苏沃雷生:修订间差异
补救23个来源,并将0个来源标记为失效。) #IABot (v2.0.9.5 |
Alexius zhou(留言 | 贡献) 内容扩充 |
||
| 第48行: | 第48行: | ||
| legal_US=Schedule IV}} |
| legal_US=Schedule IV}} |
||
'''苏沃雷生'''(Suvorexant),品牌名'''Belsomra''',是一种[[食欲素受体拮抗剂]],被用于治疗失眠症,主要用于入睡或睡眠维持困难的成年患者的治疗。<ref name=":0">{{Cite web|title=DailyMed - BELSOMRA- suvorexant tablet, film coated|url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951|access-date=2023-07-23|website=dailymed.nlm.nih.gov|archive-date=2019-07-02|archive-url=https://web.archive.org/web/20190702172706/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951|dead-url=no}}</ref><ref name=":1">{{Cite journal |last=Jacobson |first=Laura H. |last2=Callander |first2=Gabrielle E. |last3=Hoyer |first3=Daniel |date=2014-11 |title=Suvorexant for the treatment of insomnia |url=https://pubmed.ncbi.nlm.nih.gov/25318834/ |journal=Expert Review of Clinical Pharmacology |volume=7 |issue=6 |doi=10.1586/17512433.2014.966813 |issn=1751-2441 |pmid=25318834 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121525/https://pubmed.ncbi.nlm.nih.gov/25318834/ |dead-url=no }}</ref><ref name=":2">{{Cite web|title=苏沃雷生[精二]_其他镇静催眠药_镇静药与催眠药_作用于神经系统的药物_湖南药事服务网|url=https://www.hnysfww.com/mobile/goods.php?id=733|access-date=2023-07-23|website=www.hnysfww.com|archive-date=2023-07-23|archive-url=https://web.archive.org/web/20230723121526/https://www.hnysfww.com/mobile/goods.php?id=733|dead-url=no}}</ref>苏沃雷生具有加速入睡、延长睡眠时间、减少半夜醒来次数以及提高睡眠质量的作用。<ref name=":0" /><ref name=":1" /><ref name=":3">{{Cite journal |last=Kuriyama |first=Akira |last2=Tabata |first2=Hiromitsu |date=2017-10 |title=Suvorexant for the treatment of primary insomnia: A systematic review and meta-analysis |url=https://pubmed.ncbi.nlm.nih.gov/28365447/ |journal=Sleep Medicine Reviews |volume=35 |doi=10.1016/j.smrv.2016.09.004 |issn=1532-2955 |pmid=28365447 |access-date=2023-07-23 |archive-date=2023-03-13 |archive-url=https://web.archive.org/web/20230313034319/https://pubmed.ncbi.nlm.nih.gov/28365447/ |dead-url=no }}</ref>其药效中等,<ref>{{Cite web|title=Table 1: The Single Nucleotide Polymorphisms in cathepsin B protein mined from literature (PMID: 16492714).|url=http://dx.doi.org/10.7717/peerj.7425/table-1|access-date=2023-07-23|website=dx.doi.org|archive-date=2019-10-12|archive-url=https://web.archive.org/web/20191012165838/http://dx.doi.org/10.7717/peerj.7425/table-1|dead-url=no}}</ref>与其他[[食欲素受体拮抗剂]]类似,但低于[[苯二氮䓬类|苯二氮卓类药物]]与[[Z-drugs|非苯二氮卓类药物]]。<ref>{{Cite journal |last=De Crescenzo |first=Franco |last2=D'Alò |first2=Gian Loreto |last3=Ostinelli |first3=Edoardo G |last4=Ciabattini |first4=Marco |last5=Di Franco |first5=Valeria |last6=Watanabe |first6=Norio |last7=Kurtulmus |first7=Ayse |last8=Tomlinson |first8=Anneka |last9=Mitrova |first9=Zuzana |last10=Foti |first10=Francesca |last11=Del Giovane |first11=Cinzia |date=2022-07 |title=Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis |url=https://linkinghub.elsevier.com/retrieve/pii/S0140673622008789 |journal=The Lancet |language=en |volume=400 |issue=10347 |doi=10.1016/S0140-6736(22)00878-9 |access-date=2023-07-23 |archive-date=2023-06-30 |archive-url=https://web.archive.org/web/20230630023740/https://linkinghub.elsevier.com/retrieve/pii/S0140673622008789 |dead-url=no }}</ref>苏沃雷生的给药方式为口服给药。<ref name=":0" /><ref name=":1" /><ref name=":2" /><ref>{{Cite web|last=https://www.facebook.com/Drugscom|title=Generic Belsomra Availability|url=https://www.drugs.com/availability/generic-belsomra.html|access-date=2023-07-23|website=Drugs.com|language=en|archive-date=2023-04-11|archive-url=https://web.archive.org/web/20230411064618/https://www.drugs.com/availability/generic-belsomra.html|dead-url=no}}</ref> |
