三氟过氧乙酸:修订间差异
| 第71行: | 第71行: | ||
[[File:BV regiochemical and stereochemical effects.png|frameless|center|500px]] |
[[File:BV regiochemical and stereochemical effects.png|frameless|center|500px]] |
||
===杂原子的氧化=== |
|||
三氟过氧乙酸可以氧化含有低[[氧化态]][[杂原子]]的[[官能团]]。<ref name = eEROS2012 /><ref name="FinOChem"/>常见的情况包括碘的氧化(例如前面提到用碘苯来合成高价碘化合物)、氮、硫和硒。 |
|||
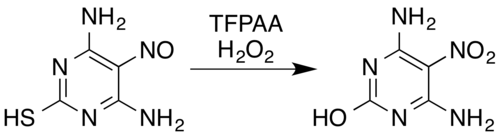
三氟过氧乙酸能把[[肟]]<ref name = eEROS2012 />和芳香[[伯胺]]<ref name = Emmons2 />氧化成[[硝基化合物]]<ref name = FinOChem />(在有[[吸电子基]]的情况下也会被氧化。举个例子,五氟苯胺会被氧化成五氟硝基苯<ref>{{cite journal|title = Aromatic polyfluoro-compounds. Part VII. The reaction of pentafluoronitrobenzene with ammonia|first1 = G. M.|last1 = Brooke|first2 = J.|last2 = Burdon|first3 = J. C.|last3 = Tatlow|journal = [[J. Chem. Soc.]]|year = 1961|pages = 802–807|doi = 10.1039/JR9610000802}}</ref>)、把[[亚硝胺]]氧化成[[硝胺]]<ref name = FinOChem /><ref name = Emmons1 />以及把[[亚硝基]]化合物氧化成硝基化合物或硝胺。<ref name = eEROS2012 />举个例子,过氧化氢和三氟过氧乙酸的混合物会把含有亚硝基的4,6-二氨基-5-亚硝基[[嘧啶]]-2-硫醇氧化成它的硝基类似物,并把[[巯基]]替换成羟基:<ref name = eEROS2012 /><ref>{{cite journal|title = A New Synthesis of 5-Nitropyrimidines|first1 = Edward C.|last1 = Taylor|first2 = Alexander|last2 = McKillop|journal = [[J. Org. Chem.]]|year = 1965|volume = 30|issue = 9|pages = 3153–3155|doi = 10.1021/jo01020a067}}</ref> |
|||
[[File:Trifluoroperacetic acid oxidation of a nitrosopyrimidinethiol.png|frameless|center|500px]] |
|||
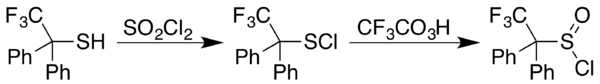
三氟过氧乙酸也能氧化[[氧族元素]]化合物。视反应条件,三氟过氧乙酸氧化硫醇(R–S–R)的产物会是[[亚砜]](R–S(O)–R)和/或[[砜]](R–S(O)<sub>2</sub>–R)。<ref name = eEROS2012 />类似的硒化合物[[硒醚]](R–Se–R)的氧化只会产生硒代砜(R–Se(O)<sub>2</sub>–R)而不产生可分离的硒代亚砜(R–Se(O)–R)。<ref name = Selenoether>{{cite book|pages = [https://archive.org/details/comprehensiveorg0000unse/page/277 277–296]|chapter = Alkyl Chalcogenides: Selenium- and Tellurium-based Functional Groups|chapter-url = https://books.google.com/books?id=BPcxrmIgLKMC&pg=PA287|title = Synthesis: Carbon with One Heteroatom Attached by a Single Bond|editor-first = Steven V.|editor-last = Ley|publisher = [[Elsevier]]|year = 1995|series = Comprehensive Organic Functional Group Transformations|isbn = 9780080423234|last1 = Kataoka|first1 = T.