卡莫氟
| 此條目的引用需要清理,使其符合格式。 (2022年10月8日) |
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | 1-hexylcarbamoyl-5-fluorouracil, HCFU, N-hexylcarbamoyl-5-fluorouracil, Yamaful, NCGC00095165-01, Hexylcarbamoyl fluorouracil, 61422-45-5, UNII-HA82M3RAB2, CCRIS 2759, C11H16FN3O3, Uracil, 5-fluoro-1-hexylcarbamoyl-, BRN 0888898, HA82M3RAB2, 1(2H)-Pyrimidinecarboxamide, 5-fluoro-N-hexyl-3,4, |
| AHFS/Drugs.com | 國際藥品名稱 |
| 給藥途徑 | Oral |
| ATC碼 | |
| 識別資訊 | |
| |
| CAS號 | 61422-45-5 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.315 |
| 化學資訊 | |
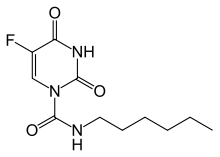
| 化學式 | C11H16FN3O3 |
| 摩爾質量 | 257.27 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
卡莫氟(INN:Carmofur)或HCFU(1-hexylcarbamoyl-5-fluorouracil)是一種嘧啶類似物,用作抗腫瘤劑。它是氟尿嘧啶的衍生物,是可口服給藥的氟尿嘧啶的親脂性掩蔽類似物。[1]
生物學[編輯]
卡莫氟前藥在腸道中被攝入和吸收,克服了二氫嘧啶脫氫酶降解氟尿嘧啶的問題。一旦進入細胞,卡莫氟前藥就會轉化為氟尿嘧啶。
作用機制[編輯]
卡莫氟前藥的作用機制傳統上被認為是產生氟尿嘧啶。[2]然而,卡莫氟是一種高效的酸性神經酰胺酶(AC)抑制劑。[2]神經酰胺影響癌細胞的存活、生長和死亡。[2]酸性神經酰胺酶活性的抑制使腫瘤細胞對抗腫瘤劑和輻射的作用敏感。[2]卡莫氟比替莫唑胺更有效,是一種能夠殺死成人和兒童膠質母細胞瘤的小分子藥物。[3][4]
醫療用途[編輯]
卡莫氟的產品營銷始於1981年。多年來,卡莫氟在中國、日本和芬蘭也被用作根治性切除的結直腸癌患者的輔助化療。[5]試驗和元分析證實,該藥物對這種癌症類型的患者有效,延長了他們的生存期。[6]
卡莫氟已被證明可抑制3C樣蛋白酶,因此是一種有前途的先導化合物,可用於開發新的COVID-19抗病毒治療。[7]
副作用[編輯]
與氟尿嘧啶一樣,卡莫氟會誘發白質腦病,其特徵是大腦中的白質進行性損傷並伴有類似中風的症狀。[8][9][10]
一項針對小肝細胞癌的臨床試驗被提前終止,因為56%的接受治療的患者有不可接受的副作用。此外,該治療對1期和2期癌症患者沒有生存優勢。[11]這可能是為什麼卡莫氟從未在美國獲得FDA批准的原因。[12]
化學合成[編輯]
Ozaki等人報道了通過用光氣和己胺處理氟尿嘧啶合成的卡莫氟。[13]Xiong等人。報道了一種合成卡莫氟的替代方法。化學製劑和結構可以在這裏找到。[1]
參考文獻[編輯]
- ^ 1.0 1.1 Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF. Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs. Chem Rev. Dec 2016, 116 (23): 14379–14455. PMC 7717319
 . PMID 27960273. doi:10.1021/acs.chemrev.6b00209.
. PMID 27960273. doi:10.1021/acs.chemrev.6b00209.
- ^ 2.0 2.1 2.2 2.3 Realini, Natalia; Solorzano, Carlos; Pagliuca, Chiara; Pizzirani, Daniela; Armirotti, Andrea; Luciani, Rosaria; Paola Costi, Maria; Bandiera, Tiziano; Piomelli, Daniele. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Scientific Reports. Jan 2013, 3 (1035): 1035. Bibcode:2013NatSR...3E1035R. PMC 3539145
 . PMID 23301156. doi:10.1038/srep01035.