'''苏沃雷生'''(Suvorexant),品牌名'''Belsomra''',是一种[[食欲素受体拮抗剂]],被用于治疗失眠症,主要用于入睡或睡眠维持困难的成年患者的治疗。<ref name=":0">{{Cite web|title=DailyMed - BELSOMRA- suvorexant tablet, film coated|url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951|access-date=2023-07-23|website=dailymed.nlm.nih.gov|archive-date=2019-07-02|archive-url=https://web.archive.org/web/20190702172706/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e5b72731-1acb-45b7-9c13-290ad12d3951|dead-url=no}}</ref><ref name=":1">{{Cite journal |last=Jacobson |first=Laura H. |last2=Callander |first2=Gabrielle E. |last3=Hoyer |first3=Daniel |date=2014-11 |title=Suvorexant for the treatment of insomnia |url=https://pubmed.ncbi.nlm.nih.gov/25318834/ |journal=Expert Review of Clinical Pharmacology |volume=7 |issue=6 |doi=10.1586/17512433.2014.966813 |issn=1751-2441 |pmid=25318834 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121525/https://pubmed.ncbi.nlm.nih.gov/25318834/ |dead-url=no }}</ref><ref name=":2">{{Cite web|title=苏沃雷生[精二]_其他镇静催眠药_镇静药与催眠药_作用于神经系统的药物_湖南药事服务网|url=https://www.hnysfww.com/mobile/goods.php?id=733|access-date=2023-07-23|website=www.hnysfww.com|archive-date=2023-07-23|archive-url=https://web.archive.org/web/20230723121526/https://www.hnysfww.com/mobile/goods.php?id=733|dead-url=no}}</ref>苏沃雷生具有加速入睡、延长睡眠时间、减少半夜醒来次数以及提高睡眠质量的作用。<ref name=":0" /><ref name=":1" /><ref name=":3">{{Cite journal |last=Kuriyama |first=Akira |last2=Tabata |first2=Hiromitsu |date=2017-10 |title=Suvorexant for the treatment of primary insomnia: A systematic review and meta-analysis |url=https://pubmed.ncbi.nlm.nih.gov/28365447/ |journal=Sleep Medicine Reviews |volume=35 |doi=10.1016/j.smrv.2016.09.004 |issn=1532-2955 |pmid=28365447 |access-date=2023-07-23 |archive-date=2023-03-13 |archive-url=https://web.archive.org/web/20230313034319/https://pubmed.ncbi.nlm.nih.gov/28365447/ |dead-url=no }}</ref>其药效中等,<ref>{{Cite web|title=Table 1: The Single Nucleotide Polymorphisms in cathepsin B protein mined from literature (PMID: 16492714).|url=http://dx.doi.org/10.7717/peerj.7425/table-1|access-date=2023-07-23|website=dx.doi.org|archive-date=2019-10-12|archive-url=https://web.archive.org/web/20191012165838/http://dx.doi.org/10.7717/peerj.7425/table-1|dead-url=no}}</ref>与其他[[食欲素受体拮抗剂]]类似,但低于[[苯二氮䓬类|苯二氮卓类药物]]与[[Z-drugs|非苯二氮卓类药物]]。<ref name=":6">{{Cite journal |last=De Crescenzo |first=Franco |last2=D'Alò |first2=Gian Loreto |last3=Ostinelli |first3=Edoardo G |last4=Ciabattini |first4=Marco |last5=Di Franco |first5=Valeria |last6=Watanabe |first6=Norio |last7=Kurtulmus |first7=Ayse |last8=Tomlinson |first8=Anneka |last9=Mitrova |first9=Zuzana |last10=Foti |first10=Francesca |last11=Del Giovane |first11=Cinzia |date=2022-07 |title=Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis |url=https://linkinghub.elsevier.com/retrieve/pii/S0140673622008789 |journal=The Lancet |language=en |volume=400 |issue=10347 |doi=10.1016/S0140-6736(22)00878-9 |access-date=2023-07-23 |archive-date=2023-06-30 |archive-url=https://web.archive.org/web/20230630023740/https://linkinghub.elsevier.com/retrieve/pii/S0140673622008789 |dead-url=no }}</ref>苏沃雷生的给药方式为口服给药。<ref name=":0" /><ref name=":1" /><ref name=":2" /><ref>{{Cite web|last=https://www.facebook.com/Drugscom|title=Generic Belsomra Availability|url=https://www.drugs.com/availability/generic-belsomra.html|access-date=2023-07-23|website=Drugs.com|language=en|archive-date=2023-04-11|archive-url=https://web.archive.org/web/20230411064618/https://www.drugs.com/availability/generic-belsomra.html|dead-url=no}}</ref> |
||
