酶:修订间差异
小 cewbot: 修正維基語法 102: PMID語法錯誤 |
Kaguya-Taketori(留言 | 贡献) 无编辑摘要 |
||
| 第2行: | 第2行: | ||
|G1=生命科学 |
|G1=生命科学 |
||
}} |
}} |
||
[[File:Glucosidase enzyme.png|thumb|400px|{{link-en|葡糖苷酶|Glucosidases}}能將一分子[[麥芽糖]]轉化爲兩分子[[葡萄糖]]。圖中活性位點以紅色表示,麥芽糖以黑色表示,輔酶[[NAD]]以黃色表示({{PDB|1OBB}})|alt=Ribbon diagram of glycosidase with an arrow showing the cleavage of the maltose sugar substrate into two glucose products.]] |
|||
[[File:GLO1 Homo sapiens small fast.gif|thumb|300px|人類乙二醛酶I的帶狀圖,其催化鋅離子顯示兩個紫色的球體。抑制劑,S-hexylglutathione,則表現為一個空間填充模型,填充兩個活性部位。綠色,紅色,藍色和黃色的球體,分別對應於碳,氧,氮和硫原子。]] |
|||
{{Biochemistry sidebar}} |
|||
'''酶'''(Enzymes({{IPAc-en|ˈ|ɛ|n|z|aɪ|m|z}} ))是一種[[大分子]][[生物]][[催化劑]]。酶能加快[[化學反應]]的速度,即具有[[催化作用]]。由酶催化的反應中,反應物稱爲[[底物]](substrates),生成的物質稱爲[[产物_(化学)|產物]]。幾乎所有細胞內[[新陳代謝|代謝過程]]都離不開酶。酶能大大加快這些過程中各化學反應進行的速率,使代謝過程能滿足生物體的需求<ref name = "Stryer_2002">{{cite book |vauthors=Stryer L, Berg JM, Tymoczko JL | title = Biochemistry | publisher = W.H. Freeman | location = San Francisco | year = 2002 | edition = 5th | isbn = 0-7167-4955-6 | url = http://www.ncbi.nlm.nih.gov/books/NBK21154/}}{{Open access}}</ref>{{rp|8.1}}。細胞中酶的類型決定了可在該細胞中發生的[[代謝途徑]]的類型。對酶進行研究的學科稱爲「酶學」(''enzymology'')。 |
|||
[[File:Purine Nucleoside Phosphorylase.jpg|thumb|230px|[[嘌呤核苷磷酸化酶]]三维结构的飘带图示,不同的[[氨基酸]]残基用不同颜色显示]] |
|||
{{TransH}} |
|||
'''酶''',又稱'''酵素''',指具有生物[[催化]]功能的高分子物質,複合球狀蛋白質。<ref>{{en}}{{cite book|author=Smith AD ''et al.'' |title=Oxford Dictionary of Biochemistry and Molecular Biology|year=1997|publisher=Oxford University Press |isbn=0-19-854768-4}}</ref>在酶的催化反应体系中,反应物分子被称为[[底物]],底物通过酶的催化转化为另一种分子。几乎所有的[[细胞]]活动进程都需要酶的参与,以提高效率。与其他非生物[[催化劑]]相似,酶藉著提供另一條[[活化能]](用''E''<sub>a</sub>或Δ''G''<sup>‡</sup>表示)需求較低的途徑来使反應進行,使更多反應粒子能擁有不少於活化能的動能,從而加快反应速率。<ref>{{cite book|author=John C. Kotz, Paul M. Treichel, John Townsend|title=Chemistry and Chemical Reactivity|year=2011|publisher=Cengage Learning|isbn=978-0840048288|pages=P.695 - 697}}</ref>大多数的酶可以将其催化的反应之速率提高上百万倍。酶作为催化剂,本身在反应过程中不被消耗,也不影响反应的[[化学平衡]]。酶有催化作用(加快反應速率),也有[[抑制剂|抑制]]作用(減慢反應速率)。<ref>{{en}}{{cite web|author=IUPAC Gold Book|title=catalyst|url=http://goldbook.iupac.org/C00876.html|publisher=IUPAC|accessdate=2014-02-26}}</ref>与其他非生物[[催化剂]]不同的是,酶具有高度的专一性,只催化特定的反应或产生特定的构型。目前已知的可以被酶催化的反应有约4000种。<ref>{{en}}{{cite journal en |url=http://www.expasy.org/NAR/enz00.pdf|author= Bairoch A.|year= 2000|title= The ENZYME database in 2000 |journal=Nucleic Acids Res|volume=28|pages=304–305|pmid=10592255 }}</ref> |
|||
Enzymes are known to catalyze more than 5,000 biochemical reaction types.<ref>{{cite journal | vauthors = Schomburg I, Chang A, Placzek S, Söhngen C, Rother M, Lang M, Munaretto C, Ulas S, Stelzer M, Grote A, Scheer M, Schomburg D | title = BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA | journal = Nucleic Acids Research | volume = 41 | issue = Database issue | pages = D764–72 | date = January 2013 | pmid = 23203881 | pmc = 3531171 | doi = 10.1093/nar/gks1049 }}</ref> Most enzymes are [[protein]]s, although a few are [[Ribozyme|catalytic RNA molecules]]. Enzymes' specificity comes from their unique [[tertiary structure|three-dimensional structures]]. |
|||
Like all catalysts, enzymes increase the [[reaction rate|rate of a reaction]] by lowering its [[activation energy]]. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is [[orotidine 5'-phosphate decarboxylase]], which allows a reaction that would otherwise take millions of years to occur in milliseconds.<ref name="radzicka">{{cite journal | vauthors = Radzicka A, Wolfenden R | title = A proficient enzyme | journal = Science | volume = 267 | issue = 5194 | pages = 90–931| date = January 1995 | pmid = 7809611 | doi=10.1126/science.7809611| bibcode = 1995Sci...267...90R }}</ref><ref name="pmid17889251">{{cite journal | vauthors = Callahan BP, Miller BG| title = OMP decarboxylase—An enigma persists | journal = Bioorganic Chemistry | volume = 35 | issue = 6 | pages = 465–9 | date = December 2007 | pmid = 17889251 | doi = 10.1016/j.bioorg.2007.07.004 }}</ref> Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the [[Chemical equilibrium|equilibrium]]of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: [[Enzyme inhibitor|inhibitors]] are molecules that decrease enzyme activity, and [[enzyme activator|activators]] are molecules that increase activity. Many [[drug]]s and [[poison]]s are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal [[temperature]] and [[pH]]. |
|||
虽然酶大多是蛋白质,但少數具有生物催化功能的分子并非為蛋白质,有一些被称为[[核酶]]的[[核糖核酸|RNA]]分子<ref>{{en}}{{cite journal en |author=Lilley D |title=Structure, folding and mechanisms of ribozymes |journal=Curr Opin Struct Biol |volume=15 |issue=3 |pages=313-23 |year=2005 |pmid=15919196}}</ref>和一些[[脱氧核糖核酸|DNA]]分子<ref>{{cite book|author=聂剑初,吴国利等|title=生物化学简明教程|year=2007|publisher=高等教育出版社|isbn=978-7-04-007259-4|pages=93}}</ref>同样具有催化功能。此外,通过人工合成所谓[[人工酶]]也具有与酶类似的催化活性。<ref>{{en}}{{cite journal en |author=Groves JT |title=Artificial enzymes. The importance of being selective |journal=Nature |volume=389 |issue=6649 |pages=329-30 |year=1997 |pmid=9311771}}</ref>有人认为酶应定义为具有[[催化]]功能的[[生物大分子]],即生物[[催化剂]],则该定义中酶包含具有催化功能的蛋白质和[[核酶]]。<ref>{{zh}}{{cite journal|author=吴诗光,周琳|year= 2002|title=对酶概念的再认识 |journal=生物学通报|volume=04期}}</ref> |
|||
Some enzymes are used commercially, for example, in the synthesis of [[antibiotics]]. Some household products use enzymes to speed up chemical reactions: enzymes in biological[[washing powder]]s break down protein, starch or [[fat]] stains on clothes, and enzymes in [[papain|meat tenderizer]] break down proteins into smaller molecules, making the meat easier to chew. |
|||
酶的催化活性可以受其他分子影响:[[酶抑制剂|抑制剂]]是可以降低酶活性的分子;[[酶激活剂|激活剂]]则是可以增加酶活性的分子。有许多[[药物]]和[[毒物|毒药]]就是酶的抑制剂。酶的活性还可以被[[温度]]、化学环境(如[[pH值]])、底物浓度以及电磁波(如微波<ref>{{en}}{{cite journal en|author= Young DD, Nichols J, Kelly RM, Deiters A|year= 2008|title=Microwave activation of enzymatic catalysis |journal=J Am Chem Soc|volume=130|pages=10048-10049|pmid= 18613673 }}</ref>)等许多因素所影响。 |
|||
== Etymology and history == |
|||
酶在工业和人们的日常生活中的应用也非常广泛。例如,药厂用特定的合成酶来合成[[抗生素]];加酶[[洗衣粉]]通过分解蛋白质和[[脂肪]]来帮助除去衣物上的污渍和油渍。 |
|||
[[Image:Eduardbuchner.jpg|alt=Photograph of Eduard Buchner.|thumb|180px|left|[[Eduard Buchner]] ]] |
|||
By the late 17th and early 18th centuries, the digestion of [[meat]] by stomach secretions<ref name="Reaumur1752">{{cite journal | vauthors = de Réaumur RA | authorlink = René Antoine Ferchault de Réaumur | year = 1752 | title = Observations sur la digestion des oiseaux|journal = Histoire de l'academie royale des sciences | volume = 1752 | pages = 266, 461 }}</ref>and the conversion of [[starch]] to [[sugar]]s by plant extracts and [[saliva]] were known but the mechanisms by which these occurred had not been identified.<ref>{{cite book | url =http://etext.lib.virginia.edu/toc/modeng/public/Wil4Sci.html | last = Williams | first = Henry Smith | title = A History of Science: in Five Volumes''. ''Volume IV: Modern Development of the Chemical and Biological Sciences | publisher = Harper and Brothers | year = 1904 | name-list-format = vanc }}</ref> |
|||
French chemist [[Anselme Payen]] was the first to discover an enzyme, [[diastase]], in 1833.<ref>{{cite journal | vauthors = Payen A, Persoz JF | year = 1833 | title = Mémoire sur la diastase, les principaux produits de ses réactions et leurs applications aux arts industriels | language = French | trans-title = Memoir on diastase, the principal products of its reactions, and their applications to the industrial arts | journal = Annales de chimie et de physique | series = 2nd | volume = 53 | url = https://books.google.com/?id=Q9I3AAAAMAAJ&pg=PA73 | pages = 73–92}}</ref> A few decades later, when studying the [[fermentation (food)|fermentation]] of sugar to [[alcohol]] by [[yeast]], [[Louis Pasteur]]concluded that this fermentation was caused by a [[Vitalism|vital force]] contained within the yeast cells called "ferments", which were thought to function only within living organisms. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."<ref>{{cite journal | vauthors = Manchester KL | title = Louis Pasteur (1822–1895)–chance and the prepared mind | journal = Trends in Biotechnology | volume = 13 | issue = 12 | pages = 511–5 |date = December 1995 | pmid = 8595136 | doi = 10.1016/S0167-7799(00)89014-9 }}</ref> |
|||
== 发现及研究史 == |
|||
[[File:Louis Pasteur, foto av Félix Nadar.jpg|thumb|left|[[法国]]科学家[[路易·巴斯德]]]] |
|||
酶的发现来源于人们对[[发酵]]机理的逐渐了解。早在18世纪末和19世纪初,人们就认识到食物在[[胃]]中被[[消化作用|消化]],<ref name="Reaumur1752">{{fr}}{{cite journal en | last = de Réaumur | first = RAF | year = 1752 | title = Observations sur la digestion des oiseaux | journal = Histoire de l'academie royale des sciences | volume = 1752|pages = 266, 461}}</ref>用植物的提取液可以将[[淀粉]]转化为[[糖]],但对于其对应的机理则并不了解。<ref>{{en}}Williams, H. S.(1904)[http://etext.lib.virginia.edu/toc/modeng/public/Wil4Sci.html A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences] Harper and Brothers (New York) Accessed 04 April 2007</ref> |
|||
In 1877, German physiologist [[Wilhelm Kühne]] (1837–1900) first used the term ''[[wiktionary:enzyme|enzyme]]'', which comes from [[Ancient Greek|Greek]] ἔνζυμον, "leavened", to describe this process.<ref>Kühne coined the word "enzyme" in: {{cite journal | vauthors = Kühne W | year = 1877 | url = https://books.google.com/?id=jzdMAAAAYAAJ&pg=PA190 |language = German | title = Über das Verhalten verschiedener organisirter und sog. ungeformter Fermente | trans-title = On the behavior of various organized and so-called unformed ferments | journal = Verhandlungen des naturhistorisch-medicinischen Vereins zu Heidelberg | series = new series | volume = 1 | issue = 3 | pages = 190–193 }} The relevant passage occurs on page 190: ''"Um Missverständnissen vorzubeugen und lästige Umschreibungen zu vermeiden schlägt Vortragender vor, die ungeformten oder nicht organisirten Fermente, deren Wirkung ohne Anwesenheit von Organismen und ausserhalb derselben erfolgen kann, als ''Enzyme'' zu bezeichnen."'' (Translation: In order to obviate misunderstandings and avoid cumbersome periphrases, [the author, a university lecturer] suggests designating as "enzymes" the unformed or not organized ferments, whose action can occur without the presence of organisms and outside of the same.)</ref> The word ''enzyme'' was used later to refer to nonliving substances such as [[pepsin]], and the word ''ferment'' was used to refer to chemical activity produced by living organisms.<ref>{{cite book | editor1-first = John L. | editor1-last = Heilbron | title = The Oxford Companion to the History of Modern Science |first1 = Frederic Lawrence | last1 = Holmes | chapter = Enzymes | page = 270 | chapterurl = https://books.google.com/books?id=abqjP-_KfzkC&pg=PA270&lpg=PA270&dq=history+of+enzymes+ferment+living+organisms&source=bl&hl=en | publisher = Oxford University Press | location = Oxford | year = 2003 | name-list-format = vanc }}</ref> |
|||
到了19世纪中叶,法国科学家[[路易·巴斯德]]对[[蔗糖]]转化为[[乙醇|酒精]]的发酵过程进行了研究,认为在[[酵母]][[细胞]]中存在一种活力物质,命名为“酵素”(ferment)。他提出发酵是这种活力物质催化的结果,并认为活力物质只存在于生命体中,细胞破裂就会失去发酵作用。<ref>{{en}}{{cite journal en |author=Dubos J.|year= 1951|title= Louis Pasteur: Free Lance of Science, Gollancz. Quoted in Manchester K. L.(1995)Louis Pasteur(1822–1895)—chance and the prepared mind.|journal= Trends Biotechnol|volume=13|issue=12|pages=511–515|id= PMID 8595136}}</ref> |
|||
[[Eduard Buchner]] submitted his first paper on the study of yeast extracts in 1897. In a series of experiments at the [[Humboldt University of Berlin|University of Berlin]], he found that sugar was fermented by yeast extracts even when there were no living yeast cells in the mixture.<ref name="urlEduard Buchner - Biographical">{{cite web | url =http://nobelprize.org/nobel_prizes/chemistry/laureates/1907/buchner-bio.html | title = Eduard Buchner | work = Nobel Laureate Biography | publisher = Nobelprize.org | accessdate = 23 February 2015 }}</ref> He named the enzyme that brought about the fermentation of sucrose "[[zymase]]".<ref name="urlEduard Buchner - Nobel Lecture: Cell-Free Fermentation">{{cite web| url = http://nobelprize.org/nobel_prizes/chemistry/laureates/1907/buchner-lecture.html | title = Eduard Buchner – Nobel Lecture: Cell-Free Fermentation | year = 1907 | work = Nobelprize.org | accessdate = 23 February 2015 }}</ref> In 1907, he received the [[Nobel Prize in Chemistry]] for "his discovery of cell-free fermentation". Following Buchner's example, enzymes are usually named according to the reaction they carry out: the suffix ''[[-ase]]'' is combined with the name of the [[substrate (biochemistry)|substrate]] (e.g.,[[lactase]] is the enzyme that cleaves [[lactose]]) or to the type of reaction (e.g., [[DNA polymerase]] forms DNA polymers).<ref>The naming of enzymes by adding the suffix "-ase" to the substrate on which the enzyme acts, has been traced to French scientist [[Émile Duclaux]] (1840–1904), who intended to honor the discoverers of [[diastase]] – the first enzyme to be isolated – by introducing this practice in his book {{cite book | author = Duclaux E | title = Traité de microbiologie: Diastases, toxines et venins | language = French |trans-title = Microbiology Treatise: diastases , toxins and venoms | year = 1899 | publisher = Masson and Co | location = Paris, France | url = https://books.google.com/books?id=Kp9EAAAAQAAJ&printsec=frontcover }} See Chapter 1, especially page 9.</ref> |
|||
1878年,[[德国]]生理学家[[威廉·屈内]]首次提出了'''酶'''(enzyme)这一概念。随后,'''酶'''被用于专指[[胃蛋白酶]]等一类非活体物质,而'''酵素'''(ferment)则被用于指由活体细胞产生的催化活性。 |
|||
The biochemical identity of enzymes was still unknown in the early 1900s. Many scientists observed that enzymatic activity was associated with proteins, but others (such as Nobel laureate [[Richard Willstätter]]) argued that proteins were merely carriers for the true enzymes and that proteins ''per se'' were incapable of catalysis.<ref name = "Willstätter_1927">{{cite journal| vauthors = Willstätter R | title = Faraday lecture. Problems and methods in enzyme research | journal = Journal of the Chemical Society (Resumed) |date = 1927 | pages = 1359 | doi = 10.1039/JR9270001359 }} quoted in {{cite journal | vauthors = Blow D | title = So do we understand how enzymes work? | journal = Structure (London, England : 1993) | volume = 8 | issue = 4 | pages = R77–R81 | date = April 2000 | pmid = 10801479 | doi = 10.1016/S0969-2126(00)00125-8 | url =http://cmgm3.stanford.edu/biochem/sb241/Herschlag_lectures/papers/Blow.pdf | format = pdf }}</ref> In 1926, [[James B. Sumner]] showed that the enzyme [[urease]] was a pure protein and crystallized it; he did likewise for the enzyme [[catalase]] in 1937. The conclusion that pure proteins can be enzymes was definitively demonstrated by [[John Howard Northrop]]and [[Wendell Meredith Stanley]], who worked on the digestive enzymes [[pepsin]] (1930), [[trypsin]] and [[chymotrypsin]]. These three scientists were awarded the 1946 Nobel Prize in Chemistry.<ref name="urlThe Nobel Prize in Chemistry 1946">{{cite web | url = http://nobelprize.org/nobel_prizes/chemistry/laureates/1946/ | title = Nobel Prizes and Laureates: The Nobel Prize in Chemistry 1946 | work = Nobelprize.org | accessdate = 23 February 2015 }}</ref> |
|||
[[File:Eduardbuchner.jpg|thumb|[[德国]]科学家[[爱德华·比希纳]]]] |
|||
这种对酶的错误认识很快得到纠正。1897年,德国科学家[[爱德华·比希纳]]开始对不含细胞的酵母提取液进行发酵研究,通过在[[柏林洪堡大學|柏林洪堡大学]]所做的一系列实验最终证明发酵过程并不需要完整的活细胞存在。<ref>{{en}}[http://nobelprize.org/nobel_prizes/chemistry/laureates/1907/buchner-bio.html 诺贝尔奖获得者爱德华·比希纳的简历]Accessed 04 April 2007</ref>他将其中能够发挥发酵作用的酶命名为[[发酵酶]](zymase)。<ref>{{en}}[http://nobelprize.org/nobel_prizes/chemistry/laureates/1907/buchner-lecture.html 爱德华·比希纳在1907年的诺贝尔奖获奖演说]Accessed 04 April 2007</ref>这一贡献打开了通向现代[[酶学]]与现代[[生物化学]]的大门,其本人也因“发现无细胞发酵及相应的生化研究”而获得了1907年的[[诺贝尔化学奖]]。在此之后,酶和酵素两个概念合二为一,并依据比希纳的命名方法,酶的发现者们根据其所催化的反应将它们命名。通常酶的英文名称是在催化底物或者反应类型的名字最后加上-ase的后缀,而对应中文命名也采用类似方法,即在名字最后加上“酶”。例如,[[乳糖酶]](lactase)是能够剪切[[乳糖]](lactose)的酶;[[DNA聚合酶]](DNA polymerase)能够催化DNA聚合反应。 |
|||
The discovery that enzymes could be crystallized eventually allowed their structures to be solved by [[x-ray crystallography]]. This was first done for [[lysozyme]], an enzyme found in tears, saliva and [[egg white]]s that digests the coating of some bacteria; the structure was solved by a group led by [[David Chilton Phillips]] and published in 1965.<ref>{{cite journal | vauthors = Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR | title = Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Ångström resolution | journal = Nature | volume = 206 | issue = 4986 | pages = 757–61 | date = May 1965 | pmid = 5891407 | doi = 10.1038/206757a0 | bibcode = 1965Natur.206..757B }}</ref> This high-resolution structure of lysozyme marked the beginning of the field of [[structural biology]] and the effort to understand how enzymes work at an atomic level of detail.<ref name="pmid10390620">{{cite journal | vauthors = Johnson LN, Petsko GA | title = David Phillips and the origin of structural enzymology | journal = Trends Biochem. Sci. | volume = 24 |issue = 7 | pages = 287–9 | year = 1999 | pmid = 10390620 | doi = 10.1016/S0968-0004(99)01423-1 }}</ref> |
|||
人们在认识到酶是一类不依赖于活体细胞的物质后,下一步工作就是鉴定其生化组成成分。许多早期研究者指出,一些蛋白质与酶的催化活性相关;但包括诺贝尔奖得主[[里夏德·维尔施泰特]]在内的部分科学家认为酶不是蛋白质,他们辩称那些蛋白质只是酶分子的携带者,蛋白质本身并不具有催化活性。1926年,[[美國|美国]]生物化学家[[詹姆斯·B·萨姆纳|詹姆斯·萨姆纳]]完成了一个决定性的实验。他首次从[[刀豆屬|刀豆]]得到[[尿素酶]]结晶,并证明了尿素酶的[[蛋白质]]本质。其后,萨姆纳在1931年在[[过氧化氢酶]]的研究中再次证实了酶为蛋白质。[[约翰·霍华德·诺思罗普]]和[[温德尔·梅雷迪思·斯坦利]]通过对胃蛋白酶、[[胰蛋白酶]]和[[胰凝乳蛋白酶]]等消化性[[蛋白酶]]的研究,最终确认蛋白质可以是酶。以后陆续发现的两千余种酶均证明酶的化学本质是蛋白质。以上三位科学家因此获得1946年度诺贝尔化学奖。<ref>{{en}}[http://nobelprize.org/nobel_prizes/chemistry/laureates/1946/ 1946年度诺贝尔化学奖获得者]Accessed 04 April 2007</ref> |
|||
== Structure == |
|||
由于蛋白质可以[[结晶]],通过[[X射线晶体学]]就可以对酶的三维结构进行研究。第一个获得结构解析的酶分子是[[溶菌酶]],一种在[[眼泪]]、[[唾液]]和[[蛋白|蛋清]]中含量丰富的酶,其功能是溶解[[细菌]]外壳。[[溶菌酶]]结构由[[大卫·菲利浦]](David Phillips)所领导的研究组解析,并于1965年发表。<ref>{{en}}{{cite journal en |author=Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR.|year= 1965|title= Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Angstrom resolution. |journal= Nature |volume=22|issue=206|pages=757–761|id= PMID 5891407}}</ref>这一成果的发表标志着[[结构生物学]]研究的开始,高分辨率的酶三维结构使得对于酶在分子水平上的工作机制的了解成为可能。 |
|||
{{multiple image |
|||
| direction = vertical |
|||
| width = 400 |
|||
| footer = |
|||
| image1 = Enzyme structure.svg |
|||
1980年代,[[托马斯·切赫]]和[[悉尼·奥尔特曼]]分别从[[四膜虫]]的[[核糖體RNA|rRNA]]前体的加工研究和细菌的[[核糖核酸酶P]][[蛋白质复合物|复合物]]的研究中都发现RNA本身具有自我催化作用,并提出了[[核酶]]的概念。这是第一次发现蛋白质以外的具有催化活性的生物分子。 |
|||
| alt1 = Lysozyme displayed as an opaque globular surface with a pronounced cleft which the substrate depicted as a stick diagram snuggly fits into |
|||
1989年,其二人也因此获得诺贝尔化学奖。<ref>{{en}}[http://nobelprize.org/chemistry/laureates/1989/ 1989年度诺贝尔化学奖]授予了[[托马斯·切赫]]和[[悉尼·奥尔特曼]]以奖励他们发现[[核糖核酸|RNA]]分子的催化性质。</ref> |
|||
| caption1 = Organisation of [[protein structure|enzyme structure]] and [[lysozyme]] example. Binding sites in blue, catalytic site in red and [[peptidoglycan]] substrate in black. ({{PDB|9LYZ}}) |
|||
| image2 = Q10 graph c.svg |
|||
== 生物学功能 == |
|||
| alt2 = A graph showing that reaction rate increases exponentially with temperature until denaturation causes it to decrease again. |
|||
在生物体内,酶发挥非常广泛的功能。[[訊息傳遞|信号转导]]和细胞活动的调控都离不开酶,特别是[[激酶]]和[[磷酸酶]]的参与。<ref>{{en}}{{cite journal en |author= Hunter T.|year= 1995|title= Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling.|journal= Cell.|volume= 80 (2)|pages= 225–236|id= PMID 7834742}}</ref>酶也能产生运动,通过催化[[肌球蛋白]]上[[三磷酸腺苷|ATP]]的水解产生[[肌肉收缩]],并且能够作为[[细胞骨架]]的一部分参与运送胞内物质。<ref>{{cite journal en |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=11294886|author= Berg JS, Powell BC, Cheney RE.|year= 2001|title= A millennial myosin census.|journal= Mol Biol Cell.|volume= 12 (4)|pages= 780–794|id= PMID 11294886}}</ref>一些位于[[细胞膜]]上的[[ATP酶]]作为[[离子泵]]参与[[主动运输]]。一些生物体中比较奇特的功能也有酶的参与,例如[[荧光素酶|-{zh:荧; zh-hans:荧; zh-hant:螢}-光素酶]]可以为[[萤火虫]]发光。<ref>{{en}}{{cite journal en |url=http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2030669|author= Meighen EA.|year= 1991|title= Molecular biology of bacterial bioluminescence.|journal= Microbiol Rev.|volume= 55 (1)|pages= 123–142|id= PMID 2030669}}</ref>[[病毒]]中也含有酶,或参与侵染细胞(如[[HIV整合酶]]和[[逆转录酶]]),或参与病毒颗粒从宿主细胞的释放(如[[流行性感冒病毒|流感病毒]]的[[神经氨酸酶]])。 |
|||
| caption2 = Enzyme activity initially increases with temperature ([[Q10 (temperature coefficient)|Q10 coefficient]]) until the enzyme's structure unfolds ([[denaturation (biochemistry)|denaturation]]), leading to an optimal [[rate of reaction]] at an intermediate temperature. |
|||
}} |
|||
{{see also|Protein structure}} |
|||
酶的一个非常重要的功能是参与在动物[[消化系统]]的工作。以[[淀粉酶]]和[[蛋白酶]]为代表的一些酶可以将进入消化道的大分子([[淀粉]]和[[蛋白质]])降解为小分子,以便于肠道吸收。淀粉不能被肠道直接吸收,而酶可以将淀粉水解为[[麥芽糖|麦芽糖]]或更进一步水解为[[葡萄糖]]等肠道可以吸收的小分子。不同的酶分解不同的食物[[底物]]。在[[草食性]][[反刍亚目|反刍动物]]的消化系统中存在一些可以产生[[纤维素酶]]的细菌,纤维素酶可以分解植物[[细胞壁]]中的[[纤维素]],从而提供可被吸收的养料。<ref>{{en}}{{cite journal en |author=Mackie RI, White BA |title=Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output |journal=J. Dairy Sci. |volume=73 |issue=10 |pages=2971–95 |date=1 October 1990 |pmid=2178174 |doi=10.3168/jds.S0022-0302(90)78986-2|last2=White }}</ref> |
|||
