碗烯
| 碗烯 | |
|---|---|

| |

| |
| IUPAC名 Dibenzo[ghi,mno]fluoranthene 二苯并[ghi,mno]熒蒽 | |
| 别名 | 心環烯 [5]環烯 |
| 识别 | |
| CAS号 | 5821-51-2 |
| PubChem | 11831840 |
| ChemSpider | 10006487 |
| SMILES |
|
| InChI |
|
| InChIKey | VXRUJZQPKRBJKH-UHFFFAOYAF |
| 性质 | |
| 化学式 | C20H10 |
| 摩尔质量 | 250.29 g/mol g·mol⁻¹ |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
碗烯(英語:Corannulene),又稱心環烯,是一种多环芳香烃,化学式為C20H10,[1]由一个环戊烷周围并五个苯环组成,它具有碗状的空间结构,可看做是富勒烯C60的一个片段。在−64℃时,碗状结构翻转的能垒是42.7kJ/mol(10.2 kcal/mol)。[2]
合成[编辑]
通过快速真空裂解技术得到的碗烯纯度比溶液法低,但同时能得到各种碗烯衍生物。
1966年,碗烯首次由多步有机合成并从产物中分离出来。[3][4][5]由[[荧]蒽]的溴代衍生物在碱催化下通过分子内的亲核取代关环得四溴代的碗烯:
再由过量的正丁基锂通过卤素-锂交换除溴,经水解得到碗烯。
有合成含不同官能团的碗烯衍生物的研究,例如含乙炔基、[2][6][7]醚键、[8]硫醚、[9]含铂官能团、[10]芳基、[11]萉[12]和茚的并环[13][14]的碗烯衍生物。
芳香性[编辑]
一种解释碗烯芳香性的模型将碗烯分成中间6电子和外围14电子的两个芳香性的共轭体系。这个模型由1966年首次合成碗烯的巴特和劳顿提出,[3]他们还给出了这个化合物的命名建议(corannulene意为核+轮烯)。
应用[编辑]
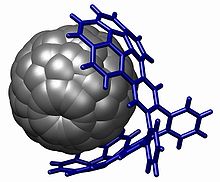
碗烯被用于主客体化学的研究,例如碗烯基团与富勒烯[17][18]和硝基苯之间的π重叠。[19]
长脂肪烃基侧链取代碗烯存在热致变的六角形柱状液晶的中间相。[20]碗烯基团也被用于树形高分子的组装,[11]或研究其作为配体的性质。[21][22][23][24][25][26][27]乙炔基碗烯衍生物对于制造蓝光发射器有潜在的应用价值。[7]
参见[编辑]
参考文献[编辑]
- ^ Scott, L. T.; Bronstein, H. E.; Preda, D. V.; Ansems, R. B. M.; Bratcher, M. S.; Hagen, Stefan. Geodesic polyarenes with exposed concave surfaces. Pure and Applied Chemistry (Walter de Gruyter GmbH). 1999-01-28, 71 (2). ISSN 1365-3075. doi:10.1351/pac199971020209.
- ^ 2.0 2.1 Scott, Lawrence T.; Hashemi, Mohammed M.; Bratcher, Matthew S. Corannulene bowl-to-bowl inversion is rapid at room temperature. Journal of the American Chemical Society (American Chemical Society (ACS)). 1992, 114 (5): 1920–1921. ISSN 0002-7863. doi:10.1021/ja00031a079.
- ^ 3.0 3.1 Barth, Wayne E.; Lawton, Richard G. Dibenzo[ghi,mno]fluoranthene. Journal of the American Chemical Society (American Chemical Society (ACS)). 1966, 88 (2): 380–381. ISSN 0002-7863. doi:10.1021/ja00954a049.
- ^ Scott, Lawrence T.; Hashemi, Mohammed M.; Meyer, Dayton T.; Warren, Hope B. Corannulene. A convenient new synthesis. Journal of the American Chemical Society (American Chemical Society (ACS)). 1991, 113 (18): 7082–7084. ISSN 0002-7863. doi:10.1021/ja00018a082.
- ^ Sygula, Andrzej; Rabideau, Peter W. A Practical, Large Scale Synthesis of the Corannulene System. Journal of the American Chemical Society (American Chemical Society (ACS)). 2000, 122 (26): 6323–6324. ISSN 0002-7863. doi:10.1021/ja0011461.
