
- ^ Rao KA. Textbook of Gynaecology. Elsevier India. November 2009: 187–. ISBN 978-81-312-1526-5.
- ^ Baird DT, Schütz G, Krattenmacher R. Organ-Selective Actions of Steroid Hormones. Springer Science & Business Media. 9 March 2013: 108–. ISBN 978-3-662-09153-1.
- ^ 4.0 4.1 Milne GW. Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. 8 May 2018: 23–. ISBN 978-1-351-78989-9.
- ^ Deshpande H. Practical Management of Ovulation Induction. JP Medical Ltd. 12 February 2016: 29–. ISBN 978-93-5250-028-4.
- ^ Van Look PF, Pérez-Palacios G, World Health Organization . Contraceptive research and development, 1984 to 1994: the road from Mexico City to Cairo and beyond. Oxford University Press. 1994: 169. ISBN 978-0-19-563630-7.
[...] lilopristone, which differs from mifepristone only in the structure of the 17a side chain, is said to have a much reduced antiglucocorticoid activity (Neef et al., 1984).
延伸閱讀
[編輯]- Puri CP, Katkam RR, D'Souza A, Elger WA, Patil RK. Effects of progesterone antagonist, lilopristone (ZK 98.734), on induction of menstruation, inhibition of nidation, and termination of pregnancy in bonnet monkeys. Biology of Reproduction. September 1990, 43 (3): 437–43. PMID 2271724. doi:10.1095/biolreprod43.3.437
 .
. - Puri CP, Patil RK, Kholkute SD, Elger WA, Swamy XR. Progesterone antagonist lilopristone: a potent abortifacient in the common marmoset. American Journal of Obstetrics and Gynecology. July 1989, 161 (1): 248–53. PMID 2502015. doi:10.1016/0002-9378(89)90274-3.
| 這是一篇關於類固醇的小作品。您可以透過編輯或修訂擴充其內容。 |