Enzymes are generally [[globular protein]]s, acting alone or in larger [[protein complex|complexes]]. Like all proteins, enzymes are linear chains of [[amino acids]] that [[protein folding|fold]] to produce a [[tertiary structure|three-dimensional structure]]. The sequence of the amino acids specifies the structure which in turn determines the catalytic activity of the enzyme.<ref>{{cite journal | vauthors = Anfinsen CB | title = Principles that govern the folding of protein chains | journal = Science | volume = 181 | issue = 4096 |pages = 223–30 | date = July 1973 | pmid = 4124164 | doi = 10.1126/science.181.4096.223 | bibcode = 1973Sci...181..223A }}</ref> Although structure determines function, a novel enzyme's activity cannot yet be predicted from its structure alone.<ref>{{cite journal | vauthors = Dunaway-Mariano D | title = Enzyme function discovery | journal = Structure (London, England : 1993) | volume = 16 | issue = 11 | pages = 1599–600 | date = November 2008 | pmid = 19000810 | doi = 10.1016/j.str.2008.10.001 }}</ref> Enzyme structures unfold ([[denaturation (biochemistry)|denature]]) when heated or exposed to chemical denaturants and this disruption to the structure typically causes a loss of activity.<ref>{{cite book |last1 = Petsko | first1 = Gregory A. | last2 = Ringe | first2 = Dagmar | title = Protein structure and function | date = 2003 | publisher = New Science | location = London | isbn=978-1405119221 | name-list-format = vanc | chapter = Chapter 1: From sequence to structure | chapterurl = https://books.google.com/books?id=2yRDWkHhN9QC&pg=PA27&dq=Protein+Denaturation+unfold+loss+of+function&hl=en | page = 27 }}</ref> Enzyme denaturation is normally linked to temperatures above a species' normal level; as a result, enzymes from bacteria living in volcanic environments such as [[hot spring]]s are prized by industrial users for their ability to function at high temperatures, allowing enzyme-catalysed reactions to be operated at a very high rate. |
|||
[[Image:Glycolysis (zh-cn).svg|thumb|left|480px|糖酵解酶及其在[[糖酵解]]的[[代谢途径]]的功能]] |
|||
Enzymes are usually much larger than their substrates. Sizes range from just 62 amino acid residues, for the [[monomer]] of [[4-Oxalocrotonate tautomerase|4-oxalocrotonate tautomerase]],<ref>{{cite journal | vauthors = Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP | title = 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer | journal = The Journal of Biological Chemistry | volume = 267 | issue = 25 | pages = 17716–21 | date = September 1992 | pmid = 1339435 }}</ref>to over 2,500 residues in the animal [[fatty acid synthase]].<ref>{{cite journal | vauthors = Smith S | title = The animal fatty acid synthase: one gene, one polypeptide, seven enzymes | journal = FASEB Journal | volume = 8 | issue = 15 | pages = 1248–59 | date = December 1994 | pmid = 8001737 }}</ref> Only a small portion of their structure (around 2–4 amino acids) is directly involved in catalysis: the catalytic site.<ref>{{ cite web | url = http://www.ebi.ac.uk/thornton-srv/databases/CSA/ | title = The Catalytic Site Atlas |publisher = The European Bioinformatics Institute | accessdate = 4 April 2007 }}</ref> This catalytic site is located next to one or more [[binding site]]s where residues orient the substrates. The catalytic site and binding site together comprise the enzyme's [[active site]]. The remaining majority of the enzyme structure serves to maintain the precise orientation and dynamics of the active site.<ref name = "Suzuki_2015_7">{{cite book | author = Suzuki H | title = How Enzymes Work: From Structure to Function | publisher = CRC Press| location = Boca Raton, FL | year = 2015 | isbn = 978-981-4463-92-8 | chapter = Chapter 7: Active Site Structure | pages = 117–140 }}</ref> |
|||
在[[代謝途徑|代谢途径]]中,多个酶以特定的顺序发挥功能:前一个酶的产物是后一个酶的底物;每个酶催化反应后,产物被传递到另一个酶。有些情况下,不同的酶可以平行地催化同一个反应,从而允许进行更为复杂的调控:比如一个酶可以以较低的活性持续地催化该反应,而另一个酶在被诱导后可以较高的活性进行催化。 |
|||
In some enzymes, no amino acids are directly involved in catalysis; instead, the enzyme contains sites to bind and orient catalytic [[cofactor (biochemistry)|cofactors]].<ref name="Suzuki_2015_7" /> Enzyme structures may also contain [[allosteric site]]s where the binding of a small molecule causes a [[conformational change]] that increases or decreases activity.<ref>{{cite book | author = Krauss G | title = Biochemistry of Signal Transduction and Regulation | date = 2003 | publisher = Wiley-VCH | location = Weinheim | isbn = 9783527605767 | edition = 3rd | pages = 89–114 | chapter = The Regulations of Enzyme Activity | chapterurl = https://books.google.com/books?id=iAvu2XRLnfYC&pg=PA91&dq=enzyme+metabolic+pathways+feedback+regulation&hl=en&redir_esc=y}}</ref> |
|||
酶的存在确定了整个代谢按正确的途径进行;而一旦没有酶的存在,代谢既不能按所需步骤进行,也无法以足够的速度完成合成以满足细胞的需要。实际上如果没有酶,代谢途径,如[[糖酵解]],无法独立进行。例如,葡萄糖可以直接与[[三磷酸腺苷|ATP]]反应使得其一个或多个碳原子被[[磷酸化]];在没有酶的催化时,这个反应进行得非常缓慢以致可以忽略;而一旦加入[[己糖激酶]],在6位上的碳原子的磷酸化反应获得极大加速,虽然其他碳原子的磷酸化反应也在缓慢进行,但在一段时间后检测可以发现,绝大多数产物为[[葡萄糖-6-磷酸]]。<ref>{{en}}{{cite web|url=http://www.ebi.ac.uk/interpro/potm/2004_2/Page1.htm|author= Jennifer McDowall|accessdate=2015-01-23|title= Enzymes of Glycolysis.}}</ref>于是每个细胞就可以通过这样一套功能性酶来完成代谢途径的整个反应网络。 |
|||
{{-}} |
|||
A small number of [[Ribonucleic acid|RNA]]-based biological catalysts called [[ribozyme]]s exist, which again can act alone or in complex with proteins. The most common of these is the [[ribosome]] which is a complex of protein and catalytic RNA components.<ref name = "Stryer_2002"/>{{rp|2.2}} |
|||
== 结构与催化机理 == |
|||
{{see also|蛋白质结构|酶促反应}} |
|||
[[File:TPI1 structure.png|thumb|300px|[[丙糖磷酸异构酶]](TIM)三维结构的飘带图和半透明的蛋白表面图显示。丙糖磷酸异构酶是典型的[[TIM桶折叠]],图中用不同颜色来表示该酶中所含有的两个TIM桶折叠[[结构域]]。]] |
|||
作为蛋白质,不同种酶之间的大小差别非常大,从62个[[氨基酸]]残基的[[4-草酰巴豆酯互变异构酶]](4-oxalocrotonate tautomerase)<ref>{{en}}{{cite journal en |author=Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP |title=4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer |journal=J. Biol. Chem. |volume=267 |issue=25 |pages=17716-21 |year=1992 |pmid=1339435}}</ref>到超过2500个残基的动物[[脂肪酸合酶]]<ref>{{en}}{{cite journal en |author=Smith S |title=The animal fatty acid synthase: one gene, one polypeptide, seven enzymes |url=http://www.fasebj.org/cgi/reprint/8/15/1248 |journal=FASEB J. |volume=8 |issue=15 |pages=1248–59 |year=1994 |pmid=8001737}}</ref>。酶的[[三维结构]]决定了它们的催化活性和机理。<ref>{{en}}{{cite journal en |author=Anfinsen C.B.|year= 1973|title= Principles that Govern the Folding of Protein Chains|journal= Science|pages= 223–230|id= PMID 4124164}}</ref>大多数的酶都要比它们的催化底物大得多,并且酶分子中只有一小部分(3-4个残基)直接参与催化反应。<ref>{{en}}[http://www.ebi.ac.uk/thornton-srv/databases/CSA/ The Catalytic Site Atlas at The European Bioinformatics Institute] Accessed 04 April 2007</ref>这些参与催化残基加上参与结合底物的残基共同形成了发生催化反应的区域,这一区域就被称为“活性中心”或“[[活化位置|活性位点]]”。有许多酶含有能够结合其催化反应所必需的[[辅因子]]的结合区域。此外,还有一些酶能够结合催化反应的直接或[[代謝途徑|间接]]产物或者底物;这种结合能够增加或降低酶活,是一种[[反馈]]调节手段。 |
|||
== Mechanism == |
|||
与其他非酶蛋白相似,酶能够[[蛋白质折叠|折叠]]形成多种三维结构类型。有一部分酶是由多个[[蛋白质亚基|亚基]]所组成的复合物酶。除了[[嗜熱生物|嗜热菌]]中的酶以外,大多数酶在高温情况下会发生去折叠,其三维结构和酶活性被破坏;对于不同的酶,这种去折叠的可逆性也有所不同。 |
|||
=== |
=== Substrate binding === |
||
Enzymes must bind their substrates before they can catalyse any chemical reaction. Enzymes are usually very specific as to what [[substrate (biochemistry)|substrates]] they bind and then the chemical reaction catalysed. Specificity is achieved by binding pockets with complementary shape, charge and [[hydrophilic]]/[[hydrophobic]] characteristics to the substrates. Enzymes can therefore distinguish between very similar substrate molecules to be [[chemoselectivity|chemoselective]], [[regioselectivity|regioselective]] and[[stereospecificity|stereospecific]].<ref>{{cite journal | vauthors = Jaeger KE, Eggert T | title = Enantioselective biocatalysis optimized by directed evolution | journal = Current Opinion in Biotechnology | volume = 15 | issue = 4 | pages = 305–13 | date = August 2004 | pmid = 15358000 | doi = 10.1016/j.copbio.2004.06.007 }}</ref> |
|||
{{FileTA|Enzymatic mechanism model|png|thumb|left|300px|三种酶催化机制模式图:A. “锁-钥匙”模式;B.诱导契合模式;C.群体移动模式。}} |
|||
通常情况下,酶对于其所催化的反应类型和底物种类具有高度的专一性。酶的活性位点和底物,它们的形状、表面电荷、[[親水性|亲]][[疏水性]]都会影响专一性。酶的催化可以具有很高的[[立体专一性]]、[[区域选择性]]和[[化学选择性]](chemoselectivity |
|||
)。<ref>{{en}}{{cite journal en |author= Jaeger KE, Eggert T.|year= 2004|title= Enantioselective biocatalysis optimized by directed evolution.| journal=Curr Opin Biotechnol.|volume= 15 (4)|pages= 305–313|id= PMID 15358000}}</ref> |
|||
具体来说,酶只对具有特定空间结构的某种或某类底物起作用。例如,[[麦芽糖酶]]只能使[[葡萄糖苷键|α-葡萄糖苷键]]断裂而对[[葡萄糖苷键|β-葡萄糖苷键]]无影响。此外,酶具有对底物[[对映异构|对映异构体]]的识别能力,只能于一种[[对映异构|对映体]]作用,而对另一对映体不起作用。例如,[[胰蛋白酶]]只能水解由[[氨基酸|L-氨基酸]]形成的[[肽键]],而不能作用于[[氨基酸|D-氨基酸]]形成的肽键;[[酵母]]中的酶只能对D-构型[[糖]](如[[葡萄糖|D-葡萄糖]])发酵,而对L-构型无效。 |
|||
Some of the enzymes showing the highest specificity and accuracy are involved in the copying and [[Gene expression|expression]] of the [[genome]]. Some of these enzymes have "[[Proofreading (biology)|proof-reading]]" mechanisms. Here, an enzyme such as [[DNA polymerase]] catalyzes a reaction in a first step and then checks that the product is correct in a second step.<ref>{{cite journal | vauthors = Shevelev IV, Hübscher U | title = The 3' 5' exonucleases | journal = Nature Reviews Molecular Cell Biology | volume = 3 | issue = 5 |pages = 364–76 | date = May 2002 | pmid = 11988770 | doi = 10.1038/nrm804 }}</ref> This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelity mammalian polymerases.<ref name = "Stryer_2002"/>{{rp|5.3.1}} Similar proofreading mechanisms are also found in [[RNA polymerase]],<ref>{{cite journal | vauthors = Zenkin N, Yuzenkova Y, Severinov K | title = Transcript-assisted transcriptional proofreading | journal = Science | volume = 313 | issue = 5786 | pages = 518–20 | date = July 2006 |pmid = 16873663 | doi = 10.1126/science.1127422 | bibcode = 2006Sci...313..518Z }}</ref> [[aminoacyl tRNA synthetase]]s<ref>{{cite journal | vauthors = Ibba M, Soll D | title = Aminoacyl-tRNA synthesis | journal = Annual Review of Biochemistry | volume = 69 | pages = 617–50 | pmid = 10966471 | doi = 10.1146/annurev.biochem.69.1.617 | year=2000}}</ref> and[[ribosome]]s.<ref>{{cite journal | vauthors = Rodnina MV, Wintermeyer W | title = Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms | journal = Annual Review of Biochemistry | volume = 70 | pages = 415–35 | pmid = 11395413 | doi = 10.1146/annurev.biochem.70.1.415 | year=2001}}</ref> |
|||
Conversely, some enzymes display [[enzyme promiscuity]], having broad specificity and acting on a range of different physiologically relevant substrates. Many enzymes possess small side activities which arose fortuitously (i.e. [[Neutral evolution|neutrally]]), which may be the starting point for the evolutionary selection of a new function.<ref name=Tawfik10>{{cite journal | vauthors = Khersonsky O, Tawfik DS | title = Enzyme promiscuity: a mechanistic and evolutionary perspective | journal = Annual Review of Biochemistry | volume = 79 |pages = 471–505 | pmid = 20235827 | pmc = | doi = 10.1146/annurev-biochem-030409-143718 | year=2010}}</ref><ref>{{cite journal | vauthors = O'Brien PJ, Herschlag D | title = Catalytic promiscuity and the evolution of new enzymatic activities | journal = Chemistry & Biology | volume = 6 | issue = 4 | pages = R91–R105 | date = April 1999 | pmid = 10099128| doi = 10.1016/S1074-5521(99)80033-7 }}</ref> |
|||
为了解释酶的专一性,研究者提出了多种可能的酶与底物的结合模式(后两种模式为大多数研究者所倾向): |
|||
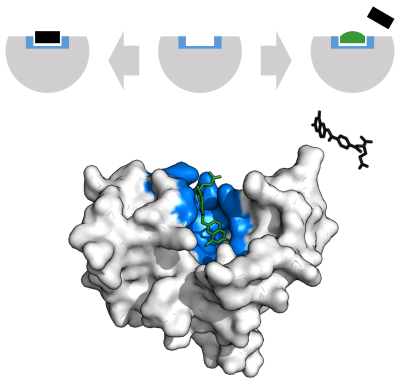
[[File:Hexokinase induced fit.svg|alt=Hexokinase displayed as an opaque surface with a pronounced open binding cleft next to unbound substrate (top) and the same enzyme with more closed cleft that surrounds the bound substrate (bottom)|thumb|400px|Enzyme changes shape by induced fit upon substrate binding to form enzyme-substrate complex. [[Hexokinase]] has a large induced fit motion that closes over the substrates [[adenosine triphosphate]] and [[xylose]]. Binding sites in blue, substrates in black and [[magnesium|Mg<sup>2+</sup>]]cofactor in yellow. ({{PDB|2E2N}}, {{PDB2|2E2Q}})]] |
|||
==== “锁-钥”模式 ==== |
|||
“锁-钥”模式(“Lock and key”,也稱為'''鎖鑰假說''')由[[赫尔曼·埃米尔·费歇尔]]于1894年提出,基于的理论是酶和底物都有一定的外形,当且仅当两者之间的外形能够精确互补时,催化反应才可以发生。<ref>{{de}}{{cite journal en |author= Fischer E.|year= 1894|title= Einfluss der Configuration auf die Wirkung der Enzyme| journal=Ber. Dt. |
|||
Chem. Ges.|volume=27|pages=2985–2993|url = http://gallica.bnf.fr/ark:/12148/bpt6k90736r/f364.chemindefer }}</ref>这一模式通常被形象地称为“锁-钥匙”模式。虽然这一模式能够解释酶的专一性,但却无法说明为什么酶能够稳定反应的过渡态。 |
|||
==== |
==== "Lock and key" model ==== |
||
To explain the observed specificity of enzymes, in 1894 [[Hermann Emil Fischer|Emil Fischer]] proposed that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.<ref>{{cite journal | vauthors = Fischer E | year = 1894 | title = Einfluss der Configuration auf die Wirkung der Enzyme | language = German |trans_title = Influence of configuration on the action of enzymes | journal=Berichte der Deutschen chemischen Gesellschaft zu Berlin | volume = 27 | issue = 3 | pages = 2985–93 | url = http://gallica.bnf.fr/ark:/12148/bpt6k90736r/f364.chemindefer|doi=10.1002/cber.18940270364 }} From page 2992: ''"Um ein Bild zu gebrauchen, will ich sagen, dass Enzym und Glucosid wie Schloss und Schlüssel zu einander passen müssen, um eine chemische Wirkung auf einander ausüben zu können."'' (To use an image, I will say that an enzyme and a glucoside [i.e., glucose derivative] must fit like a lock and key, in order to be able to exert a chemical effect on each other.)</ref> This is often referred to as "the lock and key" model.<ref name="Stryer_2002" />{{rp|8.3.2}} This early model explains enzyme specificity, but fails to explain the stabilization of the transition state that enzymes achieve.<ref name="Cooper_2000">{{cite book |author = Cooper GM | title = The Cell: a Molecular Approach | date = 2000 | publisher = ASM Press | location = Washington (DC ) | isbn = 0-87893-106-6| edition = 2nd | chapter = Chapter 2.2: The Central Role of Enzymes as Biological Catalysts | chapterurl = http://www.ncbi.nlm.nih.gov/books/NBK9921/}}</ref> |
|||
{{FileTA|Induced fit diagram|svg|thumb|400px|诱导契合模式详解图}} |
|||
==== Induced fit model ==== |
|||
诱导契合模式(Induced fit)由[[丹尼尔·科什兰]](Daniel Koshland)通过修改“锁-钥”模式,于1958年提出。基于的理论是,既然酶作为蛋白质,其结构是具有一定柔性的,因此活性位点在结合底物的过程中,通过与底物分子之间的相互作用,可以不断发生微小的形变。<ref>{{en}}{{cite journal en |url=http://www.pnas.org/cgi/reprint/44/2/98|author=Koshland D. E.|year= 1958|title= Application of a Theory of Enzyme Specificity to Protein Synthesis|journal=Proc. Natl. Acad. Sci.|volume=44|issue=2|pages=98–104|id= PMID 16590179}}</ref>在这一模式中,底物不是简单地结合到刚性的活性位点上,活性位点上的氨基酸残基的[[侧链]]可以摆动到正确的位置,使得酶能够进行催化反应。在结合过程中,活性位点不断地发生变化,直到底物完全结合,此时活性位点的形状和带电情况才会最终确定下来。<ref>{{en}}{{cite book en |last=Boyer |first=Rodney |title=Concepts in Biochemistry |origyear=2002 |accessdate=2007-04-21 |edition=2nd ed.|publisher=John Wiley & Sons, Inc. |location=New York, Chichester, Weinheim, Brisbane, Singapore, Toronto. |language=English |isbn=0-470-00379-0 |pages=137–138 |chapter=6}}</ref>在一些情况下,底物在进入活性中心时也是会发生微小形变的,如[[糖苷水解酶|糖苷酶]]的催化反应。<ref>{{en}}{{cite journal|author=Vasella A, Davies GJ, Bohm M.|year= 2002|title= Glycosidase mechanisms.|journal=Curr Opin Chem Biol.|volume=6|issue=5|pages=619–629|id= PMID 12413546}}</ref> |
|||
In 1958, [[Daniel E. Koshland, Jr.|Daniel Koshland]] suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continuously reshaped by interactions with the substrate as the substrate interacts with the enzyme.<ref>{{cite journal | vauthors = Koshland DE | title = Application of a Theory of Enzyme Specificity to Protein Synthesis | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 44 | issue = 2 | pages = 98–104 | date = February 1958 | pmid = 16590179 | pmc = 335371 | doi = 10.1073/pnas.44.2.98 | bibcode = 1958PNAS...44...98K }}</ref> As a result, the substrate does not simply bind to a rigid active site; the amino acid [[Side chain|side-chains]] that make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as [[glycosidases]], the substrate [[molecule]] also changes shape slightly as it enters the active site.<ref>{{cite journal | vauthors = Vasella A, Davies GJ, Böhm M |title = Glycosidase mechanisms | journal = Current Opinion in Chemical Biology | volume = 6 | issue = 5 | pages = 619–29 | date = October 2002 | pmid = 12413546 | doi = 10.1016/S1367-5931(02)00380-0 }}</ref> The active site continues to change until the substrate is completely bound, at which point the final shape and charge distribution is determined.<ref>{{cite book | last = Boyer | first = Rodney | title = Concepts in Biochemistry | edition = 2nd | publisher = John Wiley & Sons, Inc. | location = New York, Chichester, Weinheim, Brisbane, Singapore, Toronto. | isbn = 0-470-00379-0 | pages=137–8 | chapter = Chapter 6: Enzymes I, Reactions, Kinetics, and Inhibition | year = 2002 | oclc = 51720783 |name-list-format = vanc}}</ref> |
|||
Induced fit may enhance the fidelity of molecular recognition in the presence of competition and noise via the [[conformational proofreading]] mechanism.<ref>{{cite journal |vauthors = Savir Y, Tlusty T | title = Conformational proofreading: the impact of conformational changes on the specificity of molecular recognition | journal = PLoS ONE | volume = 2| issue = 5 | pages = e468 | year = 2007 | pmid = 17520027 | pmc = 1868595 | doi = 10.1371/journal.pone.0000468 | url = http://www.weizmann.ac.il/complex/tlusty/papers/PLoSONE2007.pdf| editor1-last = Scalas | editor1-first = Enrico |name-list-format=vanc| bibcode = 2007PLoSO...2..468S }}</ref> |
|||
=== Catalysis === |
|||
群体移动模式(Population shift)是近年来提出的一种新的酶与底物的结合模式,<ref>{{en}}{{cite journal en |author=Kumar S, Ma B, Tsai CJ, Sinha N, Nussinov R.|year= 2000|title= Folding and binding cascades: dynamic landscapes and population shifts|journal=Protein Sci.|volume=9|issue=1|pages=10–19|id= PMID 10739242}}</ref>试图解释在一些酶中所发现的底物结合前后,酶的构象有较大变化,而这是用诱导契合模式无法解释的。其基于的假设是,酶在溶液中同时存在不同构象,一种构象(构象A)为适合底物结合的构象,而另一种(构象B)则不适合,这两种构象之间保持着动态平衡。在没有底物存在的情况下,构象B占主导地位;当加入底物后,随着底物不断与构象A结合,溶液中构象A含量下降,两种构象之间的平衡被打破,导致构象B不断地转化为构象A。 |
|||
{{See also|Enzyme catalysis}} |
|||
=== 机理 === |
|||
酶催化机理多种多样,殊途同归的是最终都能够降低反应的ΔG<sup>‡</sup>:<ref>{{en}}Fersht, A (1985) ''Enzyme Structure and Mechanism''(2nd ed)p50–52 W H Freeman & co, New York ISBN 978-0-7167-1615-0</ref> |
|||
* 创造稳定[[过渡态]]的[[微环境]]。例如,通过与反应的过渡态分子更高的亲和力(与底物分子相比),提高其稳定性;或扭曲底物分子,以使得底物更趋向于转化为过渡态。 |
|||
* 提供不同的反应途径。例如,暂时性地激活底物,形成酶-底物复合物的中间态。 |
|||
* 将反应中不同底物分子结合到一起,并固定其方位至反应能够正确发生的位置,从而降低反应的“门槛”。如果只考虑反应的[[焓]]变(ΔH<sup>‡</sup>),则此作用会被忽略。有趣的是,这一作用同时也会降低反应基态的稳定性,<ref>{{en}}Jencks W.P. "Catalysis in Chemistry and Enzymology." 1987, Dover, New York</ref>因此对于催化的贡献较小。<ref>{{en}}{{cite journal en |author=Villa J, Strajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A |title=How important are entropic contributions to enzyme catalysis? |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=97 |issue=22 |pages=11899-904 |year=2000 |pmid=11050223 |url=http://www.pnas.org/cgi/content/full/97/22/11899}}</ref> |
|||
Enzymes can accelerate reactions in several ways, all of which lower the [[activation energy]] (ΔG<sup>‡</sup>, [[Gibbs free energy]])<ref name="Fersht_1985">{{cite book | author = Fersht A | title = Enzyme Structure and Mechanism | publisher = W.H. Freeman | location = San Francisco | year = 1985 | pages = 50–2 | isbn = 0-7167-1615-1}}</ref> |
|||
==== 过渡态的稳定 ==== |
|||
# By stabilizing the transition state: |
|||
对比同一反应在不受催化和受酶催化的情况,可以了解酶是如何稳定过渡态的。最有效的稳定方式是电荷相互作用,酶可以为过渡态分子上的电荷提供固定的相反电荷,<ref>{{en}}{{cite journal en |author=Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH |title=Electrostatic basis for enzyme catalysis |journal=Chem. Rev. |volume=106 |issue=8 |pages=3210-35 |year=2006 |pmid=16895325}}</ref>而这是在水溶液非催化反应体系中不存在的。 |
|||
#* Creating an environment with a charge distribution complementary to that of the transition state to lower its energy.<ref>{{cite journal | vauthors = Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH | title = Electrostatic basis for enzyme catalysis | journal = Chemical Reviews | volume = 106 | issue = 8 | pages = 3210–35 | date = August 2006 | pmid = 16895325 | doi = 10.1021/cr0503106 }}</ref> |
|||
# By providing an alternative reaction pathway: |
|||
#* Temporarily reacting with the substrate, forming a covalent intermediate to provide a lower energy transition state.<ref>{{cite book | last1 = Cox | first1 = Michael M. | last2 = Nelson | first2 = David L. | title = Lehninger Principles of Biochemistry | date = 2013 | publisher = W.H. Freeman | location = New York, N.Y. | isbn = 978-1464109621 | edition = 6th| chapter = Chapter 6.2: How enzymes work | page = 195 | chapterurl = http://www.amazon.co.uk/Lehninger-Principles-Biochemistry-David-Nelson/dp/1464109621/ref=sr_1_1?s=books&ie=UTF8&qid=1425406097&sr=1-1&keywords=9781464109621 | name-list-format = vanc }}</ref> |
|||
# By destabilising the substrate ground state: |
|||
#* Distorting bound substrate(s) into their transition state form to reduce the energy required to reach the transition state.<ref name=PMID12947189>{{cite journal | vauthors = Benkovic SJ, Hammes-Schiffer S | title = A perspective on enzyme catalysis | journal = Science | volume = 301 | issue = 5637 | pages = 1196–202 | date = August 2003 | pmid = 12947189| doi = 10.1126/science.1085515 | bibcode = 2003Sci...301.1196B }}</ref> |
|||