苏沃雷生的副作用包括[[嗜睡症|嗜睡]]、[[頭暈|头晕]]、[[白天嗜睡]]和[[镇静]]、[[頭痛|头痛]]、[[頭暈|头晕]]、[[梦境异常]]、[[口乾|口干]]和次日驾驶能力受损。<ref name=":0" /><ref name=":3" /><ref name=":4">{{Cite journal |last=Sutton |first=Eliza |date=2015-11 |title=Profile of suvorexant in the management of insomnia |url=https://www.dovepress.com/profile-of-suvorexant-in-the-management-of-insomnia-peer-reviewed-article-DDDT |journal=Drug Design, Development and Therapy |language=en |doi=10.2147/DDDT.S73224 |issn=1177-8881 |pmc=PMC4651361 |pmid=26648692 |access-date=2023-07-23 |archive-date=2018-06-02 |archive-url=https://web.archive.org/web/20180602054052/https://www.dovepress.com/profile-of-suvorexant-in-the-management-of-insomnia-peer-reviewed-article-DDDT |dead-url=no }}</ref>偶见[[睡眠瘫痪症|睡眠麻痹]],[[做梦异常]]与[[梦游症|梦游]]等复杂睡眠行为与出现[[自杀意念]]。<ref name=":0" /><ref name=":1" /><ref name=":4" />服用这种药物似乎不会产生明显的[[藥物耐受性|药物耐受性]]、[[药物依赖]]、[[藥物戒斷|戒断症状]]和[[反弹现象]]。<ref name=":0" /><ref>{{Cite journal |last=Keks |first=Nicholas A. |last2=Hope |first2=Judy |last3=Keogh |first3=Simone |date=2017-12 |title=Suvorexant: scientifically interesting, utility uncertain |url=https://pubmed.ncbi.nlm.nih.gov/28994603/ |journal=Australasian Psychiatry: Bulletin of Royal Australian and New Zealand College of Psychiatrists |volume=25 |issue=6 |doi=10.1177/1039856217734677 |issn=1440-1665 |pmid=28994603 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121525/https://pubmed.ncbi.nlm.nih.gov/28994603/ |dead-url=no }}</ref><ref>{{Cite journal |last=Muehlan |first=Clemens |last2=Vaillant |first2=Cedric |last3=Zenklusen |first3=Isabelle |last4=Kraehenbuehl |first4=Stephan |last5=Dingemanse |first5=Jasper |date=2020-11-01 |title=Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders |url=https://www.tandfonline.com/doi/full/10.1080/17425255.2020.1817380 |journal=Expert Opinion on Drug Metabolism & Toxicology |language=en |volume=16 |issue=11 |doi=10.1080/17425255.2020.1817380 |issn=1742-5255 |access-date=2023-07-23 |archive-date=2023-05-28 |archive-url=https://web.archive.org/web/20230528043810/https://www.tandfonline.com/doi/full/10.1080/17425255.2020.1817380 |dead-url=no }}</ref>苏沃雷生是一种[[双重食欲素受体激动剂]](DORA),选择性拮抗[[食欲素1型受体]](Ox1R)和[[食欲素2型受体]](Ox2R)。<ref name=":1" />该药物的达峰时间为2至3小时,清除半衰期为12小时。<ref name=":0" /><ref name=":1" />与苯二氮卓类药物与非苯二氮卓类药物不同的是,苏沃雷生并不会与 GABA 受体发生相互作用,而是通过其特殊的药理学机制发挥作用。<ref name=":1" /><ref>{{Cite journal |last=Atkin |first=Tobias |last2=Comai |first2=Stefano |last3=Gobbi |first3=Gabriella |date=2018-04 |title=Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery |url=https://pubmed.ncbi.nlm.nih.gov/29487083/ |journal=Pharmacological Reviews |volume=70 |issue=2 |doi=10.1124/pr.117.014381 |issn=1521-0081 |pmid=29487083 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121527/https://pubmed.ncbi.nlm.nih.gov/29487083/ |dead-url=no }}</ref> |
苏沃雷生的副作用包括[[嗜睡症|嗜睡]]、[[頭暈|头晕]]、[[白天嗜睡]]和[[镇静]]、[[頭痛|头痛]]、[[頭暈|头晕]]、[[梦境异常]]、[[口乾|口干]]和次日驾驶能力受损。<ref name=":0" /><ref name=":3" /><ref name=":4">{{Cite journal |last=Sutton |first=Eliza |date=2015-11 |title=Profile of suvorexant in the management of insomnia |url=https://www.dovepress.com/profile-of-suvorexant-in-the-management-of-insomnia-peer-reviewed-article-DDDT |journal=Drug Design, Development and Therapy |language=en |doi=10.2147/DDDT.S73224 |issn=1177-8881 |pmc=PMC4651361 |pmid=26648692 |access-date=2023-07-23 |archive-date=2018-06-02 |archive-url=https://web.archive.org/web/20180602054052/https://www.dovepress.com/profile-of-suvorexant-in-the-management-of-insomnia-peer-reviewed-article-DDDT |dead-url=no }}</ref>偶见[[睡眠瘫痪症|睡眠麻痹]],[[做梦异常]]与[[梦游症|梦游]]等复杂睡眠行为与出现[[自杀意念]]。<ref name=":0" /><ref name=":1" /><ref name=":4" />服用这种药物似乎不会产生明显的[[藥物耐受性|药物耐受性]]、[[药物依赖]]、[[藥物戒斷|戒断症状]]和[[反弹现象]]。