|last2 = Yoshimatsu|first2 = M.|url = https://archive.org/details/comprehensiveorg0000unse/page/277}}</ref>当R是[[芳基]]时,此反应特别有效。<ref>{{cite book|pages = [https://archive.org/details/comprehensiveorg0000unse/page/705 705–736]|chapter = Vinyl and Aryl Chalcogenides: Sulfur-, Selenium- and Tellurium-based Functional Groups|chapter-url = https://books.google.com/books?id=BPcxrmIgLKMC&pg=PA732|title = Synthesis: Carbon with One Heteroatom Attached by a Single Bond|editor-first = Steven V.|editor-last = Ley|publisher = [[Elsevier]]|year = 1995|series = Comprehensive Organic Functional Group Transformations|isbn = 9780080423234|last = Taylor|first = P. C.|url = https://archive.org/details/comprehensiveorg0000unse/page/705}}</ref>合成[[亚磺酰氯]](RS(O)Cl)的方法是先将对应的硫醇和[[磺酰氯]]({{chem|SO|2|Cl|2}})反应,产生{{le|硫基氯|sulfenyl chloride}}(RSCl)。之后用三氟过氧乙酸氧化,得到亚磺酰氯,例如下图2,2,2-三氟-1,1-二苯基[[乙硫醇]]的反应:<ref>{{cite book|pages = [https://archive.org/details/comprehensiveorg0000unse/page/113 113–276]|chapter = Alkyl Chalcogenides: Sulfur-based Functional Groups|chapter-url = https://books.google.com/books?id=BPcxrmIgLKMC&pg=PA173|title = Synthesis: Carbon with One Heteroatom Attached by a Single Bond|editor-first = Steven V.|editor-last = Ley|publisher = [[Elsevier]]|year = 1995|series = Comprehensive Organic Functional Group Transformations|isbn = 9780080423234|last1 = Page|first1 = P. C. B.|last2 = Wilkes|first2 = R. D.|last3 = Reynolds|first3 = D.|url = https://archive.org/details/comprehensiveorg0000unse/page/113}}</ref> |
|||
[[File:Conversion of F3CCPh2SH to F3CCPh2SCl and on to F3CCPh2S(O)Cl.png|frameless|600px|center]] |
|||
三氟过氧乙酸对[[噻吩]]的氧化有两种竞争途径,分别为''S''-氧化和环氧化。<ref name = ThiopheneOxidation />{{#tag:ref|这种竞争具有生化意义。例如,已知[[环利尿剂]]{{le|替宁酸|tienilic acid}}是[[细胞色素P450]]的自杀型底物,并且该过程涉及噻吩的氧化。尽管有大量的研究,但是这个氧化途径仍不清楚。<ref>{{cite journal|title = Thiophene ''S''-Oxides as new Reactive Metabolites: Formation by Cytochrome P-450 Dependent Oxidation and Reaction with Nucleophiles|first1 = Daniel|last1 = Mansuy|first2 = Philippe|last2 = Valadon|first3 = Irene|last3 = Erdelmeier|first4 = Pilar|last4 = López Garcia|first5 = Claudine|last5 = Amar|first6 = Jean-Pierre|last6 = Girault|first7 = Patrick M.