#* By orienting the substrates into a productive arrangement to reduce the reaction [[entropy]] change.<ref>{{cite book | author = Jencks WP | title = Catalysis in Chemistry and Enzymology | publisher = Dover | location = Mineola, N.Y | year = 1987 | isbn = 0-486-65460-5 }}</ref> The contribution of this mechanism to catalysis is relatively small.<ref>{{cite journal | vauthors = Villa J, Strajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A | title = How important are entropic contributions to enzyme catalysis? | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 97 | issue = 22 | pages = 11899–904 | date = October 2000 | pmid = 11050223 | pmc = 17266 | doi = 10.1073/pnas.97.22.11899 | bibcode = 2000PNAS...9711899V }}</ref> |
|||
Enzymes may use several of these mechanisms simultaneously. For example, [[protease]]s such as [[trypsin]] perform covalent catalysis using a [[catalytic triad]], stabilise charge build-up on the transition states using an [[oxyanion hole]], complete [[hydrolysis]] using an oriented water substrate. |
|||
=== Dynamics === |
|||
最近的一些研究揭示了酶内部的动态作用与其催化机制之间的联系。<ref>{{en}}{{cite journal en |author=Eisenmesser EZ, Bosco DA, Akke M, Kern D. |title=Enzyme dynamics during catalysis |journal=Science |volume=295 |issue=5559|pages=1520–3 |year=2002 |pmid=11859194}}</ref><ref>{{en}}{{cite journal en |author=Agarwal PK. |title=Role of protein dynamics in reaction rate enhancement by enzymes |journal=J. Am. Chem. Soc. |volume=127 |issue=43 |pages=15248-56 |year=2005 |pmid=16248667}}</ref>酶内部的动态作用可以描述为其内部组成元件(小的如一个氨基酸、一组氨基酸;大的如一段环区域、一个[[α螺旋]]或相邻的[[β折叠|β链]];或者可以是整个[[结构域]])的运动,这种运动可以发生在从[[飞秒]](10<sup>−15</sup>秒)到秒的不同时间尺度。通过这种动态作用,整个酶分子结构中的氨基酸残基就都可以对酶催化作用施加影响。<ref>{{en}}{{cite journal en |url=http://www.structure.org/content/article/abstract?uid=PIIS096921260500167X|author=Yang LW, Bahar I.|title=Coupling between catalytic site and collective dynamics: A requirement for mechanochemical activity of enzymes.| journal=Structure.|volume=13|pages=893–904|id=PMID 15939021|date=June 5, 2005}}</ref><ref>{{en}}{{cite journal en |url=http://www.pnas.org/cgi/content/full/99/5/2794|author=Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S.|title=Network of coupled promoting motions in enzyme catalysis.| journal=Proc. Natl. Acad. Sci. U S A.|volume=99|pages=2794–9|id=PMID 11867722|date=March 5, 2002}}</ref><ref>{{en}}Agarwal PK, Geist A, Gorin A. ''Protein dynamics and enzymatic catalysis: investigating the peptidyl-prolyl cis-trans isomerization activity of cyclophilin A.'' Biochemistry. 2004 August 24;43 (33):10605-18. PMID 15311922</ref><ref>{{en}}{{cite journal en |url=http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VRP-4D4JYMC-6&_coverDate=08%2F31%2F2004&_alid=465962916&_rdoc=1&_fmt=&_orig=search&_qd=1&_cdi=6240&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=613585a6164baa38b4f6536d8da9170a|author=Tousignant A, Pelletier JN.|title=Protein motions promote catalysis.|journal=Chem Biol.|volume=11|issue=8|pages=1037–42|id=PMID 15324804|date=Aug 2004}}</ref>蛋白质动态作用在许多酶中都起到关键作用,而是小的快速运动还是大的相对较慢的运动起作用更多是依赖于酶所催化的反应类型。对于动态作用的这些新发现,对于了解[[别构调节|别构作用]]、设计人工酶和开发新药都有重要意义。 |
|||
{{See also|Protein dynamics}} |
|||
但必须指出的是,这种时间依赖的动态进程不大可能帮助提高酶催化反应的速率,因为这种运动是随机发生的,并且速率常数取决于到达中间态的几率(P,P = exp {ΔG<sup>‡</sup>/RT})。<ref>{{en}} Olsson M.H.M., Parson W.W. and Warshel A. "Dynamical Contributions to Enzyme Catalysis: Critical Tests of A Popular Hypothesis" Chem. Rev., 2006 105: 1737-1756</ref>而且,降低ΔG<sup>‡</sup>需要相对较小的运动(与在溶液反应中的相应运动相比)以达到反应物与产物之间的过渡态。因此,这种运动或者说动态作用对于催化反应有何贡献还不清楚。 |
|||
Enzymes are not rigid, static structures; instead they have complex internal dynamic motions – that is, movements of parts of the enzyme's structure such as individual amino acid residues, groups of residues forming a [[turn (biochemistry)|protein loop]] or unit of [[protein secondary structure|secondary structure]], or even an entire [[protein domain]]. These motions give rise to a [[conformational ensemble]] of slightly different structures that interconvert with one another at [[thermodynamic equilibrium|equilibrium]]. Different states within this ensemble may be associated with different aspects of an enzyme's function. For example, different conformations of the enzyme [[dihydrofolate reductase]] are associated with the substrate binding, catalysis, cofactor release, and product release steps of the catalytic cycle.<ref>{{cite journal |vauthors=Ramanathan A, Savol A, Burger V, Chennubhotla CS, Agarwal PK |title=Protein conformational populations and functionally relevant substates |journal=Acc. Chem. Res. |volume=47 |issue=1 |pages=149–56 |year=2014|pmid=23988159 |doi=10.1021/ar400084s |url=}}</ref> |
|||
=== 别构调节 === |
|||
{{main|别构调节}} |
|||
在结合[[效应子]]的情况下,别构酶能够改变自身结构,从而达到调节酶活性的效应。这种调节作用可以是直接的,即效应子结合到别构酶上;也可以是间接的,即效应子通过结合其它能够与别构酶相互作用的蛋白来发挥调节作用。<ref>{{Cite book|author=Laurence A. Moran, Robert Horton, Gray Scrimgeour & Marc D. Perry|year=2011|title=Principles of Biochemistry (Fifth Edition)|trans_title=生物化学原理|publisher=[[培生出版集團]]|pages= 153-154|chapter= Properties of Enzymes|isbn=0-321-70733-8}}</ref> |
|||
=== |
=== Allosteric modulation === |
||
{{main |Allosteric regulation}} |
|||
一般情况下,酶在常温、常压和中性水溶液条件下可以正常发挥催化活性。在极端条件下,包括高温、过高或过低[[pH值|pH]]条件等,酶会失去催化活性,这被称为酶的失活。但也有一些酶则偏好在非常条件下发挥催化功能,如[[嗜熱生物|嗜热菌]]中的酶在高温条件下反而具有较高活性,[[嗜酸菌]]中的酶又偏好低pH条件。 |
|||
Allosteric sites are pockets on the enzyme, distinct from the active site, that bind to molecules in the cellular environment. These molecules then cause a change in the conformation or dynamics of the enzyme that is transduced to the active site and thus affects the reaction rate of the enzyme.<ref>{{cite journal |vauthors=Tsai CJ, Del Sol A, Nussinov R|title=Protein allostery, signal transmission and dynamics: a classification scheme of allosteric mechanisms |journal=Mol Biosyst |volume=5 |issue=3 |pages=207–16 |year=2009|pmid=19225609 |pmc=2898650 |doi=10.1039/b819720b |url=}}</ref> In this way, allosteric interactions can either inhibit or activate enzymes. Allosteric interactions with metabolites upstream or downstream in an enzyme's metabolic pathway cause [[feedback]] regulation, altering the activity of the enzyme according to the [[Flux (metabolism)|flux]] through the rest of the pathway.<ref>{{cite journal | vauthors = Changeux JP, Edelstein SJ | title = Allosteric mechanisms of signal transduction | journal = Science | volume = 308 | issue = 5727 |pages = 1424–8 | date = June 2005 | pmid = 15933191 | doi = 10.1126/science.1108595 | bibcode = 2005Sci...308.1424C }}</ref> |
|||
== 辅因子与辅酶 == |
|||
{{main|辅酶|辅因子}} |
|||
=== 辅因子 === |
|||
==Cofactors== |
|||
并非所有的酶自身就可以催化反应,有一些酶需要结合一些非蛋白小分子后才可以发挥或提高催化活性。<ref name="cofactor">{{en}}{{cite web|url=http://academic.brooklyn.cuny.edu/biology/bio4fv/page/coenzy_.htm |title=coenzymes and cofactors |accessdate=2008-01-13}}</ref>这些小分子被称为辅因子,它们既可以是无机分子或[[离子]](如金属离子、[[铁硫簇]]),也可以是有机化合物(如[[黄素]]、[[血紅素|血红素]])。有机辅因子通常是[[辅基]],可以与其对应的酶非常牢固地结合。这种牢固结合的辅因子与辅酶(如[[烟酰胺腺嘌呤二核苷酸]])不同的是,在整个催化反应过程中,它们一直结合在酶活性位点上而不脱落。 |
|||
[[File:Transketolase + TPP.png|thumb|400px|alt=Thiamine pyrophosphate displayed as an opaque globular surface with an open binding cleft where the substrate and cofactor both depicted as stick diagrams fit into.|Chemical structure for [[thiamine pyrophosphate]] and protein structure of [[transketolase]]. Thiamine pyrophosphate cofactor in yellow and [[xylulose 5-phosphate]] substrate in black. ({{PDB|4KXV}})]] |
|||
以含有辅因子的[[碳酸酐酶]]为例:其辅因子锌牢固地结合在活性中心,参与催化反应。<ref>{{en}}{{cite journal en |author= Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN and McKenna R.|year= 2005|title= Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II.| journal=Biochemistry.|volume= 44 (4)|pages= 1097-115|id= PMID 15667203}}</ref>黄素或血红素等辅因子可以参与催化[[氧化还原反应]],往往结合于催化此类反应的酶中。 |
|||
{{main|Cofactor (biochemistry)}} |
|||
需要辅因子结合以进行催化的酶,在不结合辅因子的情况下,被称为'''酶元'''(apoenzyme);而在结合了辅因子后,被称为'''全酶'''(holoenzyme)。大多数全酶中,辅因子都是以非[[共价键|共价]]连接方式与酶结合;也有一些有机辅因子可以与酶共价结合(如[[丙酮酸脱氢酶]]中的[[焦磷酸硫胺素]])。 |
|||
Some enzymes do not need additional components to show full activity. Others require non-protein molecules called cofactors to be bound for activity.<ref>{{cite web|url=http://www.chem.qmul.ac.uk/iupac/bioinorg/CD.html#34 | title = Glossary of Terms Used in Bioinorganic Chemistry: Cofactor | accessdate = 30 October 2007 | last = de Bolster |first = M.W.G. | year = 1997 | publisher = International Union of Pure and Applied Chemistry | name-list-format = vanc }}</ref> Cofactors can be either [[inorganic]] (e.g.,[[ion|metal ions]] and [[iron-sulfur cluster]]s) or [[organic molecules|organic compounds]] (e.g., [[flavin group|flavin]] and [[heme]]). Organic cofactors can be either[[coenzyme]]s, which are released from the enzyme's active site during the reaction, or [[prosthetic groups]], which are tightly bound to an enzyme. Organic prosthetic groups can be covalently bound (e.g., [[biotin]] in enzymes such as [[pyruvate carboxylase]]).<ref name="pmid10470036">{{cite journal | vauthors = Chapman-Smith A, Cronan JE | title = The enzymatic biotinylation of proteins: a post-translational modification of exceptional specificity | journal = Trends Biochem. Sci. | volume = 24 | issue = 9 | pages = 359–63 | year = 1999 | pmid = 10470036 | doi = 10.1016/s0968-0004(99)01438-3}}</ref> |
|||
=== 辅酶 === |
|||
[[File:NADH-3D-vdW.png|thumb|left|150px|辅酶[[烟酰胺腺嘌呤二核苷酸]]的空间填充式结构模型]] |
|||
辅酶是一类可以将化学基团从一个酶转移到另一个酶上的有机小分子,与酶较为松散地结合,对于特定酶的活性发挥是必要的。<ref name="cofactor"/>有许多[[维生素|维他命]]及其衍生物,如[[核黄素]]、[[硫胺|硫胺素]]和[[叶酸]],都属于辅酶。<ref>{{en}}{{cite web |url=http://www.elmhurst.edu/~chm/vchembook/571cofactor.html |title=Enzyme Cofactors |accessdate=2008-01-13}}</ref>这些化合物无法由人体合成,必须通过饮食补充。不同的辅酶能够携带的化学基团也不同:[[烟酰胺腺嘌呤二核苷酸]]或NADP<sup>+</sup>携带氢离子,[[辅酶A]]携带乙酰基,叶酸携带甲酰基,[[S-腺苷基蛋氨酸]]也可携带甲基。<ref>{{en}}AF Wagner, KA Folkers (1975) ''Vitamins and coenzymes.'' Interscience Publishers New York| ISBN 978-0-88275-258-7</ref> |
|||
An example of an enzyme that contains a cofactor is [[carbonic anhydrase]], which is shown in the [[ribbon diagram]] above with a zinc cofactor bound as part of its active site.<ref>{{cite journal | vauthors = Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN, McKenna R | title = Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II | journal = Biochemistry | volume = 44 | issue = 4 | pages = 1097–115 | date = February 2005 |pmid = 15667203 | doi = 10.1021/bi0480279 }}</ref> These tightly bound ions or molecules are usually found in the active site and are involved in catalysis.<ref name = "Stryer_2002"/>{{rp|8.1.1}} For example, flavin and heme cofactors are often involved in [[redox]] reactions.<ref name = "Stryer_2002"/>{{rp|17}} |
|||
由于辅酶在酶催化反应中其化学组分发生了变化,因此可以认为辅酶是一种特殊的底物或者称为“第二底物”。这种所谓的第二底物可以被许多酶所利用。例如,目前已知有约七百种酶可以利用辅酶[[烟酰胺腺嘌呤二核苷酸]]进行催化。<ref>{{en}}[http://www.brenda.uni-koeln.de/ BRENDA The Comprehensive Enzyme Information System] Accessed 04 April 2007</ref> |
|||
Enzymes that require a cofactor but do not have one bound are called ''apoenzymes'' or ''apoproteins''. An enzyme together with the cofactor(s) required for activity is called a''holoenzyme'' (or haloenzyme). The term ''holoenzyme'' can also be applied to enzymes that contain multiple protein subunits, such as the [[DNA polymerase]]s; here the holoenzyme is the complete complex containing all the subunits needed for activity.<ref name = "Stryer_2002"/>{{rp|8.1.1}} |
|||
在细胞内,反应后的辅酶可以被再生,以维持其胞内浓度在一个稳定的水平上。例如,[[NADPH]]可以通过[[磷酸戊糖途径]]再生,[[S-腺苷基蛋氨酸]]則可以通过[[甲硫氨酸腺苷基转移酶]]来再生。由于辅酶的再生对于维持酶反应体系的稳定是必要的,因此,辅酶再生系统获得了大量的实验室以及工业应用。<ref>{{zh}}{{cite journal |author=张小里,岑沛霖 |title=伴有辅酶再生的多酶反应技术进展 |journal=化工进展 |volume=6 |pages=50-52, 59 |year=1996 }}</ref> |
|||
== |
===Coenzymes=== |
||
{{see also|活化能|热力学平衡|化学平衡}} |
|||
{{FileTA|Activation Energy|png|thumb|300px|有酶或无酶催化反应体系中反应进程与能量关系图示。可以看出,当反应没有酶的催化时,底物通常需要获得较高的活化能才能到达过渡态,然后才能生成产物;而当反应体系中有酶催化时,通过酶对过渡态的稳定作用,降低了达到过渡态所需能量,从而降低了整个反应所需的能量。}} |
|||
与其他催化剂一样,酶并不改变反应的[[平衡常数]],而是通过降低反应的[[活化能]]来加快反应速率(见右图)。通常情况下,反应在酶存在或不存在的两种条件下,其反应方向是相同的,只是前者的反应速度更快一些。但必须指出的是,在酶不存在的情况下,底物可以通过其他不受催化的“自由”反应生成不同的产物,原因是这些不同产物的形成速度更快。 |
|||
Coenzymes are small organic molecules that can be loosely or tightly bound to an enzyme. Coenzymes transport chemical groups from one enzyme to another.<ref name = "Wagner_1975">{{cite book | author = Wagner AL | title = Vitamins and Coenzymes | publisher = Krieger Pub Co | year = 1975 | isbn = 0-88275-258-8}}</ref> Examples include [[Nicotinamide adenine dinucleotide|NADH]], [[Nicotinamide adenine dinucleotide phosphate|NADPH]] and [[adenosine triphosphate]] (ATP). Some coenzymes, such as [[riboflavin]], [[thiamine]] and [[folic acid]], are [[vitamins]], or compounds that cannot be synthesized by the body and must be acquired from the diet. The chemical groups carried include the [[hydride]] ion (H<sup>−</sup>) carried by [[nicotinamide adenine dinucleotide|NAD or NADP<sup>+</sup>]], the phosphate group carried by [[adenosine triphosphate]], the acetyl group carried by [[coenzyme A]], formyl, methenyl or methyl groups carried by [[folic acid]] and the methyl group carried by [[S-adenosylmethionine]].<ref name = "Wagner_1975"/> |
|||
酶可以连接两个或多个反应,因此可以用一个[[热力学]]上更容易发生的反应去“驱动”另一个热力学上不容易发生的反应。例如,细胞常常通过[[三磷酸腺苷|ATP]]被酶水解所产生的能量来驱动其他化学反应。<ref name=Nicholls>{{en}}{{cite book |author=Ferguson, S. J.; Nicholls, David; Ferguson, Stuart |title=Bioenergetics 3 |publisher=Academic |location=San Diego |year=2002 |isbn=0-12-518121-3 |edition=3rd}}</ref> |
|||
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 1000 enzymes are known to use the coenzyme NADH.<ref>{{cite web | url = http://www.brenda-enzymes.org | title = BRENDA The Comprehensive Enzyme Information System | publisher = Technische Universität Braunschweig | accessdate = 23 February 2015 }}</ref> |
|||
酶可以同等地催化正向反应和逆向反应,而并不改变反应自身的化学平衡。例如,[[碳酸酐酶]]可以催化如下两个互逆反应,催化哪一种反应则是依赖于反应物浓度。<ref>{{en}}{{cite journal en|author=Maren TH|title=Carbonic anhydrase: chemistry, physiology, and inhibition|journal=Physiol Rev|volume=47|issue=4|year=1967|pages=595-781|pmid=4964060}}</ref> |
|||
Coenzymes are usually continuously regenerated and their concentrations maintained at a steady level inside the cell. For example, NADPH is regenerated through the [[pentose phosphate pathway]] and ''S''-adenosylmethionine by [[methionine adenosyltransferase]]. This continuous regeneration means that small amounts of coenzymes can be used very intensively. For example, the human body turns over its own weight in ATP each day.<ref>{{cite journal | vauthors = Törnroth-Horsefield S, Neutze R | title = Opening and closing the metabolite gate | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 105 | issue = 50 | pages = 19565–6 | date = December 2008 |pmid = 19073922 | pmc = 2604989 | doi = 10.1073/pnas.0810654106 | bibcode = 2008PNAS..10519565T }}</ref> |
|||
: <math>\mathrm{CO_2 + H_2O |
|||
{}^\mathrm{\quad *} |
|||
\!\!\!\!\!\!\!\! |
|||
\overrightarrow{\qquad} |
|||
H_2CO_3}</math> ([[組織|组织]]内;高[[二氧化碳|CO<sub>2</sub>]]浓度下) |
|||
: <math>\mathrm{H_2CO_3 |
|||
{}^\mathrm{\quad *} |
|||
\!\!\!\!\!\!\!\! |
|||
\overrightarrow{\qquad} |
|||
CO_2 + H_2O}</math> ([[肺]]中;低CO<sub>2</sub>浓度下) |
|||
==Thermodynamics== |
|||
<span style="font-size:smaller;"> 反应式中“*”表示“碳酸酐酶”</span> |
|||
[[File:Enzyme catalysis energy levels 2.svg|thumb|400px|alt=A two dimensional plot of reaction coordinate (x-axis) vs. energy (y-axis) for catalyzed and uncatalyzed reactions. The energy of the system steadily increases from reactants (x = 0) until a maximum is reached at the transition state (x = 0.5), and steadily decreases to the products (x = 1). However, in an enzyme catalysed reaction, binding generates an enzyme-substrate complex (with slightly reduced energy) then increases up to a transition state with a smaller maximum than the uncatalysed reaction.|The energies of the stages of a [[chemical reaction]]. Uncatalysed (dashed line), substrates need a lot of [[activation energy]] to reach a [[transition state]], which then decays into lower-energy products. When enzyme catalysed (solid line), the enzyme binds the substrates (ES), then stabilizes the transition state (ES<sup>‡</sup>) to reduce the activation energy required to produce products (EP) which are finally released.]] |
|||
{{main |Activation energy|Thermodynamic equilibrium|Chemical equilibrium}} |
|||
当然,如果反应平衡极大地趋向于某一方向,比如释放高能量的反应,而逆反应不可能有效的发生,则此时酶实际上只催化热力学上允许的方向,而不催化其逆反应。 |
|||
As with all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. In the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly.<ref name = "Stryer_2002"/>{{rp|8.2.3}} For example, [[carbonic anhydrase]] catalyzes its reaction in either direction depending on the concentration of its reactants:<ref>{{cite book |vauthors=McArdle WD, Katch F, Katch VL | title = Essentials of Exercise Physiology | date = 2006 | publisher = Lippincott Williams & Wilkins | location = Baltimore, Maryland | isbn = 978-0781749916 | pages = 312–3 | edition = 3rd | chapter = Chapter 9: The Pulmonary System and Exercise | chapterurl =https://books.google.com/books?id=L4aZIDbmV3oC&pg=PA313&lpg=PA313&dq=carbonic+anhydrase+lung+tissue+low+high+carbon+dioxide+equilibrium&source=bl&ots=WmoWpbFgYQ&hl=en&sa=X&redir_esc=y#v=onepage&q=carbonic%20anhydrase%20lung%20tissue%20low%20high%20carbon%20dioxide%20equilibrium&f=false}}</ref> |
|||
== 动力学 == |
|||
{{main|酶动力学}} |
|||
{{FileTA|Simple mechanism of enzyme reaction|png|thumb|left|300px|单一底物的酶催化反应机理:酶(表示为“E”)结合底物(表示为“S”),并通过催化反应生成产物(表示为“P”)。}} |
|||
{{Unsolved|化學|为什么一些酶的表现快于扩散动力学?}} |
|||
酶动力学是研究酶结合底物能力和催化反应速率的科学。研究者通过[[酶反应分析法]](enzyme assay)来获得用于酶动力学分析的反应速率数据。 |
|||
{{NumBlk|:| <math chem>\begin{matrix}{}\\ |
|||
1902年,[[维克多·亨利]]提出了酶动力学的定量理论;<ref>{{fr}}{{cite journal en |author=Henri,V.|year=1902|title= Theorie generale de l'action de quelques diastases|journal=Compt. rend. hebd. Acad. Sci. Paris|volume= 135|pages= 916-919}}</ref>随后该理论得到他人证实并扩展为[[米氏方程]]。<ref>{{de}}{{cite journal en |author=Michaelis L., Menten M.|year=1913|title= Die Kinetik der Invertinwirkung|journal=Biochem. Z.|volume= 49|pages= 333–369}} [http://web.lemoyne.edu/~giunta/menten.html English translation] Accessed 6 April 2007</ref>亨利最大贡献在于其首次提出酶催化反应由两步组成:首先,底物可逆地结合到酶上,形成酶-底物复合物;然后,酶完成对对应化学反应的催化,并释放生成的产物(见左图)。 |
|||
\ce{{CO2} + H2O ->[\ce{Carbonic\ anhydrase}] H2CO3}\\ |
|||
{}\end{matrix}</math> (in [[Biological tissue|tissues]]; high CO<sub>2</sub> concentration)|{{EquationRef|1}}}} |
|||
{{NumBlk|:| <math chem>\begin{matrix}{}\\ |
|||
[[File:Michaelis-Menten.png|thumb|300px|酶初始反应速率(表示为“''V''”)与底物浓度(表示为“[S]”)的关系曲线。随着底物浓度不断提高,酶的反应速率也趋向于最大反应速率(表示为“''V''<sub>max</sub>”)。]] |
|||
\ce{H2CO3 ->[\ce{Carbonic\ anhydrase}] {CO2} + H2O}\\ |
|||
酶可以在一秒钟内催化数百万个反应。例如,[[乳清酸核苷5'-磷酸脱羧酶]]所催化的反应在无酶情况下,需要七千八百万年才能将一半的底物转化为产物;而同样的反应过程,如果加入这种脱羧酶,则需要的时间只有25[[数量级 (时间)|毫秒]]。<ref>{{en}}{{cite journal en |author=Radzicka A, Wolfenden R.|year= 1995|title= A proficient enzyme. |journal= Science |volume=6|issue=267|pages=90–931|id= PMID 7809611}}</ref>酶催化速率依赖于反应条件和底物浓度。如果反应条件中存在能够将蛋白解链的因素,如高温、极端的pH和高的盐浓度,都会破坏酶的活性;而提高反应体系中的底物浓度则会增加酶的活性。在酶浓度固定的情况下,随着底物浓度的不断升高,酶催化的反应速率也不断加快并趋向于最大反应速率(''V''<sub>max</sub>,见右图的饱和曲线)。出现这种现象的原因是,当反应体系中底物的浓度升高,越来越多自由状态下的酶分子结合底物形成酶-底物复合物;当所有酶分子的活性位点都被底物饱和结合,即所有酶分子形成酶-底物复合物时,催化的反应速率达到最大。当然,''V''<sub>max</sub>并不是酶唯一的动力学常数,要达到一定反应速率所需的底物浓度也是一个重要的动力学指标。这一动力学指标即[[米氏方程|米氏常数]](''K''<sub>m</sub>),指的是达到''V''<sub>max</sub>值一半的反应速率所需的底物浓度(见右图)。对于特定的底物,每一种酶都有其特征''K''<sub>m</sub>值,表示底物与酶之间的结合强度(''K''<sub>m</sub>值越低,结合越牢固,亲和力越高)。另一个重要的动力学指标是''k''<sub>cat</sub>,定义为一个酶活性位点在一秒钟内催化底物的数量,用于表示酶催化特定底物的能力。 |
|||
{}\end{matrix}</math> (in [[lung]]s; low CO<sub>2</sub> concentration)|{{EquationRef|2}}}} |
|||
酶的催化效率可以用''k''<sub>cat</sub>/''K''<sub>m</sub>来衡量。这一表示式又被称为特异性常数,其包含了催化反应中所有步骤的[[反应常数]]。由于特异性常数同时反映了酶对底物的亲和力和催化能力,因此可以用于比较不同酶对于特定底物的 |
|||
催化效率或同一种酶对于不同底物的催化效率。特异性常数的理论最大值,又称为扩散极限,约为10<sup>8</sup>至10<sup>9</sup> M<sup>−1</sup>s<sup>−1</sup>;此时,酶与底物的每一次碰撞都会导致底物被催化,因此产物的生成速率不再为反应速率所主导,而分子的扩散速率起到了决定性作用。酶的这种特性被称为“[[完美催化酶|催化完美性]]”或“动力学完美性”。相关的酶的例子有[[磷酸丙糖异构酶]]、[[碳酸酐酶]]、[[乙酰胆碱酯酶]]、[[过氧化氢酶]]、[[延胡索酸酶]]、β-内酰胺酶和[[超氧化物歧化酶]]。 |
|||
米氏方程是基于[[質量作用定律|质量作用定律]]而确立的,而该定律则基于自由扩散和热动力学驱动的碰撞这些假定。然而,由于酶/底物/产物的高浓度和相分离或者一维/二维分子运动,许多生化或细胞进程明显偏离质量作用定律的假定。<ref>{{en}}{{cite journal en |author=Ellis RJ |title=Macromolecular crowding: obvious but underappreciated |journal=Trends Biochem. Sci. |volume=26 |issue=10 |pages=597-604 |year=2001 |pmid=11590012}}</ref>在这些情况下,可以应用[[分形]]米氏方程。<ref>{{en}}{{cite journal en |author=Kopelman R |title=Fractal Reaction Kinetics |journal=Science |volume=241 |issue=4873 |pages=1620–26 |year=1988 |DOI=10.1126/science.241.4873.1620}}</ref><ref>{{en}}{{cite journal en |author=Savageau MA |title=Michaelis-Menten mechanism reconsidered: implications of fractal kinetics |journal=J. Theor. Biol. |volume=176 |issue=1 |pages=115-24 |year=1995 |pmid=7475096}}</ref><ref>{{en}}{{cite journal en |author=Schnell S, Turner TE |title=Reaction kinetics in intracellular environments with macromolecular crowding: simulations and rate laws |journal=Prog. Biophys. Mol. Biol. |volume=85 |issue=2–3 |pages=235-60 |year=2004 |pmid=15142746}}</ref><ref>{{en}}{{cite journal en |author=Xu F, Ding H |title=A new kinetic model for heterogeneous (or spatially confined) enzymatic catalysis: Contributions from the fractal and jamming (overcrowding) effects |journal=Appl. Catal. A: Gen. |volume=317 |issue=1 |pages=70–81 |year=2007 |doi=10.1016/j.apcata.2006.10.014 }}</ref> |
|||