<ref name=":0" /><ref>{{Cite journal |last=Keks |first=Nicholas A. |last2=Hope |first2=Judy |last3=Keogh |first3=Simone |date=2017-12 |title=Suvorexant: scientifically interesting, utility uncertain |url=https://pubmed.ncbi.nlm.nih.gov/28994603/ |journal=Australasian Psychiatry: Bulletin of Royal Australian and New Zealand College of Psychiatrists |volume=25 |issue=6 |doi=10.1177/1039856217734677 |issn=1440-1665 |pmid=28994603 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121525/https://pubmed.ncbi.nlm.nih.gov/28994603/ |dead-url=no }}</ref><ref>{{Cite journal |last=Muehlan |first=Clemens |last2=Vaillant |first2=Cedric |last3=Zenklusen |first3=Isabelle |last4=Kraehenbuehl |first4=Stephan |last5=Dingemanse |first5=Jasper |date=2020-11-01 |title=Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders |url=https://www.tandfonline.com/doi/full/10.1080/17425255.2020.1817380 |journal=Expert Opinion on Drug Metabolism & Toxicology |language=en |volume=16 |issue=11 |doi=10.1080/17425255.2020.1817380 |issn=1742-5255 |access-date=2023-07-23 |archive-date=2023-05-28 |archive-url=https://web.archive.org/web/20230528043810/https://www.tandfonline.com/doi/full/10.1080/17425255.2020.1817380 |dead-url=no }}</ref>苏沃雷生是一种[[双重食欲素受体激动剂]](DORA),选择性拮抗[[食欲素1型受体]](Ox1R)和[[食欲素2型受体]](Ox2R)。<ref name=":1" />该药物的达峰时间为2至3小时,清除半衰期为12小时。<ref name=":0" /><ref name=":1" />与苯二氮卓类药物与非苯二氮卓类药物不同的是,苏沃雷生并不会与 GABA 受体发生相互作用,而是通过其特殊的药理学机制发挥作用。<ref name=":1" /><ref>{{Cite journal |last=Atkin |first=Tobias |last2=Comai |first2=Stefano |last3=Gobbi |first3=Gabriella |date=2018-04 |title=Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery |url=https://pubmed.ncbi.nlm.nih.gov/29487083/ |journal=Pharmacological Reviews |volume=70 |issue=2 |doi=10.1124/pr.117.014381 |issn=1521-0081 |pmid=29487083 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723121527/https://pubmed.ncbi.nlm.nih.gov/29487083/ |dead-url=no }}</ref> |
||
苏沃雷生的药物研发始于2006年,<ref>{{Cite journal |last=Preskorn |first=Sheldon H. |date=2014-11 |title=CNS Drug Development: Lessons from the Development of Ondansetron, Aprepitant, Ramelteon, Varenicline, Lorcaserin, and Suvorexant. Part I |url=https://journals.lww.com/practicalpsychiatry/Abstract/2014/11000/CNS_Drug_Development__Lessons_from_the_Development.6.aspx |journal=Journal of Psychiatric Practice® |language=en-US |volume=20 |issue=6 |doi=10.1097/01.pra.0000456594.66363.6f |issn=1527-4160 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723132807/https://journals.lww.com/practicalpsychiatry/Abstract/2014/11000/CNS_Drug_Development__Lessons_from_the_Development.6.aspx |dead-url=no }}</ref>并于2014年上市。<ref name=":0" />由于其潜在的滥用可能性,<ref name=":0" /><ref name=":5">{{Cite journal |last=Citrome |first=L. |date=2014-12 |title=Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? |url=https://onlinelibrary.wiley.com/doi/10.1111/ijcp.12568 |journal=International Journal of Clinical Practice |language=en |volume=68 |issue=12 |doi=10.1111/ijcp.12568 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723132756/https://onlinelibrary.wiley.com/doi/10.1111/ijcp.12568 |dead-url=no }}</ref>该药物在中国大陆被列为[[二类精神药品|第二类精神药品]],<ref>{{Cite web|last=郝瑀然|title=国家药监局 公安部 国家卫生健康委关于调整麻醉药品和精神药品目录的公告_国务院部门文件_中国政府网|url=https://www.gov.cn/zhengce/zhengceku/2023-04/23/content_5752773.htm|access-date=2023-07-23|website=www.gov.cn|archive-date=2023-07-23|archive-url=https://web.archive.org/web/20230723132800/https://www.gov.cn/zhengce/zhengceku/2023-04/23/content_5752773.htm|dead-url=no}}</ref>在美国则被列为附表 IV 管控物质。<ref>{{Cite web|title=Federal Register :: Request Access|url=https://unblock.federalregister.gov/|access-date=2023-07-23|website=unblock.federalregister.gov|archive-date=2023-06-05|archive-url=https://web.archive.org/web/20230605020052/https://unblock.federalregister.gov/|dead-url=no}}</ref>在其他地方,如澳大利亚,苏沃雷生仅为处方药物而不受到管制。苏沃雷生暂无仿制药可用。