|last7 = Dansette|journal = [[J. Am. Chem. Soc.]]|year = 1991|volume = 113|issue = 20|pages = 7825–7826|doi = 10.1021/ja00020a089}}</ref><ref>{{cite book|pages = 177–260|chapter = Inhibition of Cytochrome P450 Enzymes|first1 = Maria A.|last1 = Correia|first2 = Paul F.|last2 = Hollenberg|chapter-url = https://books.google.com/books?id=abZnBwAAQBAJ&pg=PA203|title = Cytochrome P450: Structure, Mechanism, and Biochemistry|editor-first = Paul R.|editor-last = Ortiz de Montellano|publisher = Springer|year = 2015|isbn = 9783319121086|edition = 4th}}</ref><ref>{{cite book|pages = 585–614|chapter = Biotransformations Leading to Toxic Metabolites:Chemical Aspects|first1 = Anne-Christine|last1 = Macherey|first2 = Patrick M.|last2 = Dansette|chapter-url = https://books.google.com/books?id=dtScBAAAQBAJ&pg=PA603|title = The Practice of Medicinal Chemistry|editor1-first = Camille Georges|editor1-last = Wermuth|editor2-first = David|editor2-last = Aldous|editor3-first = Pierre|editor3-last = Raboisson|editor4-first = Didier|editor4-last = Rognan|publisher = [[Elsevier]]|year = 2015|edition = 4th|isbn = 9780124172135}}</ref>|name=tienilic|group=Note}}氧化的主要反应是产生亚砜,但这种亚砜会在进一步氧化之前迅速发生类似[[狄尔斯–阿尔德反应]]的[[二聚]]。反应产物中没有找到任何''S''-氧化噻吩或''S'',''S''-二氧化噻吩。<ref name = eEROS2012 /><ref name = ThiopheneOxidation>{{cite journal|title = Mechanism of the Aromatic Hydroxylation of Thiophene by Acid-Catalyzed Peracid Oxidation|first = Alexander|last = Treiber|journal = [[J. Org. Chem.]]|year = 2002|volume = 67|issue = 21|pages = 7261–7266|doi = 10.1021/jo0202177|pmid = 12375952}}</ref>这个二聚体可以继续氧化,使其中一个''S''-氧化物部分转化成''S'',''S''-二氧化物。三氟过氧乙酸对噻吩的氧化还可能发生Prilezhaev环氧化反应,<ref name = PrilezhaevRxn />产物是迅速重排成[[异构体]]噻吩-2-酮的噻吩-2,3-环氧化物。<ref name = ThiopheneOxidation />[[化学捕捉]]实验<ref>{{cite book|pages = 471–482|chapter = 8.8 Miscellaneous Experiments for Studying Mechanism|chapter-url = https://books.google.com/books?id=gY-Sxijk_tMC&pg=PA474|title = Modern Physical Organic Chemistry|first1 = Eric V.|last1 = Anslyn|first2 = Dennis A.|last2 = Dougherty|authorlink2 = Dennis A. Dougherty|publisher = University Science Books|year = 2006|isbn = 9781891389313}}</ref>证明环氧化途径不是''S''-氧化物中间体的{{le|副反应|Side reaction}},而[[同位素标记]]实验证明该环氧化物发生了[[σ迁移反应|1,2-负氢迁移]]([[NIH重排反应]]),涉及阳离子中间体。<ref name = ThiopheneOxidation />这个反应中三氟过氧乙酸的制备方法很重要,因为制备过程产生的水会抑制环氧化反应,可能是因为它充当了竞争碱。<ref name = ThiopheneOxidation /> |