The rate of a reaction is dependent on the [[activation energy]] needed to form the [[transition state]] which then decays into products. Enzymes increase reaction rates by lowering the energy of the transition state. First, binding forms a low energy enzyme-substrate complex (ES). Secondly the enzyme stabilises the transition state such that it requires less energy to achieve compared to the uncatalyzed reaction (ES<sup>‡</sup>). Finally the enzyme-product complex (EP) dissociates to release the products.<ref name = "Stryer_2002"/>{{rp|8.3}} |
|||
存在一些酶,它们的催化产物动力学速率甚至高于分子扩散速率,这种现象无法用目前公认的理论来解释。有多种理论模型被提出来解释这类现象。其中,部分情况可以用酶对底物的附加效应来解释,即一些酶被认为可以通过双偶极电场来捕捉底物以及将底物以正确方位摆放到催化活性位点。另一种理论模型引入了基于量子理论的[[量子穿隧效應|穿隧效应]],即质子或电子可以穿过激活能垒(就如同穿过隧道一般),但关于穿隧效应还有较多争议。<ref>{{en}}{{cite journal en |author= Garcia-Viloca M., Gao J., Karplus M., Truhlar D. G.|year= 2004|title= How enzymes work: analysis by modern rate theory and computer simulations.|journal= Science|volume=303|issue=5655|pages=186–195|id= PMID 14716003}}</ref><ref>{{en}}{{cite journal en |author=Olsson M. H., Siegbahn P. E., Warshel A.|year= 2004|title= Simulations of the large kinetic isotope effect and the temperature dependence of the hydrogen atom transfer in lipoxygenase|journal = J. Am. Chem. Soc.|volume=126|issue=9|pages=2820-1828|id= PMID 14995199}}</ref>有报道发现[[色胺]]中质子存在量子穿隧效应。<ref>{{en}}{{cite journal en |author=Masgrau L., Roujeinikova A., Johannissen L. O., Hothi P., Basran J., Ranaghan K. E., Mulholland A. J., Sutcliffe M. J., Scrutton N. S., Leys D.|year= 2006|title= Atomic Description of an Enzyme Reaction Dominated by Proton Tunneling|journal= Science| volume=312|issue=5771|pages=237–241|id= PMID 16614214}}</ref>因此,有研究者相信在酶催化中也存在着穿隧效应,可以直接穿过反应能垒,而不是像传统理论模型的方式通过降低能垒达到催化效果。有相关的实验报道提出在一种[[醇脫氫酶|醇脱氢酶]]的催化反应中存在穿隧效应,<ref>{{en}}{{cite journal en |author=Kohen, A., Cannio, R., Bartolucci, S., Klinman, J. P.|year= 1999|title= Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase|journal= Nature| volume=399|issue=6735|pages=496-9|id= PMID 10365965}}</ref>但穿隧效应是否在酶催化反应中普遍存在并未有定论。<ref>{{en}}{{cite journal en |author=Ball, P.|year= 2004|title= Enzymes: by chance, or by design?|journal= Nature| volume=431|issue=7007|pages=396-7|id= PMID 15385982}}</ref> |
|||
Enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavourable one so that the combined energy of the products is lower than the substrates. For example, the hydrolysis of [[Adenosine triphosphate|ATP]] is often used to drive other chemical reactions.<ref name="Nicholls">{{cite book|vauthors=Ferguson SJ, Nicholls D, Ferguson S | title = Bioenergetics 3 | publisher = Academic | location = San Diego | year = 2002 | isbn = 0-12-518121-3 | edition = 3rd}}</ref> |
|||
== 抑制作用 == |
|||
{{main|酶抑制剂}} |
|||
酶的催化活性可以被多种抑制剂所降低。<ref>{{Cite book|author=Laurence A. Moran, Robert Horton, Gray Scrimgeour & Marc D. Perry|year=2011|title=Principles of Biochemistry (Fifth Edition)|trans_title=生物化学原理|publisher=[[培生出版集團]]|pages= 148-152|chapter= Properties of Enzymes|isbn=0-321-70733-8}}</ref> |
|||
==Kinetics== |
|||
{{FileTA|Inhibition|png|thumb|400px|不同的抑制类型。分类参考自<ref>{{en}}{{cite journal en |author=Cleland, W.W.|year=1963|title= The Kinetics of Enzyme-catalyzed Reactions with two or more Substrates or Products 2. {I}nhibition: Nomenclature and Theory|journal=Biochim. Biophys. Acta|volume= 67|pages= 173-187}}</ref>。图中,“E”表示酶;“I”表示抑制剂;“S”表示底物;“P”表示产物。}} |
|||
{{multiple image |
|||
=== 可逆抑制作用 === |
|||
| direction= vertical |
|||
可逆抑制作用的类型有多种,它们的共同特点在于抑制剂对酶活性的抑制反应具有可逆性。 |
|||
| width = 325 |
|||
| footer = |
|||
| image1 = Enzyme mechanism 2.svg |
|||
==== 竞争性抑制作用 ==== |
|||
| alt1 = Schematic reaction diagrams for uncatalzyed (Substrate to Product) and catalyzed (Enzyme + Substrate to Enzyme/Substrate complex to Enzyme + Product) |
|||
抑制剂与底物竞争结合酶的活性位点(抑制剂和底物不能同时结合到活性位点),也就意味着它们不能同时结合到酶上。<ref>{{en}}{{cite journal |author=Price, NC. |year=1979 |title=What is meant by 'competitive inhibition'? |journal=Trends in Biochemical Sciences |volume=4|pages=pN272 |doi=10.1016/0968-0004(79)90205-6 |issue=11}}</ref>对于竞争性抑制作用,催化反应的最大反应速率值没有变,但是需要更高的底物浓度,反映在表观''K''<sub>m</sub>值的增加。 |
|||
| caption1 = A chemical reaction mechanism with or without [[enzyme catalysis]]. The enzyme (E) binds [[substrate (chemistry)|substrate]] (S) to produce [[product (chemistry)|product]] (P). |
|||
| image2 = Michaelis Menten curve 2.svg |
|||
==== 非竞争性抑制作用 ==== |
|||
| alt2 = A two dimensional plot of substrate concentration (x axis) vs. reaction rate (y axis). The shape of the curve is hyperbolic. The rate of the reaction is zero at zero concentration of substrate and the rate asymptotically reaches a maximum at high substrate concentration. |
|||
非竞争性抑制抑制剂可以与底物同时结合到酶上,即抑制剂不结合到活性位点。酶-抑制剂复合物(EI)或酶-抑制剂-底物复合物(EIS)都没有催化活性。与竞争性抑制作用相比,非竞争性抑制作用不能通过提高底物浓度来达到所需反应速度,即表观最大反应速率''V''<sub>max</sub>的值变小;而同时,由于抑制剂不影响底物与酶的结合,因此''K''<sub>m</sub>值保持不变。 |
|||
| caption2 = [[Michaelis–Menten kinetics|Saturation curve]] for an enzyme reaction showing the relation between the substrate concentration and reaction rate. |
|||
}} |
|||
{{main|Enzyme kinetics}} |
|||
==== 反竞争性抑制作用 ==== |
|||
反竞争性抑制作用比较少见:抑制剂不能与处于自由状态下的酶结合,而只能和酶-底物复合物(ES)结合,在酶反应动力学上表现为''V''<sub>max</sub>和''K''<sub>m</sub>值都变小。这种抑制作用可能发生在多亚基酶中。 |
|||
Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are commonly obtained from [[enzyme assay]]s. In 1913 [[Leonor Michaelis]] and [[Maud Leonora Menten]] proposed a quantitative theory of enzyme kinetics, which is referred to as [[Michaelis–Menten kinetics]].<ref>{{cite journal | vauthors = Michaelis L, Menten M | year = 1913 | title = Die Kinetik der Invertinwirkung | journal = Biochem. Z. | volume = 49 | pages = 333–369 | language = German |trans-title = The Kinetics of Invertase Action }}; {{cite journal | vauthors = Michaelis L, Menten ML, Johnson KA, Goody RS | title = The original Michaelis constant: translation of the 1913 Michaelis-Menten paper | journal = Biochemistry | volume = 50 | issue = 39 | pages = 8264–9 | year = 2011 | pmid = 21888353 | pmc = 3381512 | doi = 10.1021/bi201284u }}</ref> The major contribution of Michaelis and Menten was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis-Menten complex in their honor. The enzyme then catalyzes the chemical step in the reaction and releases the product. This work was further developed by [[George Edward Briggs|G. E. Briggs]] and [[J. B. S. Haldane]], who derived kinetic equations that are still widely used today.<ref>{{cite journal |vauthors = Briggs GE, Haldane JB | title = A Note on the Kinetics of Enzyme Action | journal = The Biochemical Journal | volume = 19 | issue = 2 | pages = 339–339 | year = 1925 |pmid = 16743508 | pmc = 1259181 | doi=10.1042/bj0190338}}</ref> |
|||
==== 复合抑制作用 ==== |
|||
这种抑制作用与非竞争性抑制作用比较相似,区别在于EIS复合物残留有部分酶的活性。在许多生物体中,这类抑制剂可以作为[[负反馈|负反馈机制]]的组成部分。若一个酶体系生产了过多的产物,那么产物就会抑制合成该产物的酶体系中第一个酶的活性,这就可以保证一旦合成足够多的产物后,该产物的合成速率会下降或停止。受这种抑制作用调控的酶通常为多亚基酶,并具有与调控产物结合的别构结合位点。这种抑制作用的反应速率与底物浓度的关系图不再是双曲线形而是S形。 |
|||
Enzyme rates depend on [[solution]] conditions and substrate [[concentration]]. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES complex. At the maximum reaction rate (''V''<sub>max</sub>) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme.<ref name = "Stryer_2002"/>{{rp|8.4}} |
|||
=== 不可逆抑制作用 === |
|||
不可逆抑制剂可以与酶结合形成[[共价键|共价连接]],而其他抑制作用中酶与抑制剂之间都是非共价结合。这种抑制作用是不可逆的,酶一旦被抑制后就无法再恢复活性状态。这类抑制剂包括[[二氟甲基鸟氨酸]](一种可用于治疗寄生虫导致的[[昏睡症]]的药物<ref name=Poulin>{{en}}Poulin R, Lu L, Ackermann B, Bey P, Pegg AE. [http://www.jbc.org/cgi/reprint/267/1/150 ''Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites.''] J Biol Chem. 1992 Jan 5;267 (1):150–8. PMID 1730582</ref>)、[[苯甲基磺酰氟]](PMSF)、[[青霉素]]和[[阿司匹林]]。这些药物都是与酶活性位点结合并被激活,然后与活性位点处的一个或多个氨基酸残基发生不可逆的反应形成共价连接。 |
|||
''V''<sub>max</sub> is only one of several important kinetic parameters. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by the[[Michaelis-Menten constant]] (''K''<sub>m</sub>), which is the substrate concentration required for an enzyme to reach one-half its maximum reaction rate; generally, each enzyme has a characteristic ''K''<sub>m</sub> for a given substrate. Another useful constant is ''k''<sub>cat</sub>, also called the ''turnover number'', which is the number of substrate molecules handled by one active site per second.<ref name = "Stryer_2002"/>{{rp|8.4}} |
|||
=== 抑制剂的用途 === |
|||
酶抑制剂常被用作药物,同样也可以被作为毒药使用。而药物和毒药之间的差别通常非常小,大多数的药物都有一定程度的毒性,正如[[帕拉塞尔苏斯]]所言:“所有东西都有毒,没有什么是无毒的”(“In all things there is a poison, and there is nothing without a poison”)。<ref>{{en}}Ball, Philip (2006) ''The Devil's Doctor: Paracelsus and the World of Renaissance Magic and Science.'' Farrar, Straus and Giroux ISBN 978-0-374-22979-5</ref>相同的,[[抗生素]]和其他抗感染药物只是特异性地对[[病原|病原体]]而不是对[[宿主]]有毒性。 |
|||
The efficiency of an enzyme can be expressed in terms of ''k''<sub>cat</sub>/''K''<sub>m</sub>. This is also called the specificity constant and incorporates the [[rate constant]]s for all steps in the reaction up to and including the first irreversible step. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 10<sup>8</sup> to 10<sup>9</sup> (M<sup>−1</sup> s<sup>−1</sup>). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called ''[[catalytically perfect enzyme|catalytically perfect]]'' or''kinetically perfect''. Example of such enzymes are [[triosephosphateisomerase|triose-phosphate isomerase]], [[carbonic anhydrase]], [[acetylcholinesterase]], [[catalase]],[[fumarase]], [[β-lactamase]], and [[superoxide dismutase]].<ref name = "Stryer_2002"/>{{rp|8.4.2}} The turnover of such enzymes can reach several million reactions per second.<ref name = "Stryer_2002"/>{{rp|9.2}} |
|||
一个获得广泛应用的抑制剂药物是[[阿司匹林]],它可以抑制[[环加氧酶]]的活性,而环加氧酶可以生产[[炎症]]反应信使[[前列腺素]],因此,阿司匹林可以起到抑制疼痛与炎症的作用。而剧毒毒药[[氰化物]]可以通过结合[[细胞色素氧化酶]]位点处的铜和铁原子不可逆地抑制酶活性,从而抑制细胞的[[呼吸作用]]。<ref>{{en}}{{cite journal en |url=http://www.jbc.org/cgi/reprint/265/14/7945|author=Yoshikawa S and Caughey WS.|volume=265|issue=14|title= Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction.|journal= J Biol Chem.|pages= 7945–7958|id= PMID 2159465|date=May 1990}}</ref> |
|||
Michaelis–Menten kinetics relies on the [[law of mass action]], which is derived from the assumptions of free [[diffusion]] and thermodynamically driven random collision. Many biochemical or cellular processes deviate significantly from these conditions, because of [[macromolecular crowding]] and constrained molecular movement.<ref>{{cite journal |vauthors = Ellis RJ | title = Macromolecular crowding: obvious but underappreciated | journal = Trends in Biochemical Sciences | volume = 26 | issue = 10 | pages = 597–604 | date = October 2001 | pmid = 11590012 | doi = 10.1016/S0968-0004(01)01938-7 }}</ref> More recent, complex extensions of the model attempt to correct for these effects.<ref>{{cite journal |vauthors = Kopelman R | title = Fractal reaction kinetics | journal = Science | volume = 241 | issue = 4873 | pages = 1620–26 | date = September 1988 | pmid = 17820893 | doi = 10.1126/science.241.4873.1620 | bibcode = 1988Sci...241.1620K }}</ref> |
|||
== 活性控制 == |
|||
==Inhibition== |
|||
细胞内有五种控制酶催化活性的机制: |
|||
# 根据外界环境的变化,细胞可以增强或减弱酶的生产(即酶相关[[基因]]的[[转录]]和[[翻译 (遗传学)|翻译]])。这属于一种[[基因调控]],被称为[[酶抑制剂|酶的诱导和抑制]]。例如,当环境中出现如[[青霉素]]这样的[[抗生素]]时,部分细菌可以对[[抗生素]]产生抗性,其原因就在于细菌体内的[[β-半乳糖苷酶]]被诱导而大量生产,这种酶可以水解青霉素分子上关键的[[β-乳胺环]]。另一个例子是在人体[[肝臟|肝脏]]中存在一类酶对于[[药物代谢]]非常重要的酶,[[细胞色素P450氧化酶]];对这一类酶的诱导或抑制,会导致{{link-en|藥物相互作用|drug interaction}}。 |
|||
# 通过将特定的酶分隔在特定的细胞组分中,细胞可以完成不同的代谢途径。例如,[[脂肪酸]]的合成是由[[细胞溶质]]、[[内质网]]和[[高尔基体]]中的一系列酶所完成,而脂肪酸的降解(以提供能量)是在线粒体中由另一系列酶通过[[β-氧化]]来完成。<ref>{{en}}{{cite journal en |url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1218279&blobtype=pdf|author=Faergeman N. J, Knudsen J.|title= Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling|journal= Biochem J|volume=323|pages=1–12|id= PMID 9173866|date=April 1997}}</ref> |
|||
# 酶可以被抑制剂与激活剂所调控。例如,一个代谢途径中的终产物常常是这一途径中第一个酶的抑制剂,从而调控这一代谢途径的产物量。这种调控机制被称为[[负反馈|负反馈机制]],因为终产物的合成量是受其自身浓度调控。负反馈机制可以根据细胞的需要,有效地调节中间代谢物的合成速率,从而使细胞的能量和物质的分配更为高效,并防止多余产物的合成。控制酶的作用,可以在生物体内维持一个稳定的内部环境(即[[體內平衡|体内平衡]])。 |
|||
# [[翻译后修饰]]也可以调控酶的活性。这些修饰包括[[磷酸化]]、[[肉豆蔻酸]]化和[[糖基化]]。例如,细胞接受[[胰岛素]]信号后,对包括[[糖原合酶]]在内的多个酶进行磷酸化,帮助控制[[糖原]]的合成或降解,使得细胞可以对[[血糖]]的变化产生反应。<ref>{{en}}{{cite journal en |url=http://jcs.biologists.org/cgi/content/full/116/7/1175|author= Doble B. W., Woodgett J. R. |title= GSK-3: tricks of the trade for a multi-tasking kinase|journal=J. Cell. Sci.|volume=116|pages=1175–1186|id= PMID 12615961|date=April 2003}}</ref>另一个翻译后修饰的例子是多肽链的剪切。[[胰凝乳蛋白酶]],一种消化性[[蛋白酶]],是产生于[[胰脏]]中的无活性的[[胰凝乳蛋白酶原]],这一蛋白通过运输到达[[胃]]后才被激活。这种方式有效地防止了胰凝乳蛋白酶在进入[[腸|肠]]之前消化胰脏或其他组织。这种无活性的酶的前体被命名为[[酶原]]。 |
|||
# 还有一些酶可以通过定位到不同环境后而被激活,比如从还原态的环境([[細胞質|细胞质]])到氧化态环境([[周质空间|细胞周质空间]]),从高pH环境到低pH环境等。[[流行性感冒病毒|流感病毒]]的[[红血球凝集素|红血球凝集素蛋白]]就是一个例子:当它接触到宿主细胞囊泡的酸性环境时,它的构象立刻发生变化,导致其获得激活。<ref>{{en}}{{cite journal en |author= Ciampor F, Cmarko D, Cmarkova J, Zavodska E.|year= 1995|title= Influenza virus M2 protein and haemagglutinin conformation changes during intracellular transport.|journal= Acta Virol.|volume=39|issue=3|pages=171 - 181|id= PMID 8579000}}</ref> |
|||
{{multiple image |
|||
== 相关疾病 == |
|||
| direction= vertical |
|||
[[Image:Phenylalanine hydroxylase brighter.jpg|thumb|200px|[[苯丙氨酸羟化酶]]. 由[http://www.rcsb.org/pdb/explore.do?structureId=1KW0 PDB 1KW0]生成。 ]] |
|||
| width = 400 |
|||
| footer = |
|||
| image1 = DHFR methotrexate inhibitor.svg |
|||
酶的活性必须严格控制以维持[[體內平衡|体内平衡]],对于能够影响一个关键酶的功能的任何基因缺陷(如[[突变]]导致活性变化,过量表达、过低表达或删除突变)都可能导致[[遺傳性疾病|遗传性疾病]]发生。许多事实显示,一种致命疾病的病因可以只是由于人体中的数千种酶中的一种发生功能故障。 |
|||
| alt1 = |
|||
| caption1 = An enzyme binding site that would normally bind substrate can alternatively bind a [[competitive inhibitor]], preventing substrate access. [[Dihydrofolate reductase]]is inhibited by [[methotrexate]] which prevents binding of its substrate, [[folic acid]]. Binding site in blue, inhibitor in green, and substrate in black. ({{PDB|4QI9}}) |
|||
| image2 = Methotrexate vs folate 2.svg |
|||
* [[苯丙酮尿症]]:此種病症是典型的酶相关病例之一。病因是[[苯丙氨酸羟化酶]](其功能是催化[[苯丙氨酸]]降解过程中的第一步)上一个氨基酸位点发生了突变,导致体内[[苯丙氨酸]]和相关产物的水平过高,如果没有得到合适的治疗,会进一步导致[[智能障碍]]。<ref>{{cite web|url=http://www.pmf.org.tw/pmf/n_s/intr01.htm|author=預防醫學基金會|accessdate=2015-01-23|title=苯酮尿症}}</ref> |
|||
| alt2 = Two dimensional representations of the chemical structure of folic acid and methotrexate highlighting the differences between these two substances (amidation of pyrimidone and methylation of secondary amine). |
|||
* [[紫質症|卟啉病]]:该病是由于[[血紅素|血红素]]生物合成途径中特定酶的酶活性过低(基因突变或其他原因导致),使得中间产物[[卟啉]]的产生和排泄异常,在一定诱因(如阳光照射)下,可导致皮肤或其他组织器官发生病变。<ref>{{cite web|url=http://www.pmf.org.tw/pmf/n_s/intr01.htm|author=台大医院|accessdate=2015-01-23|title=紫質症}}</ref> |
|||
| caption2 = The coenzyme [[folic acid]] (left) and the anti-cancer drug [[methotrexate]] (right) are very similar in structure (differences show in green). As a result, methotrexate is a competitive inhibitor of many enzymes that use folates. |
|||
* 当生殖细胞中编码[[DNA修復|DNA修复]]相关酶的基因发生突变,其结果会导致遗传性[[癌症]]综合病征,如[[著色性乾皮症|着色性干皮症]]。<ref>{{cite journal|author=谢绍安, 祝芷 |year=1984|title=着色性干皮症|journal=眼科新进展|volume= 3|url=http://www.cnki.com.cn/Article/CJFDTotal-XKJZ198403012.htm}}</ref>DNA修复酶的缺陷导致人体丧失修复突变基因的能力。发生的突变不断积累,最终使得患者有多种癌症发生。 |
|||
}} |
|||
{{main|Enzyme inhibitor}} |
|||
酶的口服给药,可用于治疗多种疾病(如胰腺功能不全和乳糖不耐受症)。由于酶作为蛋白质可能在消化道环境中失活或被降解,因此一种非侵入性的成像方法被开发用于监测作为药物的酶在消化道中的活性变化。<ref>{{en}}{{cite journal|author=Fuhrmann G, Leroux JC |year=2011|title= In vivo fluorescence imaging of exogenous enzyme activity in the gastrointestinal tract|journal= Proceedings of the National Academy of Sciences|volume= 108|pages= 9032–9037|doi=10.1073/pnas.1100285108|issue=22|pmid=21576491|pmc=3107327|last2=Leroux}}</ref> |
|||
Enzyme reaction rates can be decreased by various types of [[enzyme inhibitor]]s.<ref name = "Cornish-Bowden_2004">{{cite book | author = Cornish-Bowden A | title = Fundamentals of Enzyme Kinetics | date = 2004 | publisher = Portland Press | location = London | isbn = 1-85578-158-1 | edition = 3 }}</ref>{{rp|73–74}} |
|||
== 命名规则 == |
|||
{{see also|EC編號}} |
|||
酶通常是根据其底物性质或其所催化的化学反应类型来命名(英文中,在单词的最后要加上-ase的后缀),如[[乳糖酶]](lactase)、[[醇脫氫酶|醇脱氢酶]](alcohol dehydrogenase)和[[DNA聚合酶]](DNA polymerase)。但这样往往会导致具有相同功能的不同的酶([[同工酶]])有相同的名字,而同工酶有着不同的[[氨基酸序列]]并可以通过它们不同的最适[[pH值|pH]]和不同的反应动力学参数来区分。而且,一些酶具有两个或多个类型催化活性,这种命名方式就会使得同一种酶具有不同的名字。为了解决这些问题,[[国际生物化学与分子生物学联盟]]发展出了酶的系统命名法:<ref>{{en}}{{cite journal en|author=Bairoch A.|title=The ENZYME database in 2000|journal=Nucleic Acids Res|volume=28|pages=304-305|year=2000}}</ref> |
|||
===Types of inhibition=== |
|||
EC編號规定每种酶都由四个数字来表示,并在数字前冠以“EC”。其中,第一个数字是根据[[酶促反应]]的性质来将酶大致分为六大类: |
|||
* EC 1,[[氧化還原酶|氧化还原酶]]:催化[[底物]]进行[[氧化还原反应]]的酶类。例如,[[乳酸脱氢酶]]、[[琥珀酸脱氢酶]]、[[细胞色素氧化酶]]、[[过氧化氢酶]]、[[過氧化物酶|过氧化物酶]]等。 |
|||
* EC 2,[[轉移酶|转移酶]]:催化底物之间进行某些[[官能团|基团]]的转移或交换的酶类。例如,[[甲基转移酶]]、[[氨基转移酶]]、[[己糖激酶]]、[[磷酸化酶]]等。 |
|||
* EC 3,[[水解酶]]:催化底物发生[[水解|水解反应]]的酶类。例如,[[淀粉酶]]、[[蛋白酶]]、[[脂肪酶]]、[[磷酸酶]]等。 |
|||
* EC 4,[[裂合酶]]:催化从底物中移去一个基团并留下[[共价键|双键]]的反应或其[[可逆反應|逆反应]]的酶类。例如,[[碳酸酐酶]]、[[醛縮酶|醛缩酶]]、[[柠檬酸合酶]]等。 |
|||
* EC 5,[[異構酶|异构酶]]:催化各种[[同分異構|同分异构体]]之间的相互转化的酶类。例如,[[磷酸丙糖异构酶]]、[[消旋酶]]等。 |
|||
* EC 6,[[連接酶|连接酶]]:催化两分子底物合成为一分子[[化合物]],同时[[偶联反应|偶联]]有[[三磷酸腺苷|ATP]]的[[磷酸]]键断裂释放[[能量]]的酶类。例如,[[谷氨酰胺合成酶]]、[[氨基酸:tRNA连接酶]]等。 |
|||
;Competitive: A [[competitive inhibitor]] and substrate cannot bind to the enzyme at the same time.<ref name = "Price_1979">{{cite journal | vauthors = Price NC | year = 1979 | title = What is meant by 'competitive inhibition'? | journal = Trends in Biochemical Sciences | volume = 4 | issue=11 | pages = N272–N273 | doi = 10.1016/0968-0004(79)90205-6 }}</ref>Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example, the drug [[methotrexate]] is a competitive inhibitor of the enzyme [[dihydrofolate reductase]], which catalyzes the reduction of [[folic acid|dihydrofolate]] to tetrahydrofolate. The similarity between the structures of dihydrofolate and this drug are shown in the accompanying figure. This type of inhibition can be overcome with high substrate concentration. In some cases, the inhibitor can bind to a site other than the binding-site of the usual substrate and exert an [[#Allosteric modulation|allosteric effect]] to change the shape of the usual binding-site. |
|||
国际系统分类法除按上述六类将酶依次编号外,还根据酶所催化的[[化学键]]的特点和参加反应的基团的不同,将每一大类又进一步分类。编号中的第一个数字表示该酶属于六大类中的哪一类;第二个数字表示该酶属于哪一亚类;第三个数字表示亚-亚类;第四个数字是该酶在亚-亚类中的排序。 |
|||
;Non-competitive: A [[non-competitive inhibition|non-competitive inhibitor]] binds to a site other than where the substrate binds. The substrate still binds with its usual affinity and hence K<sub>m</sub> remains the same. However the inhibitor reduces the catalytic efficiency of the enzyme so that V<sub>max</sub> is reduced. In contrast to competitive inhibition, non-competitive inhibition cannot be overcome with high substrate concentration.<ref name = "Cornish-Bowden_2004"/>{{rp|76–78}} |
|||
; Uncompetitive: An [[uncompetitive inhibitor]] cannot bind to the free enzyme, only to the enzyme-substrate complex; hence, these types of inhibitors are most effective at high substrate concentration. In the presence of the inhibitor, the enzyme-substrate complex is inactive.<ref name = "Cornish-Bowden_2004"/>{{rp|78}} This type of inhibition is rare.<ref>{{cite journal | vauthors = Cornish-Bowden A | title = Why is uncompetitive inhibition so rare? A possible explanation, with implications for the design of drugs and pesticides |journal = FEBS Letters | volume = 203 | issue = 1 | pages = 3–6 | date = July 1986 | pmid = 3720956 | doi=10.1016/0014-5793(86)81424-7}}</ref> |
|||
;Mixed: A [[mixed inhibition|mixed inhibitor]] binds to an allosteric site and the binding of the substrate and the inhibitor affect each other. The enzyme's function is reduced but not eliminated when bound to the inhibitor. This type of inhibitor does not follow the Michaelis-Menten equation.<ref name = "Cornish-Bowden_2004"/>{{rp|76–78}} |
|||
; Irreversible: An [[irreversible inhibitor]] permanently inactivates the enzyme, usually by forming a [[covalent bond]] to the protein. [[Penicillin]]<ref>{{cite journal | vauthors = Fisher JF, Meroueh SO, Mobashery S | title = Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity | journal = Chemical Reviews | volume = 105 | issue = 2 | pages = 395–424 | date = February 2005 | pmid = 15700950 | doi = 10.1021/cr030102i }}</ref> and [[aspirin]]<ref name="Johnson">{{cite journal | vauthors = Johnson DS, Weerapana E, Cravatt BF | title = Strategies for discovering and derisking covalent, irreversible enzyme inhibitors | journal = Future Medicinal Chemistry | volume = 2 | issue = 6| pages = 949–64 | date = June 2010 | pmid = 20640225 | pmc = 2904065 | doi=10.4155/fmc.10.21}}</ref> are common drugs that act in this manner. |
|||
===Functions of inhibitors=== |
|||
{| class="wikitable" |
|||
|+ '''一些酶的命名举例''' |
|||
!编号 |
|||
!推荐命名 |
|||
!系统命名 |
|||
!催化的反应 |
|||
|- |
|||
| EC 1.4.1.3 |
|||
| [[谷氨酸脱氢酶]] |
|||
| [[穀氨酸|L-谷氨酸]]:[[烟酰胺腺嘌呤二核苷酸|NAD<sup>+</sup>]][[氧化還原酶|氧化还原酶]] |
|||
| L-谷氨酸+[[水|H<sub>2</sub>O]]+NAD<sup>+</sup>⇔[[酮戊二酸|α-酮戊二酸]]+[[氨|NH<sub>3</sub>]]+[[NADH]] |
|||
|- |
|||
| EC 2.6.1.1 |
|||
| [[天冬氨酸氨基转移酶]] |
|||
| [[天冬氨酸|L-天冬氨酸]]:α-酮戊二酸[[胺|氨基]][[轉移酶|转移酶]] |
|||
| L-天冬氨酸+α-酮戊二酸⇔[[草酰乙酸]]+L-谷氨酸 |
|||
|- |
|||
| EC 3.5.3.1 |
|||
| [[精氨酸酶]] |
|||
| [[精氨酸|L-精氨酸]][[脒基]][[水解酶]] |
|||
| L-精氨酸+H<sub>2</sub>O⇒[[鳥氨酸|L-鸟氨酸]]+[[尿素]] |
|||
|- |