<ref>{{Cite web|last=https://www.facebook.com/Drugscom|title=Generic Belsomra Availability|url=https://www.drugs.com/availability/generic-belsomra.html|access-date=2023-07-23|website=Drugs.com|language=en|archive-date=2023-04-11|archive-url=https://web.archive.org/web/20230411064618/https://www.drugs.com/availability/generic-belsomra.html|dead-url=no}}</ref><ref>{{Cite journal |last=Janto |first=Kayla |last2=Prichard |first2=J. Roxanne |last3=Pusalavidyasagar |first3=Snigdha |date=2018-08-15 |title=An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics |url=http://jcsm.aasm.org/doi/10.5664/jcsm.7282 |journal=Journal of Clinical Sleep Medicine |language=en |volume=14 |issue=08 |doi=10.5664/jcsm.7282 |issn=1550-9389 |pmc=PMC6086961 |pmid=30092886 |access-date=2023-07-23 |archive-date=2023-02-18 |archive-url=https://web.archive.org/web/20230218224811/https://jcsm.aasm.org/doi/10.5664/jcsm.7282 |dead-url=no }}</ref><ref>{{Cite journal |last=Janto |first=Kayla |last2=Prichard |first2=J. Roxanne |last3=Pusalavidyasagar |first3=Snigdha |date=2018-08-15 |title=An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics |url=http://jcsm.aasm.org/doi/10.5664/jcsm.7282 |journal=Journal of Clinical Sleep Medicine |language=en |volume=14 |issue=08 |doi=10.5664/jcsm.7282 |issn=1550-9389 |pmc=PMC6086961 |pmid=30092886 |access-date=2023-07-23 |archive-date=2023-02-18 |archive-url=https://web.archive.org/web/20230218224811/https://jcsm.aasm.org/doi/10.5664/jcsm.7282 |dead-url=no }}</ref>除苏沃雷生外,其他[[食欲素受体拮抗剂]]如[[达利雷生]]与[[莱博雷生]]亦已上市。<ref>{{Cite journal |last=Jacobson |first=Laura H. |last2=Hoyer |first2=Daniel |last3=Lecea |first3=Luis |date=2022-05 |title=Hypocretins (orexins): The ultimate translational neuropeptides |url=https://onlinelibrary.wiley.com/doi/10.1111/joim.13406 |journal=Journal of Internal Medicine |language=en |volume=291 |issue=5 |doi=10.1111/joim.13406 |issn=0954-6820 |access-date=2023-07-23 |archive-date=2023-06-21 |archive-url=https://web.archive.org/web/20230621112704/https://onlinelibrary.wiley.com/doi/10.1111/joim.13406 |dead-url=no }}</ref><ref>{{Cite journal |last=Markham |first=Anthony |date=2022-04 |title=Daridorexant: First Approval |url=https://link.springer.com/10.1007/s40265-022-01699-y |journal=Drugs |language=en |volume=82 |issue=5 |doi=10.1007/s40265-022-01699-y |issn=0012-6667 |pmc=PMC9042981 |pmid=35298826}}</ref> |
苏沃雷生的药物研发始于2006年,<ref>{{Cite journal |last=Preskorn |first=Sheldon H. |date=2014-11 |title=CNS Drug Development: Lessons from the Development of Ondansetron, Aprepitant, Ramelteon, Varenicline, Lorcaserin, and Suvorexant. Part I |url=https://journals.lww.com/practicalpsychiatry/Abstract/2014/11000/CNS_Drug_Development__Lessons_from_the_Development.6.aspx |journal=Journal of Psychiatric Practice® |language=en-US |volume=20 |issue=6 |doi=10.1097/01.pra.0000456594.66363.6f |issn=1527-4160 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723132807/https://journals.lww.com/practicalpsychiatry/Abstract/2014/11000/CNS_Drug_Development__Lessons_from_the_Development.6.aspx |dead-url=no }}</ref>并于2014年上市。<ref name=":0" />由于其潜在的滥用可能性,<ref name=":0" /><ref name=":5">{{Cite journal |last=Citrome |first=L. |date=2014-12 |title=Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? |url=https://onlinelibrary.wiley.com/doi/10.1111/ijcp.12568 |journal=International Journal of Clinical Practice |language=en |volume=68 |issue=12 |doi=10.1111/ijcp.12568 |access-date=2023-07-23 |archive-date=2023-07-23 |archive-url=https://web.archive.org/web/20230723132756/https://onlinelibrary.wiley.com/doi/10.1111/ijcp.12568 |dead-url=no }}</ref>该药物在中国大陆被列为[[二类精神药品|第二类精神药品]],<ref>{{Cite web|last=郝瑀然|title=国家药监局 公安部 国家卫生健康委关于调整麻醉药品和精神药品目录的公告_国务院部门文件_中国政府网|url=https://www.gov.cn/zhengce/zhengceku/2023-04/23/content_5752773.htm|access-date=2023-07-23|website=www.gov.cn|archive-date=2023-07-23|archive-url=https://web.archive.org/web/20230723132800/https://www.gov.cn/zhengce/zhengceku/2023-04/23/content_5752773.htm|dead-url=no}}</ref>在美国则被列为附表 IV 管控物质。