|||
[[File:Oxidation of thiophene with trifluoroperacetic acid.png|frameless|center|500px]] |
|||
==注释== |
|||
{{Reflist|group=Note}} |
|||
==参考资料== |
==参考资料== |
||
2021年12月30日 (四) 13:53的版本
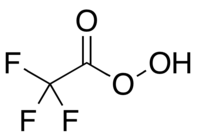
| 三氟过氧乙酸 | |
|---|---|
| |
| IUPAC名 trifluoroethaneperoxoic acid | |
| 别名 | 三氟过乙酸 TFPAA |
| 识别 | |
| CAS号 | 359-48-8 |
| PubChem | 10290812 |
| ChemSpider | 8466281 |
| SMILES |
|
| InChI |
|
| InChIKey | XYPISWUKQGWYGX-UHFFFAOYAW |
| 性质 | |
| 化学式 | CF3CO3H |
| 摩尔质量 | 130.023 g·mol⁻¹ |
| 外观 | 无色液体 |
| 沸点 | 162 °C(435 K) |
| 溶解性(水) | 反应 |
| 溶解性 | 可溶于乙腈、二氯甲烷、乙醚和环丁砜 |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
三氟过氧乙酸,简称三氟过乙酸或TFPAA,是三氟乙酸对应的过酸,化学式 CF3COOOH。它有强氧化性,可用于酮的拜耳-维立格氧化反应。[1]它可将氨基氧化为硝基,产率较高。[2]它是有机过氧酸中反应性最高的,能够将相对不活泼的烯烃氧化成环氧化物,而其他过氧酸则不能。[3]三氟过氧乙酸能氧化某些官能团中的氧族元素,例如把硒醚氧化成硒酮。[4]三氟过氧乙酸具有潜在爆炸性,[5]所以不能商购,但可以快速制备。[6]它作为实验室试剂的用途是由威廉·埃蒙斯开创和开发的。[7][8]
性质
在标准情况下,三氟过氧乙酸是沸点 162 °C的无色液体。[9]它可溶于乙腈、二氯甲烷、乙醚和环丁砜,会和水反应。[6]和其它过氧酸一样,三氟过氧乙酸有潜在的爆炸性,需要小心处理。[5]三氟过氧乙酸不可商购,但可以在实验室中合成,在-20 ℃下可以储放几个星期。[6]一些制备方法会产生有残留过氧化氢和三氟乙酸的三氟过氧乙酸,加热这种混合物极其危险,所以在加热前会使用二氧化锰来使过氧化氢发生分解反应以确保安全。[6][9]
制备
三氟过氧乙酸可以由三氟乙酸酐和90%浓过氧化氢[3]水溶液反应而成:
- CF
3COOCOCF
3 + H
2O
2 → CF
3COOOH + CF
3COOH
由于三氟乙酸酐和水反应会产生三氟乙酸,加入过量的三氟乙酸酐可以去除过氧化氢的水溶剂:[10]
- CF
3COOCOCF
3 + H
2O → 2 CF
3COOH
较稀的30%过氧化氢溶液和三氟乙酸反应,也可以合成三氟过氧乙酸。[3]
- CF
3COOH + H
2O
2 → CF
3COOOH + H
2O
尿素-过氧化氢和三氟乙酸酐的反应也可以得到三氟过氧乙酸,可以避免处理纯过氧化氢或高浓度过氧化氢溶液的危害。[6]这个制备方法不涉及水,所以产生的三氟过氧乙酸完全无水。[11]这是该反应的优势,因为水在某些氧化反应中会导致副反应。[12]
- CF
3COOCOCF
3 + H
2O
2·CO(NH
2)
2 → CF
3COOOH + CF
3COOH + CO(NH
2)
2
三氟乙酸酐和过碳酸钠 2Na
2CO
3·3H
2O
2反应,也可以产生三氟过氧乙酸。[6][13]如果合成需要缓冲溶液并且可以容忍水的存在,就可以使用这种方法。
- 3 CF
3COOCOCF
3 + 4 Na
2CO
3·1 1⁄2H
2O
2 → 6 CF
3COOOH + 4 Na
2CO
3 + 3 H
2O
三氟过氧乙酸也可以in situ合成,[14]使它能与目标底物迅速反应,而不是预先合成试剂供以后使用。
用处

6H
5I(OOCCF
3)
2
三氟过氧乙酸主要用作氧化剂。[6][8]1953年9月,《美国化学会志》发表了威廉·埃蒙斯和阿瑟·费里斯的报告,称这种in situ生成的试剂能够将苯胺氧化成硝基苯。[14]在接下来的两年中,埃蒙斯报告了该试剂的制备方法,并在该杂志上发表了六篇关于其应用的手稿。[15][16][17]埃蒙斯被人们铭记为三氟过氧乙酸作为实验室试剂的先驱[7]和开发者[8],此后三氟过氧乙酸已成为许多不同类型合成反应的有用试剂。