|||
| EC 4.1.2.13 |
|||
| [[果糖二磷酸醛缩酶]] |
|||
| [[果糖|D-果糖]]-1,6-二[[磷酸]]:[[甘油醛|D-甘油醛]]-3-磷酸[[裂合酶]] |
|||
| D-果糖-1,6-二磷酸⇔[[磷酸二羟丙酮]]+D-甘油醛-3-磷酸 |
|||
|- |
|||
| EC 5.3.1.9 |
|||
| [[磷酸葡萄糖異構酶|磷酸葡萄糖异构酶]] |
|||
| [[葡萄糖|D-葡萄糖]]-6-磷酸[[酮]][[醇]][[異構酶|异构酶]] |
|||
| D-葡萄糖-6-磷酸⇔D-果糖-6-磷酸 |
|||
|- |
|||
| EC 6.3.1.2 |
|||
| [[谷氨酰胺合成酶]] |
|||
| L-谷氨酸:[[氨]][[連接酶|连接酶]] |
|||
| [[三磷酸腺苷|ATP]]+L-谷氨酸+NH<sub>3</sub>⇒[[二磷酸腺苷|ADP]]+磷酸+[[穀氨醯胺|L-谷氨酰胺]] |
|||
|} |
|||
In many organisms, inhibitors may act as part of a [[feedback]] mechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme at the beginning of the pathway that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form of [[negative feedback]]. Major metabolic pathways such as the [[citric acid cycle]] make use of this mechanism.<ref name = "Stryer_2002" />{{rp|17.2.2}} |
|||
== 工业应用 == |
|||
酶被用于化工等各类需要高度特异性催化情况的用途。但是,酶通常能够催化的反应数量有限,而且它们在无机溶液中和高温情况下缺乏稳定性。为了提高酶的应用性,利用[[蛋白质工程]]通过合理设计或体外进化来造出具有新特点(例如耐高温)的酶已经成为一个活跃的研究领域。<ref>{{en}}{{cite journal en|author=Renugopalakrishnan V, Garduno-Juarez R, Narasimhan G, Verma CS, Wei X, Li P.|year= 2005|title= Rational design of thermally stable proteins: relevance to bionanotechnology|journal= J Nanosci Nanotechnol.|volume=5|issue=11|pages= 1759–1767|pmid= 16433409|doi= 10.1166/jnn.2005.441}}</ref><ref>{{en}}{{cite journal en|author=Hult K, Berglund P.|year= 2003|title= Engineered enzymes for improved organic synthesis|journal= Curr Opin Biotechnol.|volume=14|issue=4|pages= 395–400|pmid= 12943848|doi= 10.1016/S0958-1669(03)00095-8}}</ref>这类研究工作也有了成功的例子,一些能够催化自然界中的酶所无法催化的反应的酶已经开始被设计出来。<ref>{{en}}{{cite journal en|author=Jiang L, Althoff EA, Clemente FR, ''et al'' |title=De novo computational design of retro-aldol enzymes |journal=Science (journal) |volume=319 |issue=5868 |pages=1387–91 |pmid=18323453 |doi=10.1126/science.1152692|date=March 2008}}</ref> |
|||
Since inhibitors modulate the function of enzymes they are often used as drugs. Many such drugs are reversible competitive inhibitors that resemble the enzyme's native substrate, similar to [[methotrexate]] above; other well-known examples include [[statin]]s used to treat high [[cholesterol]],<ref name="Endo1992">{{cite journal | author = Endo A | title = The discovery and development of HMG-CoA reductase inhibitors | journal = J. Lipid Res. | volume = 33 | issue = 11 | pages = 1569–82 | date = 1 November 1992 | pmid = 1464741 | url =http://www.jlr.org/cgi/reprint/33/11/1569 | format = PDF }}</ref> and [[protease inhibitor (pharmacology)|protease inhibitors]] used to treat [[retrovirus|retroviral]] infections such as [[HIV]].<ref>{{cite journal | vauthors = Wlodawer A, Vondrasek J | title = Inhibitors of HIV-1 protease: a major success of structure-assisted drug design | journal = Annual Review of Biophysics and Biomolecular Structure | volume = 27 | pages = 249–84 | date = 1998 | pmid = 9646869 | doi = 10.1146/annurev.biophys.27.1.249 }}</ref> A common example of an irreversible inhibitor that is used as a drug is [[aspirin]], which inhibits the [[Cyclooxygenase|COX-1]] and [[Cyclooxygenase|COX-2]] enzymes that produce the [[inflammation]]messenger [[prostaglandin]].<ref name="Johnson" /> Other enzyme inhibitors are poisons. For example, the poison [[cyanide]] is an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzyme [[cytochrome c oxidase]] and blocks [[cellular respiration]].<ref>{{cite journal | vauthors = Yoshikawa S, Caughey WS | title = Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction | journal = The Journal of Biological Chemistry | volume = 265 | issue = 14 | pages = 7945–58 | date = May 1990 | pmid = 2159465 }}</ref> |
|||
== Biological function == |
|||
Enzymes serve a wide variety of [[function (biology)|functions]] inside living organisms. They are indispensable for [[signal transduction]] and cell regulation, often via[[kinase]]s and [[phosphatase]]s.<ref>{{cite journal | vauthors = Hunter T | title = Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling |journal = Cell | volume = 80 | issue = 2 | pages = 225–36 | date = January 1995 | pmid = 7834742 | doi = 10.1016/0092-8674(95)90405-0 }}</ref> They also generate movement, with[[myosin]] hydrolyzing ATP to generate [[muscle contraction]], and also transport cargo around the cell as part of the [[cytoskeleton]].<ref>{{cite journal | vauthors = Berg JS, Powell BC, Cheney RE | title = A millennial myosin census | journal = Molecular Biology of the Cell | volume = 12 | issue = 4 | pages = 780–94 | date = April 2001 | pmid = 11294886 |pmc = 32266 | doi = 10.1091/mbc.12.4.780 }}</ref> Other ATPases in the cell membrane are [[ion pump (biology)|ion pumps]] involved in [[active transport]]. Enzymes are also involved in more exotic functions, such as [[luciferase]] generating light in [[firefly|fireflies]].<ref>{{cite journal | vauthors = Meighen EA | title = Molecular biology of bacterial bioluminescence | journal = Microbiological Reviews | volume = 55 | issue = 1 | pages = 123–42 | date = March 1991 | pmid = 2030669 | pmc = 372803 }}</ref> [[Virus]]es can also contain enzymes for infecting cells, such as the [[HIV integrase]] and [[reverse transcriptase]], or for viral release from cells, like the [[influenza]] virus [[neuraminidase]].<ref name="pmid12370077">{{cite journal | vauthors = De Clercq E | title = Highlights in the development of new antiviral agents | journal = Mini Rev Med Chem | volume = 2 | issue = 2| pages = 163–75 | year = 2002 | pmid = 12370077 | doi = 10.2174/1389557024605474 }}</ref> |
|||
An important function of enzymes is in the [[digestive systems]] of animals. Enzymes such as [[amylases]] and [[proteases]] break down large molecules ([[starch]] or [[protein]]s, respectively) into smaller ones, so they can be absorbed by the intestines. Starch molecules, for example, are too large to be absorbed from the intestine, but enzymes hydrolyze the starch chains into smaller molecules such as [[maltose]] and eventually [[glucose]], which can then be absorbed. Different enzymes digest different food substances. In [[ruminants]], which have [[herbivorous]] diets, microorganisms in the gut produce another enzyme, [[cellulase]], to break down the cellulose cell walls of plant fiber.<ref>{{cite journal |vauthors = Mackie RI, White BA | title = Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output | journal = Journal of Dairy Science | volume = 73 | issue = 10 | pages = 2971–95 | date = October 1990 | pmid = 2178174 | doi = 10.3168/jds.S0022-0302(90)78986-2 }}</ref> |
|||
===Metabolism=== |
|||
[[Image:Glycolysis metabolic pathway.svg|thumb|400px|alt=Schematic diagram of the glycolytic metabolic pathway starting with glucose and ending with pyruvate via several intermediate chemicals. Each step in the pathway is catalyzed by a unique enzyme.|The [[metabolic pathway]] of [[glycolysis]] releases energy by converting [[glucose]] to [[pyruvate]] by via a series of intermediate metabolites. Each chemical modification (red box) is performed by a different enzyme.]] |
|||
Several enzymes can work together in a specific order, creating [[metabolic pathway]]s.<ref name = "Stryer_2002" />{{rp|30.1}} In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyze the same reaction in parallel; this can allow more complex regulation: with, for example, a low constant activity provided by one enzyme but an inducible high activity from a second enzyme.<ref name="Rouzer_2009">{{cite journal | vauthors = Rouzer CA, Marnett LJ | title = Cyclooxygenases: structural and functional insights | journal = J. Lipid Res. | volume = 50 Suppl |issue = | pages = S29–34 | year = 2009 | pmid = 18952571 | pmc = 2674713 | doi = 10.1194/jlr.R800042-JLR200 }}</ref> |
|||
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps and could not be regulated to serve the needs of the cell. Most central metabolic pathways are regulated at a few key steps, typically through enzymes whose activity involves the hydrolysis of [[Adenosine triphosphate|ATP]]. Because this reaction releases so much energy, other reactions that are [[endothermic|thermodynamically unfavorable]] can be coupled to ATP hydrolysis, driving the overall series of linked metabolic reactions.<ref name = "Stryer_2002" />{{rp|30.1}} |
|||
=== Control of activity === |
|||
There are five main ways that enzyme activity is controlled in the cell.<ref name = "Stryer_2002" />{{rp|30.1.1}} |
|||
;Regulation: Enzymes can be either [[enzyme activator|activated]] or [[enzyme inhibitor|inhibited]] by other molecules. For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, called committed step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called a [[negative feedback|negative feedback mechanism]], because the amount of the end product produced is regulated by its own concentration.<ref name = "Suzuki_2015_8"/>{{rp|141–48}} Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps with effective allocations of materials and energy economy, and it prevents the excess manufacture of end products. Like other [[homeostasis|homeostatic devices]], the control of enzymatic action helps to maintain a stable internal environment in living organisms.<ref name = "Suzuki_2015_8"/>{{rp|141}} |
|||
; Post-translational modification: Examples of [[post-translational modification]] include [[phosphorylation]], [[myristoylation]] and [[glycosylation]].<ref name = "Suzuki_2015_8">{{cite book | author = Suzuki H | title = How Enzymes Work: From Structure to Function | publisher = CRC Press | location = Boca Raton, FL | year = 2015 | isbn = 978-981-4463-92-8 | chapter = Chapter 8: Control of Enzyme Activity | pages = 141–69 }}</ref>{{rp|149–69}} For example, in the response to [[insulin]], the [[phosphorylation]] of multiple enzymes, including [[glycogen synthase]], helps control the synthesis or degradation of [[glycogen]] and allows the cell to respond to changes in [[blood sugar]].<ref name = "Doble_2003">{{cite journal | vauthors = Doble BW, Woodgett JR | title = GSK-3: tricks of the trade for a multi-tasking kinase | journal = Journal of Cell Science | volume = 116 |issue = Pt 7 | pages = 1175–86 | date = April 2003 | pmid = 12615961 | pmc = 3006448 | doi = 10.1242/jcs.00384 }}</ref> Another example of post-translational modification is the cleavage of the polypeptide chain. [[Chymotrypsin]], a digestive [[protease]], is produced in inactive form as [[chymotrypsinogen]] in the [[pancreas]] and transported in this form to the [[stomach]] where it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as a [[zymogen]]<ref name = "Suzuki_2015_8"/>{{rp|149–53}} or proenzyme. |
|||
; Quantity: Enzyme production ([[Transcription (genetics)|transcription]] and [[Translation (genetics)|translation]] of enzyme genes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form of [[regulation of gene expression|gene regulation]] is called [[enzyme induction]]. For example, bacteria may become[[antibiotic resistance|resistant to antibiotics]] such as [[penicillin]] because enzymes called [[beta-lactamase]]s are induced that hydrolyse the crucial [[Beta-lactam|beta-lactam ring]] within the penicillin molecule.<ref name="pmid8452343">{{cite journal | vauthors = Bennett PM, Chopra I | title = Molecular basis of beta-lactamase induction in bacteria |journal = Antimicrob. Agents Chemother. | volume = 37 | issue = 2 | pages = 153–8 | year = 1993 | pmid = 8452343 | pmc = 187630 | doi = 10.1128/aac.37.2.153| url =http://aac.asm.org/content/37/2/153.full.pdf }}</ref> Another example comes from enzymes in the [[liver]] called [[cytochrome P450 oxidase]]s, which are important in [[drug metabolism]]. Induction or inhibition of these enzymes can cause [[drug interaction]]s.<ref name = "Skett_Gibson_2001">{{cite book |vauthors=Skett P, Gibson GG | title = Introduction to Drug Metabolism | date = 2001 | publisher = Nelson Thornes Publishers | location = Cheltenham, UK | isbn = 978-0748760114 | pages = 87–118 | edition = 3 | chapter = Chapter 3: Induction and Inhibition of Drug Metabolism }}</ref> Enzyme levels can also be regulated by changing the rate of enzyme [[catabolism|degradation]].<ref name="Stryer_2002" />{{rp|30.1.1}} |
|||
; Subcellular distribution: Enzymes can be compartmentalized, with different metabolic pathways occurring in different [[cellular compartment]]s. For example, [[fatty acids]] are synthesized by one set of enzymes in the [[cytosol]], [[endoplasmic reticulum]] and [[golgi apparatus|Golgi]] and used by a different set of enzymes as a source of energy in the[[mitochondrion]], through [[β-oxidation]].<ref>{{cite journal | vauthors = Faergeman NJ, Knudsen J | title = Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling | journal = The Biochemical Journal | volume = 323 | issue = Pt 1 | pages = 1–12 | date = April 1997 | pmid = 9173866 | pmc = 1218279 }}</ref> In addition, [[protein targeting|trafficking]] of the enzyme to different compartments may change the degree of [[protonation]] ([[cytoplasm]] neutral and [[lysosome]] acidic) or oxidative state [e.g., oxidized ([[periplasm]]) or reduced ([[cytoplasm]])] which in turn affects enzyme activity.<ref name = "Suzuki_2015_4">{{cite book | author = Suzuki H | title = How Enzymes Work: From Structure to Function | publisher = CRC Press | location = Boca Raton, FL | year = 2015 | isbn = 978-981-4463-92-8 | chapter = Chapter 4: Effect of pH, Temperature, and High Pressure on Enzymatic Activity | pages = 53–74 }}</ref> |
|||
; Organ specialization: In [[multicellular]] [[eukaryote]]s, cells in different [[organ (anatomy)|organs]] and [[tissue (biology)|tissues]] have different patterns of [[gene expression]] and therefore have different sets of enzymes (known as [[isozyme]]s) available for metabolic reactions. This provides a mechanism for regulating the overall metabolism of the organism. For example, [[hexokinase]], the first enzyme in the [[glycolysis]] pathway, has a specialized form called [[glucokinase]] expressed in the [[liver]] and[[pancreas]] that has a lower [[affinity (pharmacology)|affinity]] for glucose yet is more sensitive to glucose concentration.<ref>{{cite journal | vauthors = Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y | title = Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase | journal = Structure | volume = 12 | issue = 3| pages = 429–38 | date = March 2004 | pmid = 15016359 | doi = 10.1016/j.str.2004.02.005 }}</ref> This enzyme is involved in sensing [[blood sugar]] and regulating [[insulin]]production.<ref>{{cite journal | vauthors = Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P | title = Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus | journal = The New England Journal of Medicine | volume = 328 | issue = 10 | pages = 697–702 | date = March 1993 | pmid = 8433729 | doi = 10.1056/NEJM199303113281005 }}</ref> |
|||
=== Involvement in disease === |
|||
[[File:Phenylalanine hydroxylase mutations.svg|thumb|400px|alt= Ribbon diagram of phenylalanine hydroxylase with bound cofactor, coenzyme and substrate|In [[phenylalanine hydroxylase]] over 300 different mutations throughout the structure cause [[phenylketonuria]]. [[Phenylalanine]] substrate and [[tetrahydrobiopterin]] coenzyme in black, and[[Iron|Fe<sup>2+</sup>]] cofactor in yellow. ({{PDB|1KW0}})]] |
|||
{{see also|Genetic disorder}} |
|||
Since the tight control of enzyme activity is essential for [[homeostasis]], any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to a [[genetic disease]]. The malfunction of just one type of enzyme out of the thousands of types present in the human body can be fatal. An example of a fatal [[genetic disease]] due to enzyme insufficiency is [[Tay-Sachs]] disease, in which patients lack the enzyme [[hexosaminidase]].<ref>{{cite journal | vauthors = Okada S, O'Brien JS | title = Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component | journal = Science | volume = 165 | issue = 3894 | pages = 698–700 | date = August 1969 | pmid = 5793973 | doi=10.1126/science.165.3894.698| bibcode = 1969Sci...165..698O }}</ref><ref>{{cite web | title = Learning About Tay-Sachs Disease | url = http://www.genome.gov/10001220 |publisher = U.S. National Human Genome Research Institute | accessdate = 1 March 2015 }}</ref> |
|||
One example of enzyme deficiency is the most common type of [[phenylketonuria]]. Many different single amino acid mutations in the enzyme [[phenylalanine hydroxylase]], which catalyzes the first step in the degradation of [[phenylalanine]], result in build-up of phenylalanine and related products. Some mutations are in the active site, directly disrupting binding and catalysis, but many are far from the active site and reduce activity by destabilising the protein structure, or affecting correct oligomerisation.<ref name=pmid10527663>{{cite journal | vauthors = Erlandsen H, Stevens RC | title = The structural basis of phenylketonuria | journal = Molecular Genetics and Metabolism | volume = 68 | issue = 2 | pages = 103–25 | date = October 1999 | pmid = 10527663 | doi = 10.1006/mgme.1999.2922 }}</ref><ref>{{cite journal | vauthors = Flatmark T, Stevens RC | title = Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms | journal = Chemical Reviews | volume = 99 | issue = 8 | pages = 2137–2160 | date = August 1999 | pmid = 11849022 | doi = 10.1021/cr980450y }}</ref> This can lead to [[intellectual disability]] if the disease is untreated.<ref>{{cite book | title = Genes and Disease [Internet] |chapterurl = http://www.ncbi.nlm.nih.gov/books/NBK22253/ | chapter = Phenylketonuria | publisher = National Center for Biotechnology Information (US) | location = Bethesda (MD) | year = 1998–2015 }}</ref> Another example is [[pseudocholinesterase deficiency]], in which the body's ability to break down choline ester drugs is impaired.<ref>{{cite web | title = Pseudocholinesterase deficiency | url = http://ghr.nlm.nih.gov/condition/pseudocholinesterase-deficiency | publisher = U.S. National Library of Medicine | accessdate = 5 September 2013 }}</ref> |
|||
Oral administration of enzymes can be used to treat some functional enzyme deficiencies, such as [[pancreatic insufficiency]]<ref>{{cite journal | vauthors = Fieker A, Philpott J, Armand M | title = Enzyme replacement therapy for pancreatic insufficiency: present and future | journal = Clinical and Experimental Gastroenterology | volume = 4 | pages = 55–73 |date = 2011 | pmid = 21753892 | pmc = 3132852 | doi = 10.2147/CEG.S17634 }}</ref> and [[lactose intolerance]].<ref>{{cite journal | vauthors = Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M | title = Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment | journal = United European Gastroenterology Journal | volume = 1 | issue = 3 | pages = 151–9 | date = June 2013 | pmid = 24917953 | pmc = 4040760 | doi = 10.1177/2050640613484463 }}</ref> |
|||
Another way enzyme malfunctions can cause disease comes from [[germline mutation]]s in genes coding for [[DNA repair]] enzymes. Defects in these enzymes cause cancer because cells are less able to repair mutations in their [[genome]]s. This causes a slow accumulation of mutations and results in the [[carcinogenesis|development of cancers]]. An example of such a hereditary [[cancer syndrome]] is [[xeroderma pigmentosum]], which causes the development of [[skin cancer]]s in response to even minimal exposure to [[ultraviolet light]].<ref>{{cite journal | vauthors = Cleaver JE | title = Defective repair replication of DNA in xeroderma pigmentosum | journal = Nature | volume = 218 | issue = 5142 | pages = 652–6 | date = May 1968 | pmid = 5655953 | doi = 10.1038/218652a0 | bibcode = 1968Natur.218..652C }}</ref><ref name="Andrews">{{cite book | last1 = James | first1 = William D | last2 = Elston |first2 = Dirk | last3 = Berger | first3 = Timothy G | title = Andrews' Diseases of the Skin: Clinical Dermatology | date = 2011 | publisher = Saunders/ Elsevier | location = London |isbn = 978-1437703146 | edition = 11th | page = 567 | name-list-format = vanc }}</ref> |
|||
== Naming conventions == |
|||
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in ''-ase''.<ref name="Stryer_2002" />{{rp|8.1.3}} Examples are[[lactase]], [[alcohol dehydrogenase]] and [[DNA polymerase]]. Different enzymes that catalyze the same chemical reaction are called [[isozymes]].<ref name = "Stryer_2002"/>{{rp|10.3}} |
|||
The [[International Union of Biochemistry and Molecular Biology]] have developed a [[nomenclature]] for enzymes, the [[Enzyme Commission number|EC numbers]]; each enzyme is described by a sequence of four numbers preceded by "EC". The first number broadly classifies the enzyme based on its mechanism.<ref name="url_Enzyme_Classification">{{cite web | url = http://www.chem.qmul.ac.uk/iubmb/enzyme/rules.html | title = Classification and Nomenclature of Enzymes by the Reactions they Catalyse | author = Nomenclature Committee | work = International Union of Biochemistry and Molecular Biology (NC-IUBMB) | publisher = School of Biological and Chemical Sciences, Queen Mary, University of London }}</ref> |
|||
The top-level classification is: |
|||
*EC 1, [[Oxidoreductase]]s: catalyze [[oxidation]]/reduction reactions |
|||
*EC 2, [[Transferase]]s: transfer a [[functional group]] (''e.g.'' a methyl or phosphate group) |
|||