<ref>{{Cite web|title=Federal Register :: Request Access|url=https://unblock.federalregister.gov/|access-date=2023-07-23|website=unblock.federalregister.gov|archive-date=2023-06-05|archive-url=https://web.archive.org/web/20230605020052/https://unblock.federalregister.gov/|dead-url=no}}</ref>在其他地方,如澳大利亚,苏沃雷生仅为处方药物而不受到管制。苏沃雷生暂无仿制药可用。<ref>{{Cite web|last=https://www.facebook.com/Drugscom|title=Generic Belsomra Availability|url=https://www.drugs.com/availability/generic-belsomra.html|access-date=2023-07-23|website=Drugs.com|language=en|archive-date=2023-04-11|archive-url=https://web.archive.org/web/20230411064618/https://www.drugs.com/availability/generic-belsomra.html|dead-url=no}}</ref><ref>{{Cite journal |last=Janto |first=Kayla |last2=Prichard |first2=J. Roxanne |last3=Pusalavidyasagar |first3=Snigdha |date=2018-08-15 |title=An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics |url=http://jcsm.aasm.org/doi/10.5664/jcsm.7282 |journal=Journal of Clinical Sleep Medicine |language=en |volume=14 |issue=08 |doi=10.5664/jcsm.7282 |issn=1550-9389 |pmc=PMC6086961 |pmid=30092886 |access-date=2023-07-23 |archive-date=2023-02-18 |archive-url=https://web.archive.org/web/20230218224811/https://jcsm.aasm.org/doi/10.5664/jcsm.7282 |dead-url=no }}</ref><ref>{{Cite journal |last=Janto |first=Kayla |last2=Prichard |first2=J. Roxanne |last3=Pusalavidyasagar |first3=Snigdha |date=2018-08-15 |title=An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics |url=http://jcsm.aasm.org/doi/10.5664/jcsm.7282 |journal=Journal of Clinical Sleep Medicine |language=en |volume=14 |issue=08 |doi=10.5664/jcsm.7282 |issn=1550-9389 |pmc=PMC6086961 |pmid=30092886 |access-date=2023-07-23 |archive-date=2023-02-18 |archive-url=https://web.archive.org/web/20230218224811/https://jcsm.aasm.org/doi/10.5664/jcsm.7282 |dead-url=no }}</ref>除苏沃雷生外,其他[[食欲素受体拮抗剂]]如[[达利雷生]]与[[莱博雷生]]亦已上市。<ref name=":7">{{Cite journal |last=Jacobson |first=Laura H. |last2=Hoyer |first2=Daniel |last3=Lecea |first3=Luis |date=2022-05 |title=Hypocretins (orexins): The ultimate translational neuropeptides |url=https://onlinelibrary.wiley.com/doi/10.1111/joim.13406 |journal=Journal of Internal Medicine |language=en |volume=291 |issue=5 |doi=10.1111/joim.13406 |issn=0954-6820 |access-date=2023-07-23 |archive-date=2023-06-21 |archive-url=https://web.archive.org/web/20230621112704/https://onlinelibrary.wiley.com/doi/10.1111/joim.13406 |dead-url=no }}</ref><ref>{{Cite journal |last=Markham |first=Anthony |date=2022-04 |title=Daridorexant: First Approval |url=https://link.springer.com/10.1007/s40265-022-01699-y |journal=Drugs |language=en |volume=82 |issue=5 |doi=10.1007/s40265-022-01699-y |issn=0012-6667 |pmc=PMC9042981 |pmid=35298826}}</ref> |
||
== 医疗用途 == |
== 医疗用途 == |
||
苏沃雷生的适应症为成人[[失眠症]],主要用于[[入睡]]与[[睡眠维持困难]]的治疗。<ref name=":0" /><ref name=":1" /><ref name=":2" />在15-20mg的剂量下,可以观察到服用苏沃雷生的试验组与[[安慰剂]]组相比,平均[[入睡]]时间减少达10分钟,[[睡眠维持]]时长增加约15至30分钟,总[[入睡时长]]增加约10至20分钟。<ref name=":0" />2017年对苏沃雷生治疗失眠的[[随机对照试验]]进行的[[系統綜述|系统回顾]]和[[荟萃分析]]同样发现,在为期1至3月的治疗期间,该药物改善了主观入睡时间、主观总睡眠时间和主观[[睡眠质量]]。<ref name=":3" />据报道,在批准剂量下(≤20mg),苏沃雷生用于治疗[[失眠症]]的效力 |