- ^ Wu, Yao-Ting; Bandera, Davide; Maag, Roman; Linden, Anthony; Baldridge, Kim K.; Siegel, Jay S. Multiethynyl Corannulenes: Synthesis, Structure, and Properties. Journal of the American Chemical Society (American Chemical Society (ACS)). 2008, 130 (32): 10729–10739. ISSN 0002-7863. doi:10.1021/ja802334n.
- ^ 7.0 7.1 Mack, James; Vogel, Philip; Jones, Derek; Kaval, Necati; Sutton, Art. The development of corannulene-based blue emitters. Organic & Biomolecular Chemistry (Royal Society of Chemistry (RSC)). 2007, 5 (15): 2448. ISSN 1477-0520. doi:10.1039/b705621d.
- ^ Gershoni-Poranne, Renana; Pappo, Doron; Solel, Ephrath; Keinan, Ehud. Corannulene Ethers via Ullmann Condensation. Organic Letters (American Chemical Society (ACS)). 2009-11-19, 11 (22): 5146–5149. ISSN 1523-7060. doi:10.1021/ol902352k.
- ^ Baldridge, Kim K.; Hardcastle, Kenneth I.; Seiders, T. Jon; Siegel, Jay S. Synthesis, structure and properties of decakis(phenylthio)corannulene. Org. Biomol. Chem. (Royal Society of Chemistry (RSC)). 2010, 8 (1): 53–55. ISSN 1477-0520. doi:10.1039/b919616a.
- ^ Choi, Hyunbong; Kim, Chulwoo; Park, Ki-Min; Kim, Jinho; Kang, Youngjin; Ko, Jaejung. Synthesis and structure of penta-platinum σ-bonded derivatives of corannulene. Journal of Organometallic Chemistry (Elsevier BV). 2009, 694 (22): 3529–3532. ISSN 0022-328X. doi:10.1016/j.jorganchem.2009.07.015.
- ^ 11.0 11.1 Pappo, Doron; Mejuch, Tom; Reany, Ofer; Solel, Ephrath; Gurram, Mahender; Keinan, Ehud. Diverse Functionalization of Corannulene: Easy Access to Pentagonal Superstructure. Organic Letters (American Chemical Society (ACS)). 2009-03-05, 11 (5): 1063–1066. ISSN 1523-7060. doi:10.1021/ol8028127.
- ^ Nishida, Shinsuke; Morita, Yasushi; Ueda, Akira; Kobayashi, Tadahiro; Fukui, Kozo; Ogasawara, Kanako; Sato, Kazunobu; Takui, Takeji; Nakasuji, Kazuhiro. Curve-Structured Phenalenyl Chemistry: Synthesis, Electronic Structure, and Bowl-Inversion Barrier of a Phenalenyl-Fused Corannulene Anion. Journal of the American Chemical Society (American Chemical Society (ACS)). 2008-11-12, 130 (45): 14954–14955. ISSN 0002-7863. doi:10.1021/ja806708j.
- ^ Steinberg, Brian D.; Jackson, Edward A.; Filatov, Alexander S.; Wakamiya, Atsushi; Petrukhina, Marina A.; Scott, Lawrence T. Aromatic π-Systems More Curved Than C60. The Complete Family of All Indenocorannulenes Synthesized by Iterative Microwave-Assisted Intramolecular Arylations. Journal of the American Chemical Society (American Chemical Society (ACS)). 2009-08-05, 131 (30): 10537–10545. ISSN 0002-7863. doi:10.1021/ja9031852.
- ^ Corannulenylferrocenes: towards a 1D, non-covalent metal–organic nanowire Berit Topolinski , Bernd M. Schmidt , Michael Kathan , Sergej I. Troyanov and Dieter Lentz Chem. Commun., 2012,48, 6298-6300 doi:10.1039/C2CC32275G
- ^ Sygula, Andrzej; Rabideau, Peter W. Structure and inversion barriers of corannulene, its dianion and tetraanion. An ab initio study. Journal of Molecular Structure: THEOCHEM (Elsevier BV). 1995, 333 (3): 215–226. ISSN 0166-1280. doi:10.1016/0166-1280(94)03961-j.