*EC 3, [[Hydrolase]]s: catalyze the [[hydrolysis]] of various bonds |
|||
*EC 4, [[Lyase]]s: cleave various bonds by means other than hydrolysis and oxidation |
|||
*EC 5, [[Isomerase]]s: catalyze [[isomer]]ization changes within a single molecule |
|||
*EC 6, [[Ligase]]s: join two molecules with [[covalent bond]]s. |
|||
These sections are subdivided by other features such as the substrate, products, and [[chemical mechanism]]. An enzyme is fully specified by four numerical designations. For example,[[hexokinase]] (EC 2.7.1.1) is a transferase (EC 2) that adds a phosphate group (EC 2.7) to a hexose sugar, a molecule containing an alcohol group (EC 2.7.1).<ref>{{cite web|title=EC 2.7.1.1|url=http://www.chem.qmul.ac.uk/iubmb/enzyme/EC2/7/1/1.html | author = Nomenclature Committee | work = International Union of Biochemistry and Molecular Biology (NC-IUBMB) |publisher = School of Biological and Chemical Sciences, Queen Mary, University of London }}</ref> |
|||
== Industrial applications == |
|||
Enzymes are used in the [[chemical industry]] and other industrial applications when extremely specific catalysts are required. Enzymes in general are limited in the number of reactions they have evolved to catalyze and also by their lack of stability in [[organic solvent]]s and at high temperatures. As a consequence, [[protein engineering]] is an active area of research and involves attempts to create new enzymes with novel properties, either through rational design or ''in vitro'' evolution.<ref>{{cite journal | vauthors = Renugopalakrishnan V, Garduño-Juárez R, Narasimhan G, Verma CS, Wei X, Li P | title = Rational design of thermally stable proteins: relevance to bionanotechnology | journal = Journal of Nanoscience and Nanotechnology | volume = 5 | issue = 11 | pages = 1759–1767 | date = November 2005 | pmid = 16433409 | doi = 10.1166/jnn.2005.441 }}</ref><ref>{{cite journal |vauthors = Hult K, Berglund P | title = Engineered enzymes for improved organic synthesis | journal = Current Opinion in Biotechnology | volume = 14 | issue = 4 | pages = 395–400 |date = August 2003 | pmid = 12943848 | doi = 10.1016/S0958-1669(03)00095-8 }}</ref> These efforts have begun to be successful, and a few enzymes have now been designed "from scratch" to catalyze reactions that do not occur in nature.<ref>{{cite journal | vauthors = Jiang L, Althoff EA, Clemente FR, Doyle L, Röthlisberger D, Zanghellini A, Gallaher JL, Betker JL, Tanaka F, Barbas CF, Hilvert D, Houk KN, Stoddard BL, Baker D | title = De novo computational design of retro-aldol enzymes | journal = Science | volume = 319 | issue = 5868 | pages = 1387–91 | date = March 2008 | pmid = 18323453 | pmc = 3431203 | doi = 10.1126/science.1152692 | bibcode = 2008Sci...319.1387J }}</ref> |
|||
{| class="wikitable" |
{| class="wikitable" |
||
|- style="text-align:center;" |
|||
|- |
|||
! style="width:24%; "|Application |
|||
! style="width:38%; "|Enzymes used |
|||
|width=38% align=center| '''所用酶''' |
|||
! style="width:38%; "|Uses |
|||
|- valign="top" |
|||
|- |
|||
|style="border-top: |
| style="border-top:solid 3px #aaa;" rowspan="2"|'''[[Biofuel|Biofuel industry]]''' |
||
| style="border-top:solid 3px #aaa;"|[[Cellulase]]s |
|||
[[File:Amylose.svg|thumb|180px|α-[[淀粉酶]]催化[[淀粉]]分解为[[蔗糖]]]] |
|||
| style="border-top:solid 3px #aaa;"|Break down cellulose into sugars that can be fermented to produce [[cellulosic ethanol]].<ref name="cheng">{{cite journal | vauthors = Sun Y, Cheng J | title = Hydrolysis of lignocellulosic materials for ethanol production: a review | journal = Bioresource Technology | volume = 83 | issue = 1 | pages = 1–11 | date = May 2002 | pmid = 12058826 | doi = 10.1016/S0960-8524(01)00212-7 }}</ref> |
|||
|style="border-top: solid 3px #aaaaaa;" | [[真菌]](一般为[[酵母]])α-淀粉酶 (烘烤过程中的高温环境可以破坏其结构,导致酶活丧失) |
|||
|- valign="top" |
|||
|style="border-top: solid 3px #aaaaaa;" | 催化[[麵粉|面粉]]中的淀粉分解为蔗糖。在这一过程中,酵母会产生二氧化碳。可用于[[馒头]]、[[麵包|面包]]以及其他一些中西式糕点的制作。 |
|||
| [[Ligninase]]s |
|||
|- |
|||
| Pretreatment of [[biomass]] for biofuel production.<ref name="cheng" /> |
|||
| [[蛋白酶]] |
|||
|- valign="top" |
|||
| 制作[[餅乾|饼干]]的过程中,通常用它来降低面粉中蛋白质的含量。 |
|||
| style="border-top:solid 3px #aaa;" rowspan="2"| '''[[Biological detergent]]''' |
|||
|- |
|||
| style="border-top:solid 3px #aaa;"|[[Protease]]s, [[amylase]]s, [[lipase]]s |
|||
| [[木瓜蛋白酶]] |
|||
| style="border-top:solid 3px #aaa;"|Remove protein, starch, and fat or oil stains from laundry and dishware.<ref name="Kirk">{{cite journal | vauthors = Kirk O, Borchert TV, Fuglsang CC | title = Industrial enzyme applications | journal = Current Opinion in Biotechnology | date = August 2002 | volume = 13 | issue = 4 | pages = 345–351 | doi = 10.1016/S0958-1669(02)00328-2| pmid = 12323357 }}</ref> |
|||
| 将肉嫩化,以利于烹饪。 |
|||
|- valign="top" |
|||
|- |
|||
| [[Mannanase]]s |
|||
|style="border-top: solid 3px #aaaaaa;" | '''[[婴儿食品]]''' |
|||
| Remove food stains from the common food additive [[guar gum]].<ref name="Kirk" /> |
|||
|style="border-top: solid 3px #aaaaaa;" | [[胰蛋白酶]] |
|||
|- valign="top" |
|||
|style="border-top: solid 3px #aaaaaa;" | 经过酶处理的婴儿食品更易于消化。 |
|||
| style="border-top:solid 3px #aaa;" rowspan="4"| '''[[Brewing|Brewing industry]]''' |
|||
|- |
|||
|style="border-top: |
| style="border-top:solid 3px #aaa;"|[[Amylase]], [[glucanase]]s, [[protease]]s |
||
| style="border-top:solid 3px #aaa;"|Split polysaccharides and proteins in the [[malt]].<ref name="briggs">{{cite book | last1 = Briggs | first1 = Dennis E. | title = Malts and Malting | date = 1998 | publisher = Blackie Academic | location = London | isbn = 978-0412298004 | edition = 1st | name-list-format = vanc }}</ref>{{rp|150–9}} |
|||
[[File:Sjb whiskey malt.jpg|thumb|180px|[[麦芽]]]] |
|||
|- valign="top" |
|||
|style="border-top: solid 3px #aaaaaa;" | 麦芽中的酶 |
|||
| [[Betaglucanase]]s |
|||
|style="border-top: solid 3px #aaaaaa;" | 将淀粉和蛋白质降解为糖、氨基酸和[[肽]]段,而这些原料可以被酵母发酵产生[[乙醇|酒精]]。 |
|||
| Improve the [[wort]] and beer filtration characteristics.<ref name="briggs" />{{rp|545}} |
|||
|- |
|||
|- valign="top" |
|||
| 工业生产的麦酶 |
|||
| [[Amyloglucosidase]] and [[pullulanase]]s |
|||
| 广泛用于替代天然酶进行[[啤酒]]酿造 |
|||
| Make low-calorie [[beer]] and adjust fermentability.<ref name="briggs" />{{rp|575}} |
|||
|- |
|||
|- valign="top" |
|||
| 淀粉酶、[[葡聚糖酶]]和蛋白酶 |
|||
| [[Acetolactate decarboxylase]] (ALDC) |
|||
| 分解麦芽中的多聚糖链和蛋白质。 |
|||
| Increase fermentation efficiency by reducing [[diacetyl]] formation.<ref>{{cite journal | vauthors = Dulieu C, Moll M, Boudrant J, Poncelet D | title = Improved performances and control of beer fermentation using encapsulated alpha-acetolactate decarboxylase and modeling | journal = Biotechnology Progress | volume = 16 | issue = 6 | pages = 958–65 | pmid = 11101321 | doi = 10.1021/bp000128k | year=2000}}</ref> |
|||
|- |
|||
|- valign="top" |
|||
| β-葡聚糖酶和[[阿拉伯木聚糖酶]] |
|||
| style="border-top:solid 3px #aaa;"|'''[[Cooking|Culinary uses]]''' |
|||
| 提高麦汁和啤酒的滤过性。 |
|||
| style="border-top:solid 3px #aaa;"|[[Papain]] |
|||
|- |
|||
| style="border-top:solid 3px #aaa;"|[[Tenderizer|Tenderize]] meat for cooking.<ref>{{cite book | first1 = Rodrigo | last1 =Tarté | title = Ingredients in Meat Products Properties, Functionality and Applications | date = 2008 | publisher = Springer | location = New York | isbn = 978-0-387-71327-4 | pages = 177| name-list-format = vanc }}</ref> |
|||
| [[淀粉葡糖苷酶]]和[[普鲁兰酶]] |
|||
|- valign="top" |
|||
| 制造低[[卡路里]]啤酒和调整发酵能力。 |
|||
| style="border-top:solid 3px #aaa;" rowspan="2"| '''[[Dairy|Dairy industry]]''' |
|||
|- |
|||
| style = "border-top:solid 3px #aaa;"|[[Chymosin|Rennin]] |
|||
| 蛋白酶 |
|||
| style="border-top:solid 3px #aaa;"|[[Hydrolyze]] protein in the manufacture of [[cheese]].<ref>{{cite web|url=http://www.gmo-compass.org/eng/database/enzymes/83.chymosin.html|accessdate=1 March 2015|date=10 July 2010|title=Chymosin – GMO Database|work=GMO Compass|publisher = European Union}}</ref> |
|||
| 除去在啤酒储存过程中产生的絮状物。 |
|||
|- valign="top" |
|||
|- |
|||
| [[Lipase]]s |
|||
| [[乙酰乳酸脱羧酶]](ALDC) |
|||
| Produce [[Camembert cheese]] and [[blue cheese]]s such as [[Roquefort]].<ref>{{cite journal | vauthors = Molimard P, Spinnler HE | title = Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties | journal = Journal of Dairy Science | date = February 1996 | volume = 79 | issue = 2 | pages = 169–184 | doi = 10.3168/jds.S0022-0302(96)76348-8}}</ref> |
|||
| 避免[[丁二酮]]的产生。 |
|||
|- valign="top" |
|||
|- |
|||
|style="border-top: |
| style="border-top:solid 3px #aaa;" rowspan="4"| '''[[Food processing]]''' |
||
|style="border-top: |
| style="border-top:solid 3px #aaa;"|[[Amylase]]s |
||
| style="border-top:solid 3px #aaa;"|Produce sugars from [[starch]], such as in making [[high-fructose corn syrup]].<ref>{{cite journal | vauthors = Guzmán-Maldonado H, Paredes-López O | title = Amylolytic enzymes and products derived from starch: a review | journal = Critical Reviews in Food Science and Nutrition | volume = 35 | issue = 5 | pages = 373–403 |date = September 1995 | pmid = 8573280 | doi = 10.1080/10408399509527706 }}</ref> |
|||
|style="border-top: solid 3px #aaaaaa;" | 降解果汁中的不溶物。 |
|||
|- valign="top" |
|||
|- |
|||
| [[Protease]]s |
|||
|style="border-top: solid 3px #aaaaaa;" rowspan="4" | '''[[乳品]]业''' |
|||
| Lower the protein level of [[flour]], as in [[biscuit]]-making.<ref name="GMOdatabase" /> |
|||
[[File:Roquefort cheese.jpg|thumb|180px|洛克福羊[[乳酪]]]] |
|||
|- valign="top" |
|||
|style="border-top: solid 3px #aaaaaa;" | [[凝乳酶]],提自[[反刍亚目|反刍动物]](牛、羊)幼崽的胃。 |
|||
||[[Trypsin]] |
|||
|style="border-top: solid 3px #aaaaaa;" | 制造乳酪,[[水解]]蛋白质。 |
|||
|Manufacture [[hypoallergenic]] baby foods.<ref name="GMOdatabase">{{cite web | url = http://www.gmo-compass.org/eng/database/enzymes/94.protease.html | title = Protease – GMO Database | date = 10 July 2010 | work = GMO Compass | publisher = European Union | accessdate = 28 February 2015 }}</ref> |
|||
|- |
|||
|- valign="top" |
|||
| 用微生物生产的凝乳酶 |
|||
| [[Cellulase]]s, [[pectinase]]s |
|||
| 越来越多地使用于乳品制造业。 |
|||
| Clarify [[fruit juice]]s.<ref>{{cite journal | vauthors = Alkorta I, Garbisu C, Llama MJ, Serra JL | title = Industrial applications of pectic enzymes: a review | journal = Process Biochemistry | date = January 1998 | volume = 33 | issue = 1 | pages = 21–28 | doi = 10.1016/S0032-9592(97)00046-0 }}</ref> |
|||
|- |
|||
|- valign="top" |
|||
| [[脂酶]] |
|||
| style="border-top:solid 3px #aaa;"|'''[[Molecular biology]]''' |
|||
| 洛克福羊乳酪的制作过程中添加,以加快乳酪的成熟。 |
|||
| style="border-top:solid 3px #aaa;"|[[Nuclease]]s, [[DNA ligase]] and [[polymerases]] |
|||
|- |
|||
| style="border-top:solid 3px #aaa;"|Use [[restriction enzyme|restriction digestion]] and the [[polymerase chain reaction]] to create [[recombinant DNA]].<ref name="Stryer_2002" />{{rp|6.2}} |
|||
| [[乳糖酶]] |
|||
|- valign="top" |
|||
| 将[[乳糖]]分解为[[葡萄糖]]和[[半乳糖]]。 |
|||
| style="border-top:solid 3px #aaa;"|'''[[Paper|Paper industry]]''' |
|||
|- |
|||
|style="border-top: |
| style="border-top:solid 3px #aaa;"|[[Xylanase]]s, [[hemicellulase]]s and [[lignin peroxidase]]s |
||
| style="border-top:solid 3px #aaa;"|Remove [[lignin]] from [[kraft pulp]].<ref>{{cite journal | vauthors = Bajpai P | title = Application of enzymes in the pulp and paper industry |journal = Biotechnology Progress | volume = 15 | issue = 2 | pages = 147–157 | date = March 1999 | pmid = 10194388 | doi = 10.1021/bp990013k }}</ref> |
|||
|style="border-top: solid 3px #aaaaaa;" | [[淀粉酶]]、淀粉葡糖苷酶和葡糖糖化酶 |
|||
|- valign="top" |
|||
|style="border-top: solid 3px #aaaaaa;" | 将淀粉转化为葡萄糖和各种糖浆。 |
|||
| style="border-top:solid 3px #aaa;"|'''[[Personal care]]''' |
|||
|- |
|||
| style="border-top:solid 3px #aaa;"|[[Proteases]] |
|||
| 葡萄糖异构酶 |
|||
| style="border-top:solid 3px #aaa;"|Remove proteins on [[contact lens]]es to prevent infections.<ref>{{cite journal | vauthors = Begley CG, Paragina S, Sporn A | title = An analysis of contact lens enzyme cleaners | journal = Journal of the American Optometric Association | volume = 61 | issue = 3 | pages = 190–4 | date = March 1990 | pmid = 2186082 }}</ref> |
|||
| 在生产高[[果糖]]含量的糖浆时,将葡萄糖转化为果糖。这样生产的糖浆有更好的甜度和更低的卡路里含量(与同样甜度的蔗糖相比)。 |
|||
|- valign="top" |
|||
|- |
|||
|style="border-top: |
| style="border-top:solid 3px #aaa;" rowspan="1"| '''[[Starch|Starch industry]]''' |
||
| style="border-top:solid 3px #aaa;"| [[Amylase]]s |
|||
[[File:InternationalPaper6413.jpg|160px|thumb|造纸厂]] |
|||
| style="border-top:solid 3px #aaa;"| Convert [[starch]] into [[glucose]] and various [[Inverted sugar syrup|syrups]].<ref>{{cite book | editor1-last = BeMiller | editor1-first = James N. | editor2-last = Whistler | editor2-first = Roy L. | title = Starch Chemistry and Technology | date = 2009 | publisher = Academic | location = London | isbn = 9780080926551 |edition=3rd | name-list-format = vanc | last1 = Farris | first1 = Paul L. | chapter = Economic Growth and Organization of the U.S. Starch Industry }}</ref> |
|||
|style="border-top: solid 3px #aaaaaa;" | 淀粉酶、[[木聚糖酶]]、[[纤维素酶]]和[[木质酶]] |
|||
|style="border-top: solid 3px #aaaaaa;" | 淀粉酶用于降解淀粉至低[[黏度|粘性]],加胶和给纸加膜;木聚糖酶能够降低脱色过程所需的漂白剂;纤维素酶使纤维光滑并增强纸的排水性;木质酶可以消除[[木质素]]以软化纸质。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" rowspan="2" | '''[[生質燃料|生物燃料]]生产''' |
|||
[[File:Cellulose-3D-balls.png|180px|thumb|[[纤维素]]的三维结构]] |
|||
|style="border-top: solid 3px #aaaaaa;" | [[纤维素酶]] |
|||
|style="border-top: solid 3px #aaaaaa;" | 用于降解纤维素,产生可用于发酵的蔗糖。(参见[[纤维素乙醇]])。 |
|||
|- |
|||
| {{link-en|木质酶|Ligninase}} |
|||
| 用于[[木质素]]废品的降解。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" rowspan="4" | '''生物[[清洁剂|去垢剂]]''' |
|||
|style="border-top: solid 3px #aaaaaa;" | 主要为蛋白酶(产自细菌的膜外部分) |
|||
|style="border-top: solid 3px #aaaaaa;" | 用于洗衣过程中的浸泡阶段,帮助除去衣物上的含有蛋白质的污渍。 |
|||
|- |
|||
| [[淀粉酶]] |
|||
| 除去洗衣机上的淀粉残余。 |
|||
|- |
|||
| 脂酶 |
|||
| 帮助除去衣物上的油渍。 |
|||
|- |
|||
| [[纤维素酶]] |
|||
| 作为生物纤维(如棉质衣物)的[[衣物柔顺剂|柔顺剂]]。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" | '''[[隱形眼鏡|隐形眼镜]]清洁剂''' |
|||
|style="border-top: solid 3px #aaaaaa;" | 蛋白酶 |
|||
|style="border-top: solid 3px #aaaaaa;" | 清除隐形眼镜上的蛋白质,以防止细菌滋生。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" | '''[[橡膠|橡胶]]制造业''' |
|||
|style="border-top: solid 3px #aaaaaa;" | [[过氧化氢酶]] |
|||
|style="border-top: solid 3px #aaaaaa;" | 催化[[过氧化物]]产生[[氧气]],以将[[胶乳]]转化为泡沫橡皮。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" | '''[[摄影]]业''' |
|||
|style="border-top: solid 3px #aaaaaa;" | 蛋白酶(无花果蛋白酶) |
|||
|style="border-top: solid 3px #aaaaaa;" | 溶解[[底片]]上的[[明膠]],使得[[銀|银]]成分显现。 |
|||
|- |
|||
|style="border-top: solid 3px #aaaaaa;" | '''[[分子生物学]]''' |
|||
[[File:DNA123 rotated.png|180px|thumb|DNA[[双螺旋形|双螺旋]]中的一部分]] |
|||
|style="border-top: solid 3px #aaaaaa;" | [[限制内切酶]]、[[DNA连接酶]]和[[DNA聚合酶|聚合酶]] |
|||
|style="border-top: solid 3px #aaaaaa;" | 用于在[[基因工程|遗传工程]]进行基因操纵,对于[[药理学]]、[[农业]]和[[医学]]有重要意义。在[[限制内切酶|限制性酶切]]和[[聚合酶链锁反应]]上获得广泛应用。分子生物学方法在法医学的鉴定实验上也有重要应用。 |
|||
|- |
|||
|} |
|} |
||
{{TransF}} |
|||
==參見== |
|||
{{portal|生物|分子与细胞生物学}} |
|||
== 参见 == |
|||
{{Portal box|生物学|细胞生物学|分子生物学|新陈代谢|飲食}} |
|||
* [[酶列表]] |
* [[酶列表]] |
||
* |
* 酶數據庫 |
||
** {{link-en|BRENDA|BRENDA}} |
|||
* [[酶动力学]] |
|||
** {{link-en|ExPASy|ExPASy}} |
|||
* [[酶抑制剂]] |
|||
* [[ |
** [[IntEnz]] |
||
* [[ |
** [[KEGG]] |
||
** {{link-en|MetaCyc|MetaCyc}} |
|||
* [[蛋白质]] |
|||
* [[蛋白质组学]]和[[蛋白质工程]] |
|||
== 参考文献 == |
|||
{{reflist|2}} |
|||
== 延伸阅读 == |
|||
{{refbegin|2}} |
|||
'''发现及研究史''' |
|||
* {{en}}[http://bip.cnrs-mrs.fr/bip10/buchner.htm New Beer in an Old Bottle: Eduard Buchner and the Growth of Biochemical Knowledge, edited by Athel Cornish-Bowden and published by Universitat de València (1997): ](介绍早期酶的发展历史)。ISBN 978-84-370-3328-0 |
|||
* {{en}}[http://etext.lib.virginia.edu/toc/modeng/public/Wil4Sci.html Williams, Henry Smith, 1863–1943. A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences] |
|||
* {{en}}Kleyn, J. and Hough J. The Microbiology of Brewing. ''Annual Review of Microbiology''(1971)Vol. 25: 583–608 |
|||
== 參考 == |
|||
'''酶结构与催化机理''' |
|||
{{reflist|33em}} |
|||
* {{en}}Fersht, A. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding. W. H. Freeman, 1998 ISBN 978-0-7167-3268-6 |
|||
* {{en}}Walsh, C., Enzymatic Reaction Mechanisms. W. H. Freeman and Company. 1979. ISBN 978-0-7167-0070-8 |
|||
* {{en}}Page, M. I., and Williams, A.(Eds.), 1987. Enzyme Mechanisms. Royal Society of Chemistry. ISBN 978-0-85186-947-6 |
|||
* {{en}}Bugg, T. Introduction to Enzyme and Coenzyme Chemistry, 2004, Blackwell Publishing Limited; 2nd edition. ISBN 978-1-4051-1452-3 |
|||
* {{en}}Warshel, A., Computer Modeling of Chemical Reactions in enzymes and Solutions John Wiley & Sons Inc. 1991. ISBN 978-0-471-18440-9 |
|||
== 拓展閱讀 == |
|||
'''酶热力学''' |
|||
{{Col-begin}} |
|||
* {{en}}[http://www.emc.maricopa.edu/faculty/farabee/BIOBK/BioBookEnzym.html Reactions and Enzymes],来自Estrella Mountain Community College的在线生物图书。 |
|||
{{Col-1-of-2}} |
|||
'''總論''' |
|||
* {{cite book | first1 = Jeremy M | last1 = Berg | first2 = John L | last2 = Tymoczko | first3= Lubert | last3 = Stryer | title = Biochemistry | date = 2002 | publisher = W. H. Freeman | location = New York, NY | isbn = 0-7167-3051-0 | edition = 5th | url = http://www.ncbi.nlm.nih.gov/books/NBK21154/ | name-list-format=vanc}}, A biochemistry textbook available free online through NCBI Bookshelf.{{Open access}} |
|||
'''詞源與歷史''' |
|||
'''酶反应动力学及抑制作用''' |
|||
*{{cite book | title = New Beer in an Old Bottle: Eduard Buchner and the Growth of Biochemical Knowledge | url = http://bip.cnrs-mrs.fr/bip10/buchner.htm | editor-first = Athel |editor-last = Cornish-Bowden | publisher = Universitat de València | year = 1997 | isbn = 84-370-3328-4 | name-list-format = vanc }}, A history of early enzymology. |
|||
* {{en}}Athel Cornish-Bowden, ''Fundamentals of Enzyme Kinetics''.(3rd edition), Portland Press (2004), ISBN 978-1-85578-158-0. |
|||
* {{en}}Irwin H. Segel, ''Enzyme Kinetics: Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems''. Wiley-Interscience; New Ed edition (1993), ISBN 978-0-471-30309-1. |
|||
* {{en}}John W. Baynes, ''Medical Biochemistry'', Elsevier-Mosby; 2nd Edition (2005), ISBN 978-0-7234-3341-5, p. 57. |
|||
* {{zh}}颜思旭,蔡红玉编著。《酶催化动力学原理与方法》。厦门大学出版社。1987年。ISBN 978-7-5615-0030-9 |
|||
{{Col-2-of-2}} |
|||
'''酶在细胞中的功能和调控''' |
|||
* {{en}}Price, N. and Stevens, L., ''Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins'', Oxford University Press,(1999), ISBN 978-0-19-850229-6 |
|||
* {{en}}[http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=gnd.chapter.86 Nutritional and Metabolic Diseases],来自[[美国国家生物技术信息中心|NCBI]]的在线图书''Introduction to Genes and Disease''。 |
|||
'''酶 |
'''酶結構及作用機理'' |
||
* {{cite book | author = Suzuki H | title = How Enzymes Work: From Structure to Function | publisher = CRC Press | location = Boca Raton, FL | year = 2015 | isbn = 978-981-4463-92-8}} |
|||
* {{en}}[http://www.chem.qmul.ac.uk/iubmb/enzyme/ Enzyme Nomenclature],国际生物化学与分子生物学联盟命名委员会推荐的酶的名字。 |
|||
* {{en}}Koshland D. ''The Enzymes'', v. I, ch. 7, Acad. Press, New York,(1959) |
|||
''' |
'''動力學及抑制''' |
||
* {{cite book | last1 = Cornish-Bowden | first1 = Athel | title = Fundamentals of Enzyme Kinetics | date = 2012 | publisher = Wiley-VCH | location = Weinheim | isbn = 978-3527330744 |edition = 4th | name-list-format=vanc }} |
|||
* {{en}}[http://www.mapsenzymes.com/History_of_Enzymes.asp History of industrial enzymes],包含介绍工业用酶的历史(从20世纪末至今)的文章。 |
|||
* {{zh}}梁传伟,张苏勤主编。《酶工程》。化学工业出版社。2006年。ISBN 978-7-5025-7872-5 |
|||
{{refend}} |
|||
{{Col-end}} |
|||
== 外部链接 == |
|||
{{Commonscat|Enzymes}} |
|||
{{refbegin|2}} |
|||
* {{en}}[http://www.ebi.ac.uk/intenz/spotlight.jsp Enzyme spotlight],由[[欧州生物信息学研究所]]每月选出一种酶进行介绍。 |
|||
* {{en}}[http://www.amfep.org AMFEP],Association of Manufacturers and Formulators of Enzyme Products |
|||
* {{en}}[http://www.brenda-enzymes.org BRENDA]数据库,包含所有已知酶的详细信息和对应文献,商业用户需付费使用。 |
|||
* {{en}}[http://www.ebi.ac.uk/thornton-srv/databases/enzymes/ 酶结构数据库],含有已知三维结构的酶,可以链接到[[蛋白质数据库]]。 |
|||
* {{en}}[http://us.expasy.org/enzyme/ ExPASy的酶数据库],链接到{{lang-en|Swiss-Prot|Swiss-Prot}}序列数据以及其他相关数据库和文献。 |
|||
* {{en}}[http://www.genome.jp/kegg/ KEGG],京都基因与基因组百科辞典(Kyoto Encyclopedia of Genes and Genomes),含有介绍生化途径和相关酶的图像和基于超链接的信息。 |
|||
* {{en}}[http://www-mitchell.ch.cam.ac.uk/macie MACiE数据库],关于酶反应机理。 |
|||
* {{en}}[http://metacyc.org/ MetaCyc数据库],关于代谢途径和相关酶。 |
|||
* {{en}}[http://www.vega.org.uk/video/programme/19 与诺贝尔奖获得者John Cornforth面对面交谈]由Vega Science Trust提供的免费视频。 |
|||
{{refend}} |
|||
* {{en}}[http://www.creative-enzymes.com/],Creative Enzymes uses its expertise in enzyme manufacturing to supply customers enzymes using for life science research and production |
|||
{{-}} |
|||
{{酶}} |
|||
{{食品化学}} |
|||
{{Food chemistry}} |
|||
{{Enzymes}} |
|||
{{Authority control}} |
{{Authority control}} |
||
[[Category:生物分子]] |
[[Category:生物分子]] |
||
2016年8月16日 (二) 08:58的版本

| 生物學的一部分 |
| 生物化学 |
|---|
 |
| 关键部分 |
| 歷史和主題 |
| 詞彙 |
| 生物化學主题 |
酶(Enzymes(/ˈɛnzaɪmz/ ))是一種大分子生物催化劑。酶能加快化學反應的速度,即具有催化作用。由酶催化的反應中,反應物稱爲底物(substrates),生成的物質稱爲產物。幾乎所有細胞內代謝過程都離不開酶。酶能大大加快這些過程中各化學反應進行的速率,使代謝過程能滿足生物體的需求[1]:8.1。細胞中酶的類型決定了可在該細胞中發生的代謝途徑的類型。對酶進行研究的學科稱爲「酶學」(enzymology)。
Enzymes are known to catalyze more than 5,000 biochemical reaction types.[2] Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.
Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds.[3][4] Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibriumof a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.
Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biologicalwashing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.
Etymology and history

By the late 17th and early 18th centuries, the digestion of meat by stomach secretions[5]and the conversion of starch to sugars by plant extracts and saliva were known but the mechanisms by which these occurred had not been identified.[6]
French chemist Anselme Payen was the first to discover an enzyme, diastase, in 1833.[7] A few decades later, when studying the fermentation of sugar to alcohol by yeast, Louis Pasteurconcluded that this fermentation was caused by a vital force contained within the yeast cells called "ferments", which were thought to function only within living organisms. He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells."[8]
In 1877, German physiologist Wilhelm Kühne (1837–1900) first used the term enzyme, which comes from Greek ἔνζυμον, "leavened", to describe this process.[9] The word enzyme was used later to refer to nonliving substances such as pepsin, and the word ferment was used to refer to chemical activity produced by living organisms.[10]
Eduard Buchner submitted his first paper on the study of yeast extracts in 1897. In a series of experiments at the University of Berlin, he found that sugar was fermented by yeast extracts even when there were no living yeast cells in the mixture.[11] He named the enzyme that brought about the fermentation of sucrose "zymase".[12] In 1907, he received the Nobel Prize in Chemistry for "his discovery of cell-free fermentation". Following Buchner's example, enzymes are usually named according to the reaction they carry out: the suffix -ase is combined with the name of the substrate (e.g.,lactase is the enzyme that cleaves lactose) or to the type of reaction (e.g., DNA polymerase forms DNA polymers).[13]
The biochemical identity of enzymes was still unknown in the early 1900s. Many scientists observed that enzymatic activity was associated with proteins, but others (such as Nobel laureate Richard Willstätter) argued that proteins were merely carriers for the true enzymes and that proteins per se were incapable of catalysis.[14] In 1926, James B. Sumner showed that the enzyme urease was a pure protein and crystallized it; he did likewise for the enzyme catalase in 1937. The conclusion that pure proteins can be enzymes was definitively demonstrated by John Howard Northropand Wendell Meredith Stanley, who worked on the digestive enzymes pepsin (1930), trypsin and chymotrypsin. These three scientists were awarded the 1946 Nobel Prize in Chemistry.[15]
The discovery that enzymes could be crystallized eventually allowed their structures to be solved by x-ray crystallography. This was first done for lysozyme, an enzyme found in tears, saliva and egg whites that digests the coating of some bacteria; the structure was solved by a group led by David Chilton Phillips and published in 1965.[16] This high-resolution structure of lysozyme marked the beginning of the field of structural biology and the effort to understand how enzymes work at an atomic level of detail.[17]
Structure
Enzymes are generally globular proteins, acting alone or in larger complexes. Like all proteins, enzymes are linear chains of amino acids that fold to produce a three-dimensional structure. The sequence of the amino acids specifies the structure which in turn determines the catalytic activity of the enzyme.[18] Although structure determines function, a novel enzyme's activity cannot yet be predicted from its structure alone.[19] Enzyme structures unfold (denature) when heated or exposed to chemical denaturants and this disruption to the structure typically causes a loss of activity.[20] Enzyme denaturation is normally linked to temperatures above a species' normal level; as a result, enzymes from bacteria living in volcanic environments such as hot springs are prized by industrial users for their ability to function at high temperatures, allowing enzyme-catalysed reactions to be operated at a very high rate.
Enzymes are usually much larger than their substrates. Sizes range from just 62 amino acid residues, for the monomer of 4-oxalocrotonate tautomerase,[21]to over 2,500 residues in the animal fatty acid synthase.[22] Only a small portion of their structure (around 2–4 amino acids) is directly involved in catalysis: the catalytic site.[23] This catalytic site is located next to one or more binding sites where residues orient the substrates. The catalytic site and binding site together comprise the enzyme's active site. The remaining majority of the enzyme structure serves to maintain the precise orientation and dynamics of the active site.[24]
In some enzymes, no amino acids are directly involved in catalysis; instead, the enzyme contains sites to bind and orient catalytic cofactors.[24] Enzyme structures may also contain allosteric sites where the binding of a small molecule causes a conformational change that increases or decreases activity.[25]
A small number of RNA-based biological catalysts called ribozymes exist, which again can act alone or in complex with proteins. The most common of these is the ribosome which is a complex of protein and catalytic RNA components.[1]:2.2
Mechanism
Substrate binding
Enzymes must bind their substrates before they can catalyse any chemical reaction. Enzymes are usually very specific as to what substrates they bind and then the chemical reaction catalysed. Specificity is achieved by binding pockets with complementary shape, charge and hydrophilic/hydrophobic characteristics to the substrates. Enzymes can therefore distinguish between very similar substrate molecules to be chemoselective, regioselective andstereospecific.[26]
Some of the enzymes showing the highest specificity and accuracy are involved in the copying and expression of the genome. Some of these enzymes have "proof-reading" mechanisms. Here, an enzyme such as DNA polymerase catalyzes a reaction in a first step and then checks that the product is correct in a second step.[27] This two-step process results in average error rates of less than 1 error in 100 million reactions in high-fidelity mammalian polymerases.[1]:5.3.1 Similar proofreading mechanisms are also found in RNA polymerase,[28] aminoacyl tRNA synthetases[29] andribosomes.[30]
Conversely, some enzymes display enzyme promiscuity, having broad specificity and acting on a range of different physiologically relevant substrates. Many enzymes possess small side activities which arose fortuitously (i.e. neutrally), which may be the starting point for the evolutionary selection of a new function.[31][32]
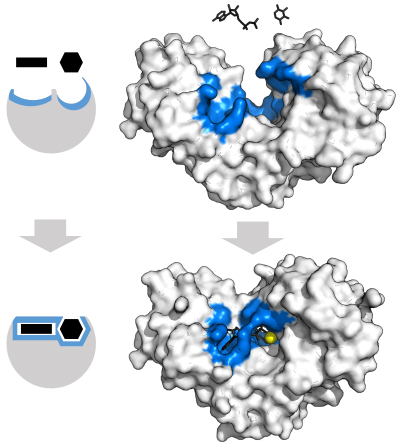
"Lock and key" model
To explain the observed specificity of enzymes, in 1894 Emil Fischer proposed that both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.[33] This is often referred to as "the lock and key" model.[1]:8.3.2 This early model explains enzyme specificity, but fails to explain the stabilization of the transition state that enzymes achieve.[34]
Induced fit model
In 1958, Daniel Koshland suggested a modification to the lock and key model: since enzymes are rather flexible structures, the active site is continuously reshaped by interactions with the substrate as the substrate interacts with the enzyme.[35] As a result, the substrate does not simply bind to a rigid active site; the amino acid side-chains that make up the active site are molded into the precise positions that enable the enzyme to perform its catalytic function. In some cases, such as glycosidases, the substrate molecule also changes shape slightly as it enters the active site.[36] The active site continues to change until the substrate is completely bound, at which point the final shape and charge distribution is determined.[37] Induced fit may enhance the fidelity of molecular recognition in the presence of competition and noise via the conformational proofreading mechanism.[38]
Catalysis
Enzymes can accelerate reactions in several ways, all of which lower the activation energy (ΔG‡, Gibbs free energy)[39]
- By stabilizing the transition state:
- Creating an environment with a charge distribution complementary to that of the transition state to lower its energy.[40]
- By providing an alternative reaction pathway:
- Temporarily reacting with the substrate, forming a covalent intermediate to provide a lower energy transition state.[41]
- By destabilising the substrate ground state:
- Distorting bound substrate(s) into their transition state form to reduce the energy required to reach the transition state.[42]
- By orienting the substrates into a productive arrangement to reduce the reaction entropy change.[43] The contribution of this mechanism to catalysis is relatively small.[44]
Enzymes may use several of these mechanisms simultaneously. For example, proteases such as trypsin perform covalent catalysis using a catalytic triad, stabilise charge build-up on the transition states using an oxyanion hole, complete hydrolysis using an oriented water substrate.
Dynamics
Enzymes are not rigid, static structures; instead they have complex internal dynamic motions – that is, movements of parts of the enzyme's structure such as individual amino acid residues, groups of residues forming a protein loop or unit of secondary structure, or even an entire protein domain. These motions give rise to a conformational ensemble of slightly different structures that interconvert with one another at equilibrium. Different states within this ensemble may be associated with different aspects of an enzyme's function. For example, different conformations of the enzyme dihydrofolate reductase are associated with the substrate binding, catalysis, cofactor release, and product release steps of the catalytic cycle.[45]
Allosteric modulation
Allosteric sites are pockets on the enzyme, distinct from the active site, that bind to molecules in the cellular environment. These molecules then cause a change in the conformation or dynamics of the enzyme that is transduced to the active site and thus affects the reaction rate of the enzyme.[46] In this way, allosteric interactions can either inhibit or activate enzymes. Allosteric interactions with metabolites upstream or downstream in an enzyme's metabolic pathway cause feedback regulation, altering the activity of the enzyme according to the flux through the rest of the pathway.[47]
Cofactors

Some enzymes do not need additional components to show full activity. Others require non-protein molecules called cofactors to be bound for activity.[48] Cofactors can be either inorganic (e.g.,metal ions and iron-sulfur clusters) or organic compounds (e.g., flavin and heme). Organic cofactors can be eithercoenzymes, which are released from the enzyme's active site during the reaction, or prosthetic groups, which are tightly bound to an enzyme. Organic prosthetic groups can be covalently bound (e.g., biotin in enzymes such as pyruvate carboxylase).[49]
An example of an enzyme that contains a cofactor is carbonic anhydrase, which is shown in the ribbon diagram above with a zinc cofactor bound as part of its active site.[50] These tightly bound ions or molecules are usually found in the active site and are involved in catalysis.[1]:8.1.1 For example, flavin and heme cofactors are often involved in redox reactions.[1]:17
Enzymes that require a cofactor but do not have one bound are called apoenzymes or apoproteins. An enzyme together with the cofactor(s) required for activity is called aholoenzyme (or haloenzyme). The term holoenzyme can also be applied to enzymes that contain multiple protein subunits, such as the DNA polymerases; here the holoenzyme is the complete complex containing all the subunits needed for activity.[1]:8.1.1
Coenzymes
Coenzymes are small organic molecules that can be loosely or tightly bound to an enzyme. Coenzymes transport chemical groups from one enzyme to another.[51] Examples include NADH, NADPH and adenosine triphosphate (ATP). Some coenzymes, such as riboflavin, thiamine and folic acid, are vitamins, or compounds that cannot be synthesized by the body and must be acquired from the diet. The chemical groups carried include the hydride ion (H−) carried by NAD or NADP+, the phosphate group carried by adenosine triphosphate, the acetyl group carried by coenzyme A, formyl, methenyl or methyl groups carried by folic acid and the methyl group carried by S-adenosylmethionine.[51]
Since coenzymes are chemically changed as a consequence of enzyme action, it is useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different enzymes. For example, about 1000 enzymes are known to use the coenzyme NADH.[52]
Coenzymes are usually continuously regenerated and their concentrations maintained at a steady level inside the cell. For example, NADPH is regenerated through the pentose phosphate pathway and S-adenosylmethionine by methionine adenosyltransferase. This continuous regeneration means that small amounts of coenzymes can be used very intensively. For example, the human body turns over its own weight in ATP each day.[53]
Thermodynamics
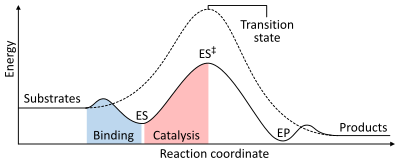
As with all catalysts, enzymes do not alter the position of the chemical equilibrium of the reaction. In the presence of an enzyme, the reaction runs in the same direction as it would without the enzyme, just more quickly.[1]:8.2.3 For example, carbonic anhydrase catalyzes its reaction in either direction depending on the concentration of its reactants:[54]
| (in tissues; high CO2 concentration) | () |
| (in lungs; low CO2 concentration) | () |
The rate of a reaction is dependent on the activation energy needed to form the transition state which then decays into products. Enzymes increase reaction rates by lowering the energy of the transition state. First, binding forms a low energy enzyme-substrate complex (ES). Secondly the enzyme stabilises the transition state such that it requires less energy to achieve compared to the uncatalyzed reaction (ES‡). Finally the enzyme-product complex (EP) dissociates to release the products.[1]:8.3
Enzymes can couple two or more reactions, so that a thermodynamically favorable reaction can be used to "drive" a thermodynamically unfavourable one so that the combined energy of the products is lower than the substrates. For example, the hydrolysis of ATP is often used to drive other chemical reactions.[55]
Kinetics
Enzyme kinetics is the investigation of how enzymes bind substrates and turn them into products. The rate data used in kinetic analyses are commonly obtained from enzyme assays. In 1913 Leonor Michaelis and Maud Leonora Menten proposed a quantitative theory of enzyme kinetics, which is referred to as Michaelis–Menten kinetics.[56] The major contribution of Michaelis and Menten was to think of enzyme reactions in two stages. In the first, the substrate binds reversibly to the enzyme, forming the enzyme-substrate complex. This is sometimes called the Michaelis-Menten complex in their honor. The enzyme then catalyzes the chemical step in the reaction and releases the product. This work was further developed by G. E. Briggs and J. B. S. Haldane, who derived kinetic equations that are still widely used today.[57]
Enzyme rates depend on solution conditions and substrate concentration. To find the maximum speed of an enzymatic reaction, the substrate concentration is increased until a constant rate of product formation is seen. This is shown in the saturation curve on the right. Saturation happens because, as substrate concentration increases, more and more of the free enzyme is converted into the substrate-bound ES complex. At the maximum reaction rate (Vmax) of the enzyme, all the enzyme active sites are bound to substrate, and the amount of ES complex is the same as the total amount of enzyme.[1]:8.4
Vmax is only one of several important kinetic parameters. The amount of substrate needed to achieve a given rate of reaction is also important. This is given by theMichaelis-Menten constant (Km), which is the substrate concentration required for an enzyme to reach one-half its maximum reaction rate; generally, each enzyme has a characteristic Km for a given substrate. Another useful constant is kcat, also called the turnover number, which is the number of substrate molecules handled by one active site per second.[1]:8.4
The efficiency of an enzyme can be expressed in terms of kcat/Km. This is also called the specificity constant and incorporates the rate constants for all steps in the reaction up to and including the first irreversible step. Because the specificity constant reflects both affinity and catalytic ability, it is useful for comparing different enzymes against each other, or the same enzyme with different substrates. The theoretical maximum for the specificity constant is called the diffusion limit and is about 108 to 109 (M−1 s−1). At this point every collision of the enzyme with its substrate will result in catalysis, and the rate of product formation is not limited by the reaction rate but by the diffusion rate. Enzymes with this property are called catalytically perfect orkinetically perfect. Example of such enzymes are triose-phosphate isomerase, carbonic anhydrase, acetylcholinesterase, catalase,fumarase, β-lactamase, and superoxide dismutase.[1]:8.4.2 The turnover of such enzymes can reach several million reactions per second.[1]:9.2
Michaelis–Menten kinetics relies on the law of mass action, which is derived from the assumptions of free diffusion and thermodynamically driven random collision. Many biochemical or cellular processes deviate significantly from these conditions, because of macromolecular crowding and constrained molecular movement.[58] More recent, complex extensions of the model attempt to correct for these effects.[59]
Inhibition
Enzyme reaction rates can be decreased by various types of enzyme inhibitors.[60]:73–74
Types of inhibition
- Competitive
- A competitive inhibitor and substrate cannot bind to the enzyme at the same time.[61]Often competitive inhibitors strongly resemble the real substrate of the enzyme. For example, the drug methotrexate is a competitive inhibitor of the enzyme dihydrofolate reductase, which catalyzes the reduction of dihydrofolate to tetrahydrofolate. The similarity between the structures of dihydrofolate and this drug are shown in the accompanying figure. This type of inhibition can be overcome with high substrate concentration. In some cases, the inhibitor can bind to a site other than the binding-site of the usual substrate and exert an allosteric effect to change the shape of the usual binding-site.
- Non-competitive
- A non-competitive inhibitor binds to a site other than where the substrate binds. The substrate still binds with its usual affinity and hence Km remains the same. However the inhibitor reduces the catalytic efficiency of the enzyme so that Vmax is reduced. In contrast to competitive inhibition, non-competitive inhibition cannot be overcome with high substrate concentration.[60]:76–78
- Uncompetitive
- An uncompetitive inhibitor cannot bind to the free enzyme, only to the enzyme-substrate complex; hence, these types of inhibitors are most effective at high substrate concentration. In the presence of the inhibitor, the enzyme-substrate complex is inactive.[60]:78 This type of inhibition is rare.[62]
- Mixed
- A mixed inhibitor binds to an allosteric site and the binding of the substrate and the inhibitor affect each other. The enzyme's function is reduced but not eliminated when bound to the inhibitor. This type of inhibitor does not follow the Michaelis-Menten equation.[60]:76–78
- Irreversible
- An irreversible inhibitor permanently inactivates the enzyme, usually by forming a covalent bond to the protein. Penicillin[63] and aspirin[64] are common drugs that act in this manner.
Functions of inhibitors
In many organisms, inhibitors may act as part of a feedback mechanism. If an enzyme produces too much of one substance in the organism, that substance may act as an inhibitor for the enzyme at the beginning of the pathway that produces it, causing production of the substance to slow down or stop when there is sufficient amount. This is a form of negative feedback. Major metabolic pathways such as the citric acid cycle make use of this mechanism.[1]:17.2.2
Since inhibitors modulate the function of enzymes they are often used as drugs. Many such drugs are reversible competitive inhibitors that resemble the enzyme's native substrate, similar to methotrexate above; other well-known examples include statins used to treat high cholesterol,[65] and protease inhibitors used to treat retroviral infections such as HIV.[66] A common example of an irreversible inhibitor that is used as a drug is aspirin, which inhibits the COX-1 and COX-2 enzymes that produce the inflammationmessenger prostaglandin.[64] Other enzyme inhibitors are poisons. For example, the poison cyanide is an irreversible enzyme inhibitor that combines with the copper and iron in the active site of the enzyme cytochrome c oxidase and blocks cellular respiration.[67]
Biological function
Enzymes serve a wide variety of functions inside living organisms. They are indispensable for signal transduction and cell regulation, often viakinases and phosphatases.[68] They also generate movement, withmyosin hydrolyzing ATP to generate muscle contraction, and also transport cargo around the cell as part of the cytoskeleton.[69] Other ATPases in the cell membrane are ion pumps involved in active transport. Enzymes are also involved in more exotic functions, such as luciferase generating light in fireflies.[70] Viruses can also contain enzymes for infecting cells, such as the HIV integrase and reverse transcriptase, or for viral release from cells, like the influenza virus neuraminidase.[71]
An important function of enzymes is in the digestive systems of animals. Enzymes such as amylases and proteases break down large molecules (starch or proteins, respectively) into smaller ones, so they can be absorbed by the intestines. Starch molecules, for example, are too large to be absorbed from the intestine, but enzymes hydrolyze the starch chains into smaller molecules such as maltose and eventually glucose, which can then be absorbed. Different enzymes digest different food substances. In ruminants, which have herbivorous diets, microorganisms in the gut produce another enzyme, cellulase, to break down the cellulose cell walls of plant fiber.[72]
Metabolism

Several enzymes can work together in a specific order, creating metabolic pathways.[1]:30.1 In a metabolic pathway, one enzyme takes the product of another enzyme as a substrate. After the catalytic reaction, the product is then passed on to another enzyme. Sometimes more than one enzyme can catalyze the same reaction in parallel; this can allow more complex regulation: with, for example, a low constant activity provided by one enzyme but an inducible high activity from a second enzyme.[73]
Enzymes determine what steps occur in these pathways. Without enzymes, metabolism would neither progress through the same steps and could not be regulated to serve the needs of the cell. Most central metabolic pathways are regulated at a few key steps, typically through enzymes whose activity involves the hydrolysis of ATP. Because this reaction releases so much energy, other reactions that are thermodynamically unfavorable can be coupled to ATP hydrolysis, driving the overall series of linked metabolic reactions.[1]:30.1
Control of activity
There are five main ways that enzyme activity is controlled in the cell.[1]:30.1.1
- Regulation
- Enzymes can be either activated or inhibited by other molecules. For example, the end product(s) of a metabolic pathway are often inhibitors for one of the first enzymes of the pathway (usually the first irreversible step, called committed step), thus regulating the amount of end product made by the pathways. Such a regulatory mechanism is called a negative feedback mechanism, because the amount of the end product produced is regulated by its own concentration.[74]:141–48 Negative feedback mechanism can effectively adjust the rate of synthesis of intermediate metabolites according to the demands of the cells. This helps with effective allocations of materials and energy economy, and it prevents the excess manufacture of end products. Like other homeostatic devices, the control of enzymatic action helps to maintain a stable internal environment in living organisms.[74]:141
- Post-translational modification
- Examples of post-translational modification include phosphorylation, myristoylation and glycosylation.[74]:149–69 For example, in the response to insulin, the phosphorylation of multiple enzymes, including glycogen synthase, helps control the synthesis or degradation of glycogen and allows the cell to respond to changes in blood sugar.[75] Another example of post-translational modification is the cleavage of the polypeptide chain. Chymotrypsin, a digestive protease, is produced in inactive form as chymotrypsinogen in the pancreas and transported in this form to the stomach where it is activated. This stops the enzyme from digesting the pancreas or other tissues before it enters the gut. This type of inactive precursor to an enzyme is known as a zymogen[74]:149–53 or proenzyme.
- Quantity
- Enzyme production (transcription and translation of enzyme genes) can be enhanced or diminished by a cell in response to changes in the cell's environment. This form of gene regulation is called enzyme induction. For example, bacteria may becomeresistant to antibiotics such as penicillin because enzymes called beta-lactamases are induced that hydrolyse the crucial beta-lactam ring within the penicillin molecule.[76] Another example comes from enzymes in the liver called cytochrome P450 oxidases, which are important in drug metabolism. Induction or inhibition of these enzymes can cause drug interactions.[77] Enzyme levels can also be regulated by changing the rate of enzyme degradation.[1]:30.1.1
- Subcellular distribution
- Enzymes can be compartmentalized, with different metabolic pathways occurring in different cellular compartments. For example, fatty acids are synthesized by one set of enzymes in the cytosol, endoplasmic reticulum and Golgi and used by a different set of enzymes as a source of energy in themitochondrion, through β-oxidation.[78] In addition, trafficking of the enzyme to different compartments may change the degree of protonation (cytoplasm neutral and lysosome acidic) or oxidative state [e.g., oxidized (periplasm) or reduced (cytoplasm)] which in turn affects enzyme activity.[79]
- Organ specialization
- In multicellular eukaryotes, cells in different organs and tissues have different patterns of gene expression and therefore have different sets of enzymes (known as isozymes) available for metabolic reactions. This provides a mechanism for regulating the overall metabolism of the organism. For example, hexokinase, the first enzyme in the glycolysis pathway, has a specialized form called glucokinase expressed in the liver andpancreas that has a lower affinity for glucose yet is more sensitive to glucose concentration.[80] This enzyme is involved in sensing blood sugar and regulating insulinproduction.[81]
Involvement in disease
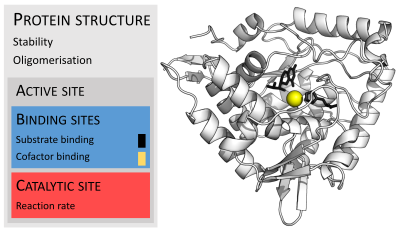
Since the tight control of enzyme activity is essential for homeostasis, any malfunction (mutation, overproduction, underproduction or deletion) of a single critical enzyme can lead to a genetic disease. The malfunction of just one type of enzyme out of the thousands of types present in the human body can be fatal. An example of a fatal genetic disease due to enzyme insufficiency is Tay-Sachs disease, in which patients lack the enzyme hexosaminidase.[82][83]
One example of enzyme deficiency is the most common type of phenylketonuria. Many different single amino acid mutations in the enzyme phenylalanine hydroxylase, which catalyzes the first step in the degradation of phenylalanine, result in build-up of phenylalanine and related products. Some mutations are in the active site, directly disrupting binding and catalysis, but many are far from the active site and reduce activity by destabilising the protein structure, or affecting correct oligomerisation.[84][85] This can lead to intellectual disability if the disease is untreated.[86] Another example is pseudocholinesterase deficiency, in which the body's ability to break down choline ester drugs is impaired.[87] Oral administration of enzymes can be used to treat some functional enzyme deficiencies, such as pancreatic insufficiency[88] and lactose intolerance.[89]
Another way enzyme malfunctions can cause disease comes from germline mutations in genes coding for DNA repair enzymes. Defects in these enzymes cause cancer because cells are less able to repair mutations in their genomes. This causes a slow accumulation of mutations and results in the development of cancers. An example of such a hereditary cancer syndrome is xeroderma pigmentosum, which causes the development of skin cancers in response to even minimal exposure to ultraviolet light.[90][91]
Naming conventions
An enzyme's name is often derived from its substrate or the chemical reaction it catalyzes, with the word ending in -ase.[1]:8.1.3 Examples arelactase, alcohol dehydrogenase and DNA polymerase. Different enzymes that catalyze the same chemical reaction are called isozymes.[1]:10.3
The International Union of Biochemistry and Molecular Biology have developed a nomenclature for enzymes, the EC numbers; each enzyme is described by a sequence of four numbers preceded by "EC". The first number broadly classifies the enzyme based on its mechanism.[92]
The top-level classification is:
- EC 1, Oxidoreductases: catalyze oxidation/reduction reactions
- EC 2, Transferases: transfer a functional group (e.g. a methyl or phosphate group)
- EC 3, Hydrolases: catalyze the hydrolysis of various bonds
- EC 4, Lyases: cleave various bonds by means other than hydrolysis and oxidation
- EC 5, Isomerases: catalyze isomerization changes within a single molecule
- EC 6, Ligases: join two molecules with covalent bonds.
These sections are subdivided by other features such as the substrate, products, and chemical mechanism. An enzyme is fully specified by four numerical designations. For example,hexokinase (EC 2.7.1.1) is a transferase (EC 2) that adds a phosphate group (EC 2.7) to a hexose sugar, a molecule containing an alcohol group (EC 2.7.1).[93]
Industrial applications
Enzymes are used in the chemical industry and other industrial applications when extremely specific catalysts are required. Enzymes in general are limited in the number of reactions they have evolved to catalyze and also by their lack of stability in organic solvents and at high temperatures. As a consequence, protein engineering is an active area of research and involves attempts to create new enzymes with novel properties, either through rational design or in vitro evolution.[94][95] These efforts have begun to be successful, and a few enzymes have now been designed "from scratch" to catalyze reactions that do not occur in nature.[96]
| Application | Enzymes used | Uses |
|---|---|---|
| Biofuel industry | Cellulases | Break down cellulose into sugars that can be fermented to produce cellulosic ethanol.[97] |
| Ligninases | Pretreatment of biomass for biofuel production.[97] | |
| Biological detergent | Proteases, amylases, lipases | Remove protein, starch, and fat or oil stains from laundry and dishware.[98] |
| Mannanases | Remove food stains from the common food additive guar gum.[98] | |
| Brewing industry | Amylase, glucanases, proteases | Split polysaccharides and proteins in the malt.[99]:150–9 |
| Betaglucanases | Improve the wort and beer filtration characteristics.[99]:545 | |
| Amyloglucosidase and pullulanases | Make low-calorie beer and adjust fermentability.[99]:575 | |
| Acetolactate decarboxylase (ALDC) | Increase fermentation efficiency by reducing diacetyl formation.[100] | |
| Culinary uses | Papain | Tenderize meat for cooking.[101] |
| Dairy industry | Rennin | Hydrolyze protein in the manufacture of cheese.[102] |
| Lipases | Produce Camembert cheese and blue cheeses such as Roquefort.[103] | |
| Food processing | Amylases | Produce sugars from starch, such as in making high-fructose corn syrup.[104] |
| Proteases | Lower the protein level of flour, as in biscuit-making.[105] | |
| Trypsin | Manufacture hypoallergenic baby foods.[105] | |
| Cellulases, pectinases | Clarify fruit juices.[106] | |
| Molecular biology | Nucleases, DNA ligase and polymerases | Use restriction digestion and the polymerase chain reaction to create recombinant DNA.[1]:6.2 |
| Paper industry | Xylanases, hemicellulases and lignin peroxidases | Remove lignin from kraft pulp.[107] |
| Personal care | Proteases | Remove proteins on contact lenses to prevent infections.[108] |
| Starch industry | Amylases | Convert starch into glucose and various syrups.[109] |
參見
參考
- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 Stryer L, Berg JM, Tymoczko JL. Biochemistry 5th. San Francisco: W.H. Freeman. 2002. ISBN 0-7167-4955-6.