苏沃雷生的适应症为成人[[失眠症]],主要用于[[入睡]]与[[睡眠维持困难]]的治疗。<ref name=":0" /><ref name=":1" /><ref name=":2" />在15-20mg的剂量下,可以观察到服用苏沃雷生的试验组与[[安慰剂]]组相比,平均[[入睡]]时间减少达10分钟,[[睡眠维持]]时长增加约15至30分钟,总[[入睡时长]]增加约10至20分钟。<ref name=":0" />2017年对苏沃雷生治疗失眠的[[随机对照试验]]进行的[[系統綜述|系统回顾]]和[[荟萃分析]]同样发现,在为期1至3月的治疗期间,该药物改善了主观入睡时间、主观总睡眠时间和主观[[睡眠质量]]。<ref name=":3" />据报道,在批准剂量下(≤20mg),苏沃雷生用于治疗[[失眠症]]的效力为中等水平。<ref name=":4" /><ref>{{Cite journal |last=Keks |first=Nicholas A |last2=Hope |first2=Judy |last3=Keogh |first3=Simone |date=2017-12 |title=Suvorexant: scientifically interesting, utility uncertain |url=http://journals.sagepub.com/doi/10.1177/1039856217734677 |journal=Australasian Psychiatry |language=en |volume=25 |issue=6 |doi=10.1177/1039856217734677 |issn=1039-8562 |access-date=2023-07-24 |archive-date=2023-02-18 |archive-url=https://web.archive.org/web/20230218224813/https://journals.sagepub.com/doi/10.1177/1039856217734677 |dead-url=no }}</ref><ref>{{Cite journal |last=Kripke |first=Daniel F. |date=2015 |title=Is suvorexant a better choice than alternative hypnotics? |url=https://pubmed.ncbi.nlm.nih.gov/26594338/ |journal=F1000Research |volume=4 |doi=10.12688/f1000research.6845.1 |issn=2046-1402 |pmc=4648222 |pmid=26594338}}</ref><ref>{{Cite web|date=2013-06-14|title=Letter to the FDA Opposing Approval of the Sleep Medicine Suvorexant|url=https://www.citizen.org/article/letter-to-the-fda-opposing-approval-of-the-sleep-medicine-suvorexant/|access-date=2023-07-24|website=Public Citizen|language=en|archive-date=2023-04-15|archive-url=https://web.archive.org/web/20230415012203/https://www.citizen.org/article/letter-to-the-fda-opposing-approval-of-the-sleep-medicine-suvorexant/|dead-url=no}}</ref> |
||
[[元分析|网络荟萃分析]]评估了苏沃雷生的促眠效果,并将其与其他食欲素受体拮抗剂如[[达利雷生]]与[[莱博雷生]],以及包括苯二氮卓类药物、非苯二氮卓类药物、抗组胺药、镇静类抗抑郁药物(如多虑平、米氮平、阿米替林、曲唑酮)和褪黑素受体激动剂在内的其他助眠类药物的效果进行了比较。<ref>{{Cite journal |last=McElroy |first=Heather |last2=O’Leary |first2=Beth |last3=Adena |first3=Michael |last4=Campbell |first4=Renee |last5=Monfared |first5=Amir Abbas Tahami |last6=Meier |first6=Genevieve |date=2021-09 |title=Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis |url=https://www.jmcp.org/doi/10.18553/jmcp.2021.21011 |journal=Journal of Managed Care & Specialty Pharmacy |volume=27 |issue=9 |doi=10.18553/jmcp.2021.21011 |issn=2376-0540 |access-date=2023-07-24 |archive-date=2023-05-28 |archive-url=https://web.archive.org/web/20230528043811/https://www.jmcp.org/doi/10.18553/jmcp.2021.21011 |dead-url=no }}</ref><ref>{{Cite journal |last=Wang |first=Lu |last2=Pan |first2=Yundan |last3=Ye |first3=Chunyan |last4=Guo |first4=Lizhe |last5=Luo |first5=Sumei |last6=Dai |first6=Sisi |last7=Chen |first7=Na |last8=Wang |first8=E. |date=2021-12-01 |title=A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults |url=https://www.sciencedirect.com/science/article/pii/S0149763421004176 |journal=Neuroscience & Biobehavioral Reviews |language=en |volume=131 |doi=10.1016/j.neubiorev.2021.09.035 |issn=0149-7634}}</ref><ref>{{Cite journal |last=Wang |first=Lu |last2=Pan |first2=Yundan |last3=Ye |first3=Chunyan |last4=Guo |first4=Lizhe |last5=Luo |first5=Sumei |last6=Dai |first6=Sisi |last7=Chen |first7=Na |last8=Wang |first8=E. |date=2021-12-01 |title=A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults |url=https://www.sciencedirect.com/science/article/pii/S0149763421004176 |journal=Neuroscience & Biobehavioral Reviews |language=en |volume=131 |doi=10.1016/j.neubiorev.2021.09.035 |issn=0149-7634 |access-date=2023-07-24 |archive-date=2023-07-24 |archive-url=https://web.archive.org/web/20230724094821/https://www.sciencedirect.com/science/article/pii/S0149763421004176 |dead-url=no }}</ref><ref>{{Cite journal |last=Xue |first=Tao |last2=Wu |first2=Xin |last3=Chen |first3=Shujun |last4=Yang |first4=Yanbo |last5=Yan |first5=Zeya |last6=Song |first6=Zhaoming |last7=Zhang |first7=Wei |last8=Zhang |first8=Jianguo |last9=Chen |first9=Zhouqing |last10=Wang |first10=Zhong |date=2022-02-01 |title=The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis |url=https://www.sciencedirect.com/science/article/pii/S1087079221001581 |journal=Sleep Medicine Reviews |language=en |volume=61 |doi=10.1016/j.smrv.2021.101573 |issn=1087-0792}}</ref> |