- ^ Monaco, Guglielmo; Scott, Lawrence T.; Zanasi, Riccardo. Magnetic Euripi in Corannulene. The Journal of Physical Chemistry A (American Chemical Society (ACS)). 2008-09-04, 112 (35): 8136–8147. ISSN 1089-5639. doi:10.1021/jp8038779.
- ^ Sygula, Andrzej; Fronczek, Frank R.; Sygula, Renata; Rabideau, Peter W.; Olmstead, Marilyn M. A Double Concave Hydrocarbon Buckycatcher. Journal of the American Chemical Society (American Chemical Society (ACS)). 2007, 129 (13): 3842–3843. ISSN 0002-7863. doi:10.1021/ja070616p.
- ^ Wong, Bryan M. Noncovalent interactions in supramolecular complexes: A study on corannulene and the double concave buckycatcher. Journal of Computational Chemistry (Wiley-Blackwell). 2009-01-15, 30 (1): 51–56. ISSN 0192-8651. doi:10.1002/jcc.21022.
- ^ Kobryn, Lesya; Henry, William P.; Fronczek, Frank R.; Sygula, Renata; Sygula, Andrzej. Molecular clips and tweezers with corannulene pincers. Tetrahedron Letters (Elsevier BV). 2009, 50 (51): 7124–7127. ISSN 0040-4039. doi:10.1016/j.tetlet.2009.09.177.
- ^ Miyajima, Daigo; Tashiro, Kentaro; Araoka, Fumito; Takezoe, Hideo; Kim, Jungeun; Kato, Kenichi; Takata, Masaki; Aida, Takuzo. Liquid Crystalline Corannulene Responsive to Electric Field. Journal of the American Chemical Society (American Chemical Society (ACS)). 2009-01-14, 131 (1): 44–45. ISSN 0002-7863. doi:10.1021/ja808396b.
- ^ Hexahapto Metal Coordination to Curved Polyaromatic Hydrocarbon Surfaces: The First Transition Metal Corannulene Complex T. Jon Seiders, Kim K. Baldridge, Joseph M. O'Connor, and Jay S. Siegel J. Am. Chem. Soc., 1997, 119 (20), pp 4781–4782 doi:10.1021/ja964380t
- ^ d8 Rhodium and Iridium Complexes of Corannulene Jay S. Siegel, Kim K. Baldridge, Anthony Linden, and Reto Dorta J. Am. Chem. Soc., 2006, 128 (33), pp 10644–10645 doi:10.1021/ja062110x
- ^ Petrukhina, Marina A. Coordination of Buckybowls: The First Concave-Bound Metal Complex. Angewandte Chemie International Edition (Wiley-Blackwell). 2008-02-15, 47 (9): 1550–1552. ISSN 1433-7851. doi:10.1002/anie.200704783.
- ^ Zhu, Bolin; Ellern, Arkady; Sygula, Andrzej; Sygula, Renata; Angelici, Robert J. η6-Coordination of the Curved Carbon Surface of Corannulene (C20H10) to (η6-arene)M2+(M = Ru, Os). Organometallics (American Chemical Society (ACS)). 2007, 26 (7): 1721–1728. ISSN 0276-7333. doi:10.1021/om0610795.
- ^ Petrukhina, Marina A.; Sevryugina, Yulia; Rogachev, Andrey Yu.; Jackson, Edward A.; Scott, Lawrence T. Corannulene: A Preference forexo-Metal Binding. X-ray Structural Characterization of [Ru2(O2CCF3)2(CO)4·(η2-C20H10)2]. Organometallics (American Chemical Society (ACS)). 2006, 25 (22): 5492–5495. ISSN 0276-7333. doi:10.1021/om060350f.
- ^ Siegel, Jay S.; Baldridge, Kim K.; Linden, Anthony; Dorta, Reto. d8Rhodium and Iridium Complexes of Corannulene. Journal of the American Chemical Society (American Chemical Society (ACS)). 2006, 128 (33): 10644–10645. ISSN 0002-7863. doi:10.1021/ja062110x.
- ^ Bandera, D., Baldridge, K. K., Linden, A., Dorta, R. and Siegel, J. S. (2011), Stereoselective Coordination of C5-Symmetric Corannulene Derivatives with an Enantiomerically Pure [RhI(nbd*)] Metal Complex. Angewandte Chemie International Edition, 50: 865–867. doi:10.1002/anie.201006877
| |||||||||||||||||||||||||||||||||||||