三氟过氧乙酸可以合成超价碘化合物二(三氟乙酸)碘苯 (CF
3COO)
2IC
6H
5,它可在酸性条件下引起霍夫曼降解反应。[18]这种超价分子可以通过两种方法制备,一种方法是从二(乙酸)碘苯制备,[19]另一种方法则是碘苯和三氟过氧乙酸和三氟乙酸的混合物反应:[18]
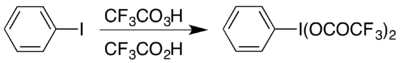
拜耳-维立格氧化反应

由于其相对于类似的过氧酸和过氧化物的高酸性,三氟过氧乙酸是拜耳-维立格氧化反应中最强的试剂之一。[20]:17这个反应以于1899年报告此反应的阿道夫·冯·拜尔和维克多·维立格命名,可以把酮氧化成酯或内酯。[1]该反应被认为是通过克里格中间体进行的,[6]并且显示出对氧原子插入位置的良好区域选择性和化学选择性,以及在相邻位置保留立体化学结构。为了防止强酸性的三氟乙酸副产物造成酯产物的水解[21]和酯交换反应[22],会加入缓冲物质磷酸一氢钠 Na
2HPO
4。[3]

杂原子的氧化
三氟过氧乙酸可以氧化含有低氧化态杂原子的官能团。[6][8]常见的情况包括碘的氧化(例如前面提到用碘苯来合成高价碘化合物)、氮、硫和硒。
三氟过氧乙酸能把肟[6]和芳香伯胺[16]氧化成硝基化合物[8](在有吸电子基的情况下也会被氧化。举个例子,五氟苯胺会被氧化成五氟硝基苯[23])、把亚硝胺氧化成硝胺[8][15]以及把亚硝基化合物氧化成硝基化合物或硝胺。[6]举个例子,过氧化氢和三氟过氧乙酸的混合物会把含有亚硝基的4,6-二氨基-5-亚硝基嘧啶-2-硫醇氧化成它的硝基类似物,并把巯基替换成羟基:[6][24]
三氟过氧乙酸也能氧化氧族元素化合物。视反应条件,三氟过氧乙酸氧化硫醇(R–S–R)的产物会是亚砜(R–S(O)–R)和/或砜(R–S(O)2–R)。[6]类似的硒化合物硒醚(R–Se–R)的氧化只会产生硒代砜(R–Se(O)2–R)而不产生可分离的硒代亚砜(R–Se(O)–R)。[4]当R是芳基时,此反应特别有效。[25]合成亚磺酰氯(RS(O)Cl)的方法是先将对应的硫醇和磺酰氯(SO
2Cl
2)反应,产生硫基氯(RSCl)。之后用三氟过氧乙酸氧化,得到亚磺酰氯,例如下图2,2,2-三氟-1,1-二苯基乙硫醇的反应:[26]
三氟过氧乙酸对噻吩的氧化有两种竞争途径,分别为S-氧化和环氧化。[27][Note 1]氧化的主要反应是产生亚砜,但这种亚砜会在进一步氧化之前迅速发生类似狄尔斯–阿尔德反应的二聚。反应产物中没有找到任何S-氧化噻吩或S,S-二氧化噻吩。[6][27]这个二聚体可以继续氧化,使其中一个S-氧化物部分转化成S,S-二氧化物。三氟过氧乙酸对噻吩的氧化还可能发生Prilezhaev环氧化反应,[31]产物是迅速重排成异构体噻吩-2-酮的噻吩-2,3-环氧化物。[27]化学捕捉实验[32]证明环氧化途径不是S-氧化物中间体的副反应,而同位素标记实验证明该环氧化物发生了1,2-负氢迁移(NIH重排反应),涉及阳离子中间体。[27]这个反应中三氟过氧乙酸的制备方法很重要,因为制备过程产生的水会抑制环氧化反应,可能是因为它充当了竞争碱。[27]

注释
参考资料
- ^ 1.0 1.1 Kürti, László; Czakó, Barbara. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier Academic Press. 2005: 28. ISBN 9780124297852.
- ^ 化工引擎. [2011-09-17]. (原始内容存档于2013-04-25).
- ^ 3.0 3.1 3.2 3.3 Hiyama, Tamejiro. 8.2 Trifluoroacetic acid and Trifluoroperacetic acid. Organofluorine Compounds: Chemistry and Applications. Springer Science & Business Media. 2000: 255–257. ISBN 9783662041642.
- ^ 4.0 4.1 Kataoka, T.; Yoshimatsu, M. Alkyl Chalcogenides: Selenium- and Tellurium-based Functional Groups. Ley, Steven V. (编). Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. 1995: 277–296. ISBN 9780080423234.
- ^ 5.0 5.1 Carey, Francis A.; Sundberg, Richard J. 5.5 Addition Reactions Involving Epoxides. Advanced Organic Chemistry: Part A: Structure and Mechanisms 5th. Springer Science & Business Media. 2007: 503–514. ISBN 9780387448978.