- ^ Schomburg I, Chang A, Placzek S, Söhngen C, Rother M, Lang M, Munaretto C, Ulas S, Stelzer M, Grote A, Scheer M, Schomburg D. BRENDA in 2013: integrated reactions, kinetic data, enzyme function data, improved disease classification: new options and contents in BRENDA. Nucleic Acids Research. January 2013, 41 (Database issue): D764–72. PMC 3531171
 . PMID 23203881. doi:10.1093/nar/gks1049.
. PMID 23203881. doi:10.1093/nar/gks1049.
- ^ Radzicka A, Wolfenden R. A proficient enzyme. Science. January 1995, 267 (5194): 90–931. Bibcode:1995Sci...267...90R. PMID 7809611. doi:10.1126/science.7809611.
- ^ Callahan BP, Miller BG. OMP decarboxylase—An enigma persists. Bioorganic Chemistry. December 2007, 35 (6): 465–9. PMID 17889251. doi:10.1016/j.bioorg.2007.07.004.
- ^ de Réaumur RA. Observations sur la digestion des oiseaux. Histoire de l'academie royale des sciences. 1752, 1752: 266, 461.
- ^ Williams HS. A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences. Harper and Brothers. 1904.
- ^ Payen A, Persoz JF. Mémoire sur la diastase, les principaux produits de ses réactions et leurs applications aux arts industriels [Memoir on diastase, the principal products of its reactions, and their applications to the industrial arts]. Annales de chimie et de physique. 2nd. 1833, 53: 73–92 (French).
- ^ Manchester KL. Louis Pasteur (1822–1895)–chance and the prepared mind. Trends in Biotechnology. December 1995, 13 (12): 511–5. PMID 8595136. doi:10.1016/S0167-7799(00)89014-9.
- ^ Kühne coined the word "enzyme" in: Kühne W. Über das Verhalten verschiedener organisirter und sog. ungeformter Fermente [On the behavior of various organized and so-called unformed ferments]. Verhandlungen des naturhistorisch-medicinischen Vereins zu Heidelberg. new series. 1877, 1 (3): 190–193 (German). The relevant passage occurs on page 190: "Um Missverständnissen vorzubeugen und lästige Umschreibungen zu vermeiden schlägt Vortragender vor, die ungeformten oder nicht organisirten Fermente, deren Wirkung ohne Anwesenheit von Organismen und ausserhalb derselben erfolgen kann, als Enzyme zu bezeichnen." (Translation: In order to obviate misunderstandings and avoid cumbersome periphrases, [the author, a university lecturer] suggests designating as "enzymes" the unformed or not organized ferments, whose action can occur without the presence of organisms and outside of the same.)
- ^ Holmes FL. Enzymes. Heilbron JL (编). The Oxford Companion to the History of Modern Science. Oxford: Oxford University Press. 2003: 270.
- ^ Eduard Buchner. Nobel Laureate Biography. Nobelprize.org. [23 February 2015].
- ^ Eduard Buchner – Nobel Lecture: Cell-Free Fermentation. Nobelprize.org. 1907 [23 February 2015].
- ^ The naming of enzymes by adding the suffix "-ase" to the substrate on which the enzyme acts, has been traced to French scientist Émile Duclaux (1840–1904), who intended to honor the discoverers of diastase – the first enzyme to be isolated – by introducing this practice in his book Duclaux E. Traité de microbiologie: Diastases, toxines et venins [Microbiology Treatise: diastases , toxins and venoms]. Paris, France: Masson and Co. 1899 (French). See Chapter 1, especially page 9.
- ^ Willstätter R. Faraday lecture. Problems and methods in enzyme research. Journal of the Chemical Society (Resumed). 1927: 1359. doi:10.1039/JR9270001359. quoted in Blow D. So do we understand how enzymes work? (pdf). Structure (London, England : 1993). April 2000, 8 (4): R77–R81. PMID 10801479. doi:10.1016/S0969-2126(00)00125-8.
- ^ Nobel Prizes and Laureates: The Nobel Prize in Chemistry 1946. Nobelprize.org. [23 February 2015].
- ^ Blake CC, Koenig DF, Mair GA, North AC, Phillips DC, Sarma VR. Structure of hen egg-white lysozyme. A three-dimensional Fourier synthesis at 2 Ångström resolution. Nature. May 1965, 206 (4986): 757–61. Bibcode:1965Natur.206..757B. PMID 5891407. doi:10.1038/206757a0.
- ^ Johnson LN, Petsko GA. David Phillips and the origin of structural enzymology. Trends Biochem. Sci. 1999, 24 (7): 287–9. PMID 10390620. doi:10.1016/S0968-0004(99)01423-1.
- ^ Anfinsen CB. Principles that govern the folding of protein chains. Science. July 1973, 181 (4096): 223–30. Bibcode:1973Sci...181..223A. PMID 4124164. doi:10.1126/science.181.4096.223.
- ^ Dunaway-Mariano D. Enzyme function discovery. Structure (London, England : 1993). November 2008, 16 (11): 1599–600. PMID 19000810. doi:10.1016/j.str.2008.10.001.
- ^ Petsko GA, Ringe D. Chapter 1: From sequence to structure. Protein structure and function. London: New Science. 2003: 27. ISBN 978-1405119221.
- ^ Chen LH, Kenyon GL, Curtin F, Harayama S, Bembenek ME, Hajipour G, Whitman CP. 4-Oxalocrotonate tautomerase, an enzyme composed of 62 amino acid residues per monomer. The Journal of Biological Chemistry. September 1992, 267 (25): 17716–21. PMID 1339435.
- ^ Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB Journal. December 1994, 8 (15): 1248–59. PMID 8001737.
- ^ The Catalytic Site Atlas. The European Bioinformatics Institute. [4 April 2007].
- ^ 24.0 24.1 Suzuki H. Chapter 7: Active Site Structure. How Enzymes Work: From Structure to Function. Boca Raton, FL: CRC Press. 2015: 117–140. ISBN 978-981-4463-92-8.
- ^ Krauss G. The Regulations of Enzyme Activity. Biochemistry of Signal Transduction and Regulation 3rd. Weinheim: Wiley-VCH. 2003: 89–114. ISBN 9783527605767.
- ^ Jaeger KE, Eggert T. Enantioselective biocatalysis optimized by directed evolution. Current Opinion in Biotechnology. August 2004, 15 (4): 305–13. PMID 15358000. doi:10.1016/j.copbio.2004.06.007.
- ^ Shevelev IV, Hübscher U. The 3' 5' exonucleases. Nature Reviews Molecular Cell Biology. May 2002, 3 (5): 364–76. PMID 11988770. doi:10.1038/nrm804.
- ^ Zenkin N, Yuzenkova Y, Severinov K. Transcript-assisted transcriptional proofreading. Science. July 2006, 313 (5786): 518–20. Bibcode:2006Sci...313..518Z. PMID 16873663. doi:10.1126/science.1127422.
- ^ Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annual Review of Biochemistry. 2000, 69: 617–50. PMID 10966471. doi:10.1146/annurev.biochem.69.1.617.
- ^ Rodnina MV, Wintermeyer W. Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms. Annual Review of Biochemistry. 2001, 70: 415–35. PMID 11395413. doi:10.1146/annurev.biochem.70.1.415.
- ^ Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Annual Review of Biochemistry. 2010, 79: 471–505. PMID 20235827. doi:10.1146/annurev-biochem-030409-143718.
- ^ O'Brien PJ, Herschlag D. Catalytic promiscuity and the evolution of new enzymatic activities. Chemistry & Biology. April 1999, 6 (4): R91–R105. PMID 10099128. doi:10.1016/S1074-5521(99)80033-7.
- ^ Fischer E. Einfluss der Configuration auf die Wirkung der Enzyme [Influence of configuration on the action of enzymes]. Berichte der Deutschen chemischen Gesellschaft zu Berlin. 1894, 27 (3): 2985–93. doi:10.1002/cber.18940270364 (German). From page 2992: "Um ein Bild zu gebrauchen, will ich sagen, dass Enzym und Glucosid wie Schloss und Schlüssel zu einander passen müssen, um eine chemische Wirkung auf einander ausüben zu können." (To use an image, I will say that an enzyme and a glucoside [i.e., glucose derivative] must fit like a lock and key, in order to be able to exert a chemical effect on each other.)
- ^ Cooper GM. Chapter 2.2: The Central Role of Enzymes as Biological Catalysts. The Cell: a Molecular Approach 2nd. Washington (DC ): ASM Press. 2000. ISBN 0-87893-106-6.
- ^ Koshland DE. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proceedings of the National Academy of Sciences of the United States of America. February 1958, 44 (2): 98–104. Bibcode:1958PNAS...44...98K. PMC 335371
 . PMID 16590179. doi:10.1073/pnas.44.2.98.
. PMID 16590179. doi:10.1073/pnas.44.2.98.
- ^ Vasella A, Davies GJ, Böhm M. Glycosidase mechanisms. Current Opinion in Chemical Biology. October 2002, 6 (5): 619–29. PMID 12413546. doi:10.1016/S1367-5931(02)00380-0.
- ^ Boyer R. Chapter 6: Enzymes I, Reactions, Kinetics, and Inhibition. Concepts in Biochemistry 2nd. New York, Chichester, Weinheim, Brisbane, Singapore, Toronto.: John Wiley & Sons, Inc. 2002: 137–8. ISBN 0-470-00379-0. OCLC 51720783.
- ^ Savir Y, Tlusty T. Scalas E , 编. Conformational proofreading: the impact of conformational changes on the specificity of molecular recognition (PDF). PLoS ONE. 2007, 2 (5): e468. Bibcode:2007PLoSO...2..468S. PMC 1868595
 . PMID 17520027. doi:10.1371/journal.pone.0000468.
. PMID 17520027. doi:10.1371/journal.pone.0000468.
- ^ Fersht A. Enzyme Structure and Mechanism. San Francisco: W.H. Freeman. 1985: 50–2. ISBN 0-7167-1615-1.
- ^ Warshel A, Sharma PK, Kato M, Xiang Y, Liu H, Olsson MH. Electrostatic basis for enzyme catalysis. Chemical Reviews. August 2006, 106 (8): 3210–35. PMID 16895325. doi:10.1021/cr0503106.
- ^ Cox MM, Nelson DL. Chapter 6.2: How enzymes work. Lehninger Principles of Biochemistry 6th. New York, N.Y.: W.H. Freeman. 2013: 195. ISBN 978-1464109621.
- ^ Benkovic SJ, Hammes-Schiffer S. A perspective on enzyme catalysis. Science. August 2003, 301 (5637): 1196–202. Bibcode:2003Sci...301.1196B. PMID 12947189. doi:10.1126/science.1085515.
- ^ Jencks WP. Catalysis in Chemistry and Enzymology. Mineola, N.Y: Dover. 1987. ISBN 0-486-65460-5.
- ^ Villa J, Strajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A. How important are entropic contributions to enzyme catalysis?. Proceedings of the National Academy of Sciences of the United States of America. October 2000, 97 (22): 11899–904. Bibcode:2000PNAS...9711899V. PMC 17266
 . PMID 11050223. doi:10.1073/pnas.97.22.11899.
. PMID 11050223. doi:10.1073/pnas.97.22.11899.
- ^ Ramanathan A, Savol A, Burger V, Chennubhotla CS, Agarwal PK. Protein conformational populations and functionally relevant substates. Acc. Chem. Res. 2014, 47 (1): 149–56. PMID 23988159. doi:10.1021/ar400084s.
- ^ Tsai CJ, Del Sol A, Nussinov R. Protein allostery, signal transmission and dynamics: a classification scheme of allosteric mechanisms. Mol Biosyst. 2009, 5 (3): 207–16. PMC 2898650
 . PMID 19225609. doi:10.1039/b819720b.
. PMID 19225609. doi:10.1039/b819720b.
- ^ Changeux JP, Edelstein SJ. Allosteric mechanisms of signal transduction. Science. June 2005, 308 (5727): 1424–8. Bibcode:2005Sci...308.1424C. PMID 15933191. doi:10.1126/science.1108595.
- ^ de Bolster MW. Glossary of Terms Used in Bioinorganic Chemistry: Cofactor. International Union of Pure and Applied Chemistry. 1997 [30 October 2007].
- ^ Chapman-Smith A, Cronan JE. The enzymatic biotinylation of proteins: a post-translational modification of exceptional specificity. Trends Biochem. Sci. 1999, 24 (9): 359–63. PMID 10470036. doi:10.1016/s0968-0004(99)01438-3.
- ^ Fisher Z, Hernandez Prada JA, Tu C, Duda D, Yoshioka C, An H, Govindasamy L, Silverman DN, McKenna R. Structural and kinetic characterization of active-site histidine as a proton shuttle in catalysis by human carbonic anhydrase II. Biochemistry. February 2005, 44 (4): 1097–115. PMID 15667203. doi:10.1021/bi0480279.
- ^ 51.0 51.1 Wagner AL. Vitamins and Coenzymes. Krieger Pub Co. 1975. ISBN 0-88275-258-8.
- ^ BRENDA The Comprehensive Enzyme Information System. Technische Universität Braunschweig. [23 February 2015].
- ^ Törnroth-Horsefield S, Neutze R. Opening and closing the metabolite gate. Proceedings of the National Academy of Sciences of the United States of America. December 2008, 105 (50): 19565–6. Bibcode:2008PNAS..10519565T. PMC 2604989
 . PMID 19073922. doi:10.1073/pnas.0810654106.
. PMID 19073922. doi:10.1073/pnas.0810654106.
- ^ McArdle WD, Katch F, Katch VL. Chapter 9: The Pulmonary System and Exercise. Essentials of Exercise Physiology 3rd. Baltimore, Maryland: Lippincott Williams & Wilkins. 2006: 312–3. ISBN 978-0781749916.
- ^ Ferguson SJ, Nicholls D, Ferguson S. Bioenergetics 3 3rd. San Diego: Academic. 2002. ISBN 0-12-518121-3.
- ^ Michaelis L, Menten M. Die Kinetik der Invertinwirkung [The Kinetics of Invertase Action]. Biochem. Z. 1913, 49: 333–369 (German).; Michaelis L, Menten ML, Johnson KA, Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry. 2011, 50 (39): 8264–9. PMC 3381512
 . PMID 21888353. doi:10.1021/bi201284u.
. PMID 21888353. doi:10.1021/bi201284u.
- ^ Briggs GE, Haldane JB. A Note on the Kinetics of Enzyme Action. The Biochemical Journal. 1925, 19 (2): 339–339. PMC 1259181
 . PMID 16743508. doi:10.1042/bj0190338.
. PMID 16743508. doi:10.1042/bj0190338.
- ^ Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends in Biochemical Sciences. October 2001, 26 (10): 597–604. PMID 11590012. doi:10.1016/S0968-0004(01)01938-7.
- ^ Kopelman R. Fractal reaction kinetics. Science. September 1988, 241 (4873): 1620–26. Bibcode:1988Sci...241.1620K. PMID 17820893. doi:10.1126/science.241.4873.1620.
- ^ 60.0 60.1 60.2 60.3 Cornish-Bowden A. Fundamentals of Enzyme Kinetics 3. London: Portland Press. 2004. ISBN 1-85578-158-1.
- ^ Price NC. What is meant by 'competitive inhibition'?. Trends in Biochemical Sciences. 1979, 4 (11): N272–N273. doi:10.1016/0968-0004(79)90205-6.
- ^ Cornish-Bowden A. Why is uncompetitive inhibition so rare? A possible explanation, with implications for the design of drugs and pesticides. FEBS Letters. July 1986, 203 (1): 3–6. PMID 3720956. doi:10.1016/0014-5793(86)81424-7.
- ^ Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to beta-lactam antibiotics: compelling opportunism, compelling opportunity. Chemical Reviews. February 2005, 105 (2): 395–424. PMID 15700950. doi:10.1021/cr030102i.
- ^ 64.0 64.1 Johnson DS, Weerapana E, Cravatt BF. Strategies for discovering and derisking covalent, irreversible enzyme inhibitors. Future Medicinal Chemistry. June 2010, 2 (6): 949–64. PMC 2904065
 . PMID 20640225. doi:10.4155/fmc.10.21.
. PMID 20640225. doi:10.4155/fmc.10.21.
- ^ Endo A. The discovery and development of HMG-CoA reductase inhibitors (PDF). J. Lipid Res. 1 November 1992, 33 (11): 1569–82. PMID 1464741.
- ^ Wlodawer A, Vondrasek J. Inhibitors of HIV-1 protease: a major success of structure-assisted drug design. Annual Review of Biophysics and Biomolecular Structure. 1998, 27: 249–84. PMID 9646869. doi:10.1146/annurev.biophys.27.1.249.
- ^ Yoshikawa S, Caughey WS. Infrared evidence of cyanide binding to iron and copper sites in bovine heart cytochrome c oxidase. Implications regarding oxygen reduction. The Journal of Biological Chemistry. May 1990, 265 (14): 7945–58. PMID 2159465.
- ^ Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. January 1995, 80 (2): 225–36. PMID 7834742. doi:10.1016/0092-8674(95)90405-0.
- ^ Berg JS, Powell BC, Cheney RE. A millennial myosin census. Molecular Biology of the Cell. April 2001, 12 (4): 780–94. PMC 32266
 . PMID 11294886. doi:10.1091/mbc.12.4.780.
. PMID 11294886. doi:10.1091/mbc.12.4.780.
- ^ Meighen EA. Molecular biology of bacterial bioluminescence. Microbiological Reviews. March 1991, 55 (1): 123–42. PMC 372803
 . PMID 2030669.
. PMID 2030669.
- ^ De Clercq E. Highlights in the development of new antiviral agents. Mini Rev Med Chem. 2002, 2 (2): 163–75. PMID 12370077. doi:10.2174/1389557024605474.
- ^ Mackie RI, White BA. Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output. Journal of Dairy Science. October 1990, 73 (10): 2971–95. PMID 2178174. doi:10.3168/jds.S0022-0302(90)78986-2.
- ^ Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J. Lipid Res. 2009,. 50 Suppl: S29–34. PMC 2674713
 . PMID 18952571. doi:10.1194/jlr.R800042-JLR200.
. PMID 18952571. doi:10.1194/jlr.R800042-JLR200.
- ^ 74.0 74.1 74.2 74.3 Suzuki H. Chapter 8: Control of Enzyme Activity. How Enzymes Work: From Structure to Function. Boca Raton, FL: CRC Press. 2015: 141–69. ISBN 978-981-4463-92-8.
- ^ Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. April 2003, 116 (Pt 7): 1175–86. PMC 3006448
 . PMID 12615961. doi:10.1242/jcs.00384.
. PMID 12615961. doi:10.1242/jcs.00384.
- ^ Bennett PM, Chopra I. Molecular basis of beta-lactamase induction in bacteria (PDF). Antimicrob. Agents Chemother. 1993, 37 (2): 153–8. PMC 187630
 . PMID 8452343. doi:10.1128/aac.37.2.153.
. PMID 8452343. doi:10.1128/aac.37.2.153.
- ^ Skett P, Gibson GG. Chapter 3: Induction and Inhibition of Drug Metabolism. Introduction to Drug Metabolism 3. Cheltenham, UK: Nelson Thornes Publishers. 2001: 87–118. ISBN 978-0748760114.
- ^ Faergeman NJ, Knudsen J. Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. The Biochemical Journal. April 1997, 323 (Pt 1): 1–12. PMC 1218279
 . PMID 9173866.
. PMID 9173866.
- ^ Suzuki H. Chapter 4: Effect of pH, Temperature, and High Pressure on Enzymatic Activity. How Enzymes Work: From Structure to Function. Boca Raton, FL: CRC Press. 2015: 53–74. ISBN 978-981-4463-92-8.
- ^ Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. March 2004, 12 (3): 429–38. PMID 15016359. doi:10.1016/j.str.2004.02.005.
- ^ Froguel P, Zouali H, Vionnet N, Velho G, Vaxillaire M, Sun F, Lesage S, Stoffel M, Takeda J, Passa P. Familial hyperglycemia due to mutations in glucokinase. Definition of a subtype of diabetes mellitus. The New England Journal of Medicine. March 1993, 328 (10): 697–702. PMID 8433729. doi:10.1056/NEJM199303113281005.
- ^ Okada S, O'Brien JS. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. August 1969, 165 (3894): 698–700. Bibcode:1969Sci...165..698O. PMID 5793973. doi:10.1126/science.165.3894.698.
- ^ Learning About Tay-Sachs Disease. U.S. National Human Genome Research Institute. [1 March 2015].
- ^ Erlandsen H, Stevens RC. The structural basis of phenylketonuria. Molecular Genetics and Metabolism. October 1999, 68 (2): 103–25. PMID 10527663. doi:10.1006/mgme.1999.2922.
- ^ Flatmark T, Stevens RC. Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms. Chemical Reviews. August 1999, 99 (8): 2137–2160. PMID 11849022. doi:10.1021/cr980450y.
- ^ Phenylketonuria. Genes and Disease [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). 1998–2015.
- ^ Pseudocholinesterase deficiency. U.S. National Library of Medicine. [5 September 2013].
- ^ Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clinical and Experimental Gastroenterology. 2011, 4: 55–73. PMC 3132852
 . PMID 21753892. doi:10.2147/CEG.S17634.
. PMID 21753892. doi:10.2147/CEG.S17634.
- ^ Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterology Journal. June 2013, 1 (3): 151–9. PMC 4040760
 . PMID 24917953. doi:10.1177/2050640613484463.
. PMID 24917953. doi:10.1177/2050640613484463.
- ^ Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. May 1968, 218 (5142): 652–6. Bibcode:1968Natur.218..652C. PMID 5655953. doi:10.1038/218652a0.
- ^ James WD, Elston D, Berger TG. Andrews' Diseases of the Skin: Clinical Dermatology 11th. London: Saunders/ Elsevier. 2011: 567. ISBN 978-1437703146.
- ^ Nomenclature Committee. Classification and Nomenclature of Enzymes by the Reactions they Catalyse. International Union of Biochemistry and Molecular Biology (NC-IUBMB). School of Biological and Chemical Sciences, Queen Mary, University of London.
- ^ Nomenclature Committee. EC 2.7.1.1. International Union of Biochemistry and Molecular Biology (NC-IUBMB). School of Biological and Chemical Sciences, Queen Mary, University of London.
- ^ Renugopalakrishnan V, Garduño-Juárez R, Narasimhan G, Verma CS, Wei X, Li P. Rational design of thermally stable proteins: relevance to bionanotechnology. Journal of Nanoscience and Nanotechnology. November 2005, 5 (11): 1759–1767. PMID 16433409. doi:10.1166/jnn.2005.441.
- ^ Hult K, Berglund P. Engineered enzymes for improved organic synthesis. Current Opinion in Biotechnology. August 2003, 14 (4): 395–400. PMID 12943848. doi:10.1016/S0958-1669(03)00095-8.
- ^ Jiang L, Althoff EA, Clemente FR, Doyle L, Röthlisberger D, Zanghellini A, Gallaher JL, Betker JL, Tanaka F, Barbas CF, Hilvert D, Houk KN, Stoddard BL, Baker D. De novo computational design of retro-aldol enzymes. Science. March 2008, 319 (5868): 1387–91. Bibcode:2008Sci...319.1387J. PMC 3431203
 . PMID 18323453. doi:10.1126/science.1152692.
. PMID 18323453. doi:10.1126/science.1152692.
- ^ 97.0 97.1 Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresource Technology. May 2002, 83 (1): 1–11. PMID 12058826. doi:10.1016/S0960-8524(01)00212-7.
- ^ 98.0 98.1 Kirk O, Borchert TV, Fuglsang CC. Industrial enzyme applications. Current Opinion in Biotechnology. August 2002, 13 (4): 345–351. PMID 12323357. doi:10.1016/S0958-1669(02)00328-2.
- ^ 99.0 99.1 99.2 Briggs DE. Malts and Malting 1st. London: Blackie Academic. 1998. ISBN 978-0412298004.
- ^ Dulieu C, Moll M, Boudrant J, Poncelet D. Improved performances and control of beer fermentation using encapsulated alpha-acetolactate decarboxylase and modeling. Biotechnology Progress. 2000, 16 (6): 958–65. PMID 11101321. doi:10.1021/bp000128k.
- ^ Tarté R. Ingredients in Meat Products Properties, Functionality and Applications. New York: Springer. 2008: 177. ISBN 978-0-387-71327-4.
- ^ Chymosin – GMO Database. GMO Compass. European Union. 10 July 2010 [1 March 2015].
- ^ Molimard P, Spinnler HE. Review: Compounds Involved in the Flavor of Surface Mold-Ripened Cheeses: Origins and Properties. Journal of Dairy Science. February 1996, 79 (2): 169–184. doi:10.3168/jds.S0022-0302(96)76348-8.
- ^ Guzmán-Maldonado H, Paredes-López O. Amylolytic enzymes and products derived from starch: a review. Critical Reviews in Food Science and Nutrition. September 1995, 35 (5): 373–403. PMID 8573280. doi:10.1080/10408399509527706.
- ^ 105.0 105.1 Protease – GMO Database. GMO Compass. European Union. 10 July 2010 [28 February 2015].
- ^ Alkorta I, Garbisu C, Llama MJ, Serra JL. Industrial applications of pectic enzymes: a review. Process Biochemistry. January 1998, 33 (1): 21–28. doi:10.1016/S0032-9592(97)00046-0.
- ^ Bajpai P. Application of enzymes in the pulp and paper industry. Biotechnology Progress. March 1999, 15 (2): 147–157. PMID 10194388. doi:10.1021/bp990013k.
- ^ Begley CG, Paragina S, Sporn A. An analysis of contact lens enzyme cleaners. Journal of the American Optometric Association. March 1990, 61 (3): 190–4. PMID 2186082.
- ^ Farris PL. Economic Growth and Organization of the U.S. Starch Industry. BeMiller JN, Whistler RL (编). Starch Chemistry and Technology 3rd. London: Academic. 2009. ISBN 9780080926551.
拓展閱讀
|
總論
詞源與歷史
|
'酶結構及作用機理
動力學及抑制
|
| |||||||||||||||||||||||||||
| ||||||||||||

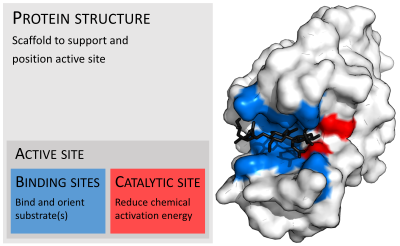

![{\displaystyle {\begin{matrix}{}\\{\ce {{CO2}+H2O->[{\ce {Carbonic\ anhydrase}}]H2CO3}}\\{}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d17f8a66c1a14f8d4718f1efb0cb0fa604bcfb15)
![{\displaystyle {\begin{matrix}{}\\{\ce {H2CO3->[{\ce {Carbonic\ anhydrase}}]{CO2}+H2O}}\\{}\end{matrix}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3bbc8ea9147d44fd500c9dcb477229fd2828109b)