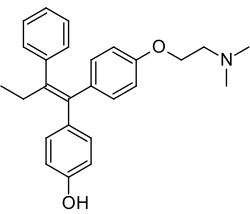
阿非昔芬
外观
 | |
| 臨床資料 | |
|---|---|
| 商品名 | TamoGel |
| 其他名稱 | 4-羟基他莫昔芬、4-OHT、4-HT、OHTAM |
| 给药途径 | 外用(凝胶) |
| 识别信息 | |
| |
| CAS号 | 68392-35-8 |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.120 |
| 化学信息 | |
| 化学式 | C26H29NO2 |
| 摩尔质量 | 387.52 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
阿非昔芬(INN:afimoxifene),也叫4-羟基他莫昔芬(简称4-OHT),是三苯乙烯基的选择性雌激素受体调节剂(SERM),也是他莫昔芬的活性代谢物。[1][2][3]该药物正在开发中,暂定品牌为TamoGel,作为治疗乳腺增生的外用凝胶。[1][4]它已经完成了针对周期性乳腺痛的II期临床试验,[5]但阿非昔芬获批用于该适应症并上市之前还需要进一步研究。[4]
阿非昔芬是一种SERM,因此可作为雌激素受体ERα和ERβ的组织选择性激动剂或拮抗剂,根据组织的不同,具有混合的雌激素和抗雌激素活性。它也是G蛋白偶联雌激素受体(GPER)的激动剂,具有相对较低的亲和力(100-1,000 nM,对比雌二醇的3-6 nM)。[6]除了其雌激素和抗雌激素活性外,阿非昔芬还被发现可作为雌激素相关受体ERRβ和ERRγ的拮抗剂。[7][8][9]
参考资料
[编辑]- ^ 1.0 1.1 Afimoxifene - BHR Pharma. AdisInsight. Springer Nature Switzerland AG.
- ^ Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. The Journal of Pharmacology and Experimental Therapeutics. September 2004, 310 (3): 1062–1075. PMID 15159443. S2CID 21413981. doi:10.1124/jpet.104.065607.
- ^ Statement on a nonproprietary name adopted by the USAN council: Afimoxifene (PDF). American Medical Association. [2008-03-26].
- ^ 4.0 4.1 Goyal A, Mansel RE. Mastalgia. Jatoi I, Rody A (编). Management of Breast Diseases. Springer. 16 November 2016: 77–. ISBN 978-3-319-46356-8.
- ^ Mansel R, Goyal A, Nestour EL, Masini-Etévé V, O'Connell K. A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Research and Treatment. December 2007, 106 (3): 389–397. PMID 17351746. S2CID 22382077. doi:10.1007/s10549-007-9507-x.
- ^ Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators. Pharmacological Reviews. July 2015, 67 (3): 505–540. PMC 4485017
 . PMID 26023144. doi:10.1124/pr.114.009712.
. PMID 26023144. doi:10.1124/pr.114.009712.
- ^ Levine AC. Hormones and Cancer: Breast and Prostate, An Issue of Endocrinology and Metabolism Clinics of North America. Elsevier Health Sciences. 3 October 2011: 271–. ISBN 978-1-4557-1239-7.
- ^ Khetan SK. Anti-Androgenic Chemicals. Endocrine Disruptors in the Environment. Wiley. 23 May 2014: 104–. ISBN 978-1-118-89115-5.
- ^ Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Current Topics in Medicinal Chemistry. 2006, 6 (3): 203–215. PMID 16515477. doi:10.2174/1568026610606030203.