- ^ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 Caster, Kenneth C.; Rao, A. Somasekar; Mohan, H. Rama; McGrath, Nicholas A.; Brichacek, Matthew. Encyclopedia of Reagents for Organic Synthesis. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2012. ISBN 978-0471936237. doi:10.1002/047084289X.rt254.pub2.
|chapter=被忽略 (帮助) - ^ 7.0 7.1 Freeman, Jeremiah P. William D. Emmons: November 18, 1924 – December 8, 2001 (PDF). Org. Synth. November 14, 2002, 80: xxvii–xxix [January 21, 2017]. (原始内容 (PDF)存档于March 16, 2015).
- ^ 8.0 8.1 8.2 8.3 8.4 8.5 Chambers, Richard D. Functional Compounds Containing Oxygen, Sulphur or Nitrogen and their Derivatives. Fluorine in Organic Chemistry. CRC Press. 2004: 242–243. ISBN 9780849317903.
- ^ 9.0 9.1 Luxon, S. G. Hazards in the Chemical Laboratory 5th. Royal Society of Chemistry. 1992: 627. ISBN 9780851862293.
- ^ (1968) "2,3,4,5,6,6-Hexamethyl-2,4-cyclohexadien-1-one". Org. Synth. 48; Coll. Vol. 5: 598.
- ^ Cooper, Mark S.; Heaney, Harry; Newbold, Amanda J.; Sanderson, William R. Oxidation Reactions Using Urea–Hydrogen Peroxide; A Safe Alternative to Anhydrous Hydrogen Peroxide. Synlett. 1990, 1990 (9): 533–535. doi:10.1055/s-1990-21156.
- ^ Ziegler, Fredrick E.; Metcalf, Chester A.; Nangia, Ashwini; Schulte, Gayle. Structure and total synthesis of sporol and neosporol. J. Am. Chem. Soc. 1993, 115 (7): 2581–2589. doi:10.1021/ja00060a006.
- ^ Kang, Ho-Jung; Jeong, Hee-Sun. New Method of Generating Trifluoroperoxyacetic acid for the Baeyer-Villiger Reaction. Bull. Korean Chem. Soc. 1996, 17 (1): 5–6.
- ^ 14.0 14.1 Emmons, William D.; FerrisEmmons, Arthur F. Oxidation Reactions with Pertrifluoroacetic Acid. J. Am. Chem. Soc. 1953, 75 (18): 4623–4624. doi:10.1021/ja01114a539.
- ^ 15.0 15.1 Emmons, William D. Peroxytrifluoroacetic Acid. I. The Oxidation of Nitrosamines to Nitramines. J. Am. Chem. Soc. 1954, 76 (13): 3468–3470. doi:10.1021/ja01642a029.
- ^ 16.0 16.1 Emmons, William D. Peroxytrifluoroacetic Acid. II. The Oxidation of Anilines to Nitrobenzenes. J. Am. Chem. Soc. 1954, 76 (13): 3470–3472. doi:10.1021/ja01642a030.
- ^ Emmons, William D.; Pagano, Angelo S.; Freeman, Jeremiah P. Peroxytrifluoroacetic Acid. III. The Hydroxylation of Olefins. J. Am. Chem. Soc. 1954, 76 (13): 3472–3474. doi:10.1021/ja01642a031.
Emmons, William D.; Pagano, Angelo S. Peroxytrifluoroacetic Acid. IV. The Epoxidation of Olefins. J. Am. Chem. Soc. 1955, 77 (1): 89–92. doi:10.1021/ja01606a029.
Emmons, William D.; Lucas, George B. Peroxytrifluoroacetic Acid. V. The Oxidation of Ketones to Esters. J. Am. Chem. Soc. 1955, 77 (8): 2287–2288. doi:10.1021/ja01613a077.