. PMID 23301156. doi:10.1038/srep01035.
- ^ Doan, Ninh B.; Nguyen, Ha S.; Montoure, Andrew; Al-Gizawiy, Mona M.; Mueller, Wade M.; Kurpad, Shekar; Rand, Scott D.; Connelly, Jennifer M.; Chitambar, Christopher R. Acid ceramidase is a novel drug target for pediatric brain tumors. Oncotarget. 2017-03-01, 8 (15): 24753–24761. ISSN 1949-2553. PMC 5421885
 . PMID 28445970. doi:10.18632/oncotarget.15800.
. PMID 28445970. doi:10.18632/oncotarget.15800.
- ^ Doan, Ninh B.; Alhajala, Hisham; Al-Gizawiy, Mona M.; Mueller, Wade M.; Rand, Scott D.; Connelly, Jennifer M.; Cochran, Elizabeth J.; Chitambar, Christopher R.; Clark, Paul. Acid ceramidase and its inhibitors: a de novo drug target and a new class of drugs for killing glioblastoma cancer stem cells with high efficiency. Oncotarget. 2017-11-23, 8 (68): 112662–112674. ISSN 1949-2553. PMC 5762539
 . PMID 29348854. doi:10.18632/oncotarget.22637.
. PMID 29348854. doi:10.18632/oncotarget.22637.
- ^ Sakamoto J, Oba K, Matsui T, Kobayashi M. Efficacy of oral anticancer agents for colorectal cancer. Dis. Colon Rectum. Oct 2006, 49 (10 Suppl): S82–91. PMID 17106820. S2CID 30655861. doi:10.1007/s10350-006-0601-7.
- ^ Sakamoto, J; Hamada, C; Rahman, M; Kodaira, S; Ito, K; Nakazato, H; Ohashi, Y; Yasutomi, M. An Individual Patient Data Meta-analysis of Adjuvant Therapy with Carmofur in Patients with Curatively Resected Colon Cancer. Japanese Journal of Clinical Oncology. 2005, 35 (9): 536–544. PMID 16155120. doi:10.1093/jjco/hyi147
 .
.
- ^ Jin, Zhenming; Zhao, Yao; Sun, Yuan; Zhang, Bing; Wang, Haofeng; Wu, Yan; Zhu, Yan; Zhu, Chen; Hu, Tianyu; Du, Xiaoyu; Duan, Yinkai; Yu, Jing; Yang, Xiaobao; Yang, Xiuna; Yang, Kailin; Liu, Xiang; Guddat, Luke W.; Xiao, Gengfu; Zhang, Leike; Yang, Haitao; Rao, Zihe. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nature Structural and Molecular Biology. Dec 2020, 27 (6): 529–532. PMID 32382072. doi:10.1038/s41594-020-0440-6
 .
.
- ^ Yamada T, Okamura S, Okazaki T, et al. Leukoencephalopathy following treatment with carmofur: a case report and review of the Japanese literature. Asia-Oceania Journal of Obstetrics and Gynaecology. June 1989, 15 (2): 161–8. PMID 2667512. doi:10.1111/j.1447-0756.1989.tb00171.x.
- ^ Mizutani T. [Leukoencephalopathy caused by antineoplastic drugs]. Brain Nerve. February 2008, 60 (2): 137–41. PMID 18306661 (日語).
- ^ Baehring JM, Fulbright RK. Delayed leukoencephalopathy with stroke-like presentation in chemotherapy recipients. J Neurol Neurosurg Psychiatry. May 2008, 79 (5): 535–9. PMID 17682013. S2CID 38293604. doi:10.1136/jnnp.2007.123737.
- ^ Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. Mar 1996, 83 (3): 336–40. PMID 8665186. S2CID 28134419. doi:10.1002/bjs.1800830313.
- ^ 引用錯誤:沒有為名為
Shelton 20162的參考文獻提供內容 - ^ Ozaki S, Nagase T, Ahmad S, Tamai H, Hoshi A, Iigo M. Synthesis and antitumor activity of 1- or 3-(alpha-hetero substituted)alkyl-5-fluorouracil derivatives. Nucleic Acids Symp Ser. 1987, 18 (18): 1–4. PMID 3697106.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