Emmons, William D.; Pagano, Angelo S. Peroxytrifluoroacetic Acid. VI. The Oxidation of Oximes to Nitroparaffins. J. Am. Chem. Soc. 1955, 77 (17): 4557–4559. doi:10.1021/ja01622a036. - ^ 18.0 18.1 Aubé, Jeffrey; Fehl, Charlie; Liu, Ruzhang; McLeod, Michael C.; Motiwala, Hashim F. 6.15 Hofmann, Curtius, Schmidt, Lossen, and Related Reactions. Heteroatom Manipulations. Comprehensive Organic Synthesis II 6. 1993: 598–635. ISBN 9780080977430. doi:10.1016/B978-0-08-097742-3.00623-6.
- ^ (1988) "Hofmann Rearrangement Under Mildly Acidic Conditions Using [I,I-Bis(Trifluoroacetoxy)Iodobenzene: Cyclobutylamine Hydrochloride from Cyclobutanecarboxamide]". Org. Synth. 66; Coll. Vol. 8: 132.
- ^ Myers, Andrew G. Chemistry 115 Handouts: Oxidation (PDF). Harvard University. [10 January 2017].
- ^ Carruthers, William. 6.3 Oxidation of Olefins. Some Modern Methods of Organic Synthesis. Cambridge University Press. 1971: 259–280. ISBN 9780521096430.
- ^ Carruthers, William. 6.5 Baeyer–Villiger oxidation of ketones. Some Modern Methods of Organic Synthesis. Cambridge University Press. 1971: 287–290. ISBN 9780521096430.
- ^ Brooke, G. M.; Burdon, J.; Tatlow, J. C. Aromatic polyfluoro-compounds. Part VII. The reaction of pentafluoronitrobenzene with ammonia. J. Chem. Soc. 1961: 802–807. doi:10.1039/JR9610000802.
- ^ Taylor, Edward C.; McKillop, Alexander. A New Synthesis of 5-Nitropyrimidines. J. Org. Chem. 1965, 30 (9): 3153–3155. doi:10.1021/jo01020a067.
- ^ Taylor, P. C. Vinyl and Aryl Chalcogenides: Sulfur-, Selenium- and Tellurium-based Functional Groups. Ley, Steven V. (编). Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. 1995: 705–736. ISBN 9780080423234.
- ^ Page, P. C. B.; Wilkes, R. D.; Reynolds, D. Alkyl Chalcogenides: Sulfur-based Functional Groups. Ley, Steven V. (编). Synthesis: Carbon with One Heteroatom Attached by a Single Bond. Comprehensive Organic Functional Group Transformations. Elsevier. 1995: 113–276. ISBN 9780080423234.
- ^ 27.0 27.1 27.2 27.3 27.4 Treiber, Alexander. Mechanism of the Aromatic Hydroxylation of Thiophene by Acid-Catalyzed Peracid Oxidation. J. Org. Chem. 2002, 67 (21): 7261–7266. PMID 12375952. doi:10.1021/jo0202177.
- ^ Mansuy, Daniel; Valadon, Philippe; Erdelmeier, Irene; López Garcia, Pilar; Amar, Claudine; Girault, Jean-Pierre; Dansette, Patrick M. Thiophene S-Oxides as new Reactive Metabolites: Formation by Cytochrome P-450 Dependent Oxidation and Reaction with Nucleophiles. J. Am. Chem. Soc. 1991, 113 (20): 7825–7826. doi:10.1021/ja00020a089.
- ^ Correia, Maria A.; Hollenberg, Paul F. Inhibition of Cytochrome P450 Enzymes. Ortiz de Montellano, Paul R. (编). Cytochrome P450: Structure, Mechanism, and Biochemistry 4th. Springer. 2015: 177–260. ISBN 9783319121086.
- ^ Macherey, Anne-Christine; Dansette, Patrick M. Biotransformations Leading to Toxic Metabolites:Chemical Aspects. Wermuth, Camille Georges; Aldous, David; Raboisson, Pierre; Rognan, Didier (编). The Practice of Medicinal Chemistry 4th. Elsevier. 2015: 585–614. ISBN 9780124172135.
- ^ 引用错误:没有为名为
PrilezhaevRxn的参考文献提供内容 - ^ Anslyn, Eric V.; Dougherty, Dennis A. 8.8 Miscellaneous Experiments for Studying Mechanism. Modern Physical Organic Chemistry. University Science Books. 2006: 471–482. ISBN 9781891389313.