[[元分析|网络荟萃分析]]评估了苏沃雷生的促眠效果,并将其与其他食欲素受体拮抗剂如[[达利雷生]]与[[莱博雷生]],以及包括苯二氮卓类药物、非苯二氮卓类药物、抗组胺药、镇静类抗抑郁药物(如多虑平、米氮平、阿米替林、曲唑酮)和褪黑素受体激动剂在内的其他助眠类药物的效果进行了比较。<ref>{{Cite journal |last=McElroy |first=Heather |last2=O’Leary |first2=Beth |last3=Adena |first3=Michael |last4=Campbell |first4=Renee |last5=Monfared |first5=Amir Abbas Tahami |last6=Meier |first6=Genevieve |date=2021-09 |title=Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis |url=https://www.jmcp.org/doi/10.18553/jmcp.2021.21011 |journal=Journal of Managed Care & Specialty Pharmacy |volume=27 |issue=9 |doi=10.18553/jmcp.2021.21011 |issn=2376-0540 |access-date=2023-07-24 |archive-date=2023-05-28 |archive-url=https://web.archive.org/web/20230528043811/https://www.jmcp.org/doi/10.18553/jmcp.2021.21011 |dead-url=no }}</ref><ref>{{Cite journal |last=Wang |first=Lu |last2=Pan |first2=Yundan |last3=Ye |first3=Chunyan |last4=Guo |first4=Lizhe |last5=Luo |first5=Sumei |last6=Dai |first6=Sisi |last7=Chen |first7=Na |last8=Wang |first8=E. |date=2021-12-01 |title=A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults |url=https://www.sciencedirect.com/science/article/pii/S0149763421004176 |journal=Neuroscience & Biobehavioral Reviews |language=en |volume=131 |doi=10.1016/j.neubiorev.2021.09.035 |issn=0149-7634}}</ref><ref>{{Cite journal |last=Wang |first=Lu |last2=Pan |first2=Yundan |last3=Ye |first3=Chunyan |last4=Guo |first4=Lizhe |last5=Luo |first5=Sumei |last6=Dai |first6=Sisi |last7=Chen |first7=Na |last8=Wang |first8=E. |date=2021-12-01 |title=A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults |url=https://www.sciencedirect.com/science/article/pii/S0149763421004176 |journal=Neuroscience & Biobehavioral Reviews |language=en |volume=131 |doi=10.1016/j.neubiorev.2021.09.035 |issn=0149-7634 |access-date=2023-07-24 |archive-date=2023-07-24 |archive-url=https://web.archive.org/web/20230724094821/https://www.sciencedirect.com/science/article/pii/S0149763421004176 |dead-url=no }}</ref><ref>{{Cite journal |last=Xue |first=Tao |last2=Wu |first2=Xin |last3=Chen |first3=Shujun |last4=Yang |first4=Yanbo |last5=Yan |first5=Zeya |last6=Song |first6=Zhaoming |last7=Zhang |first7=Wei |last8=Zhang |first8=Jianguo |last9=Chen |first9=Zhouqing |last10=Wang |first10=Zhong |date=2022-02-01 |title=The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis |url=https://www.sciencedirect.com/science/article/pii/S1087079221001581 |journal=Sleep Medicine Reviews |language=en |volume=61 |doi=10.1016/j.smrv.2021.101573 |issn=1087-0792}}</ref>一项于2022年发表的大规模系统回顾和[[网络荟萃分析]]研究发现,苏沃雷生用于治疗[[失眠症]]时,与[[對照組|对照组]]相比其标准化[[效应值]](SMD)为0.31(95% CI为0.01至0.62)。<ref name=":6" />在治疗失眠症时间为4周时,苏沃雷生的疗效似乎和莱博雷生(SMD 0.36,95% CI 0.08至0.63)与达利雷生(SMD 0.23,95% CI -0.01至0.48)相当,而[[苯二氮卓类药物]]与[[Z-drugs|非苯二氮卓类药物]]则显示出了更为强大的作用效果(SMD为0.45至0.83),与抗组胺类药物(如[[多虑平]]、[[多西拉敏]]与[[曲米帕明]])相似(SMD为0.30至0.55)。<ref name=":6" /> |
||
[[食欲素受体拮抗剂]](如苏沃雷生)主要通过增加[[快速動眼期|快速动眼期睡眠]](REM)来增加总睡眠时间,而其对[[非快速動眼睡眠|非快速动眼期睡眠]](NREM)没有影响甚至可能减少其持续时间。而这与大多数其他安眠药不同,后者要么不影响REM睡眠,要么减少其持续时间。这些差异造成的影响尚未完全阐明<ref name=":7" />。与如[[苯二氮䓬类|苯二氮䓬类药物]]和[[Z-drugs|非苯二氮卓类药物]]等其他[[安眠药]]不同的是,[[食欲素受体拮抗剂]]不会干扰[[睡眠结构]],这可能可以为患者提供更为宁静的睡眠。 |
|||
由于在临床试验阶段就被排除在外,目前尚不清楚苏沃雷生在有[[物質濫用|物质滥用]]与[[酗酒]]史的人群中的使用是否安全。一项[[Cochrane回顾]]发现,苏沃雷生在用于[[失智症|痴呆症]]人群失眠问题的短期治疗时有效且副作用较少。<ref>{{Cite journal |last=McCleery |first=Jenny |last2=Sharpley |first2=Ann L |date=2020-11-15 |editor-last=Cochrane Dementia and Cognitive Improvement Group |title=Pharmacotherapies for sleep disturbances in dementia |url=http://doi.wiley.com/10.1002/14651858.CD009178.pub4 |journal=Cochrane Database of Systematic Reviews |language=en |volume=2020 |issue=11 |doi=10.1002/14651858.CD009178.pub4 |pmc=PMC8094738 |pmid=33189083}}</ref>因未在儿童与亲少年人群中进行研究,目前尚不清楚苏沃雷生对其睡眠问题是否有效且安全。 |
|||
苏沃雷生已被美国食品药品监督管理局在5-20mg剂量下和[[澳大利亚政府药物管理局|澳大利亚政府药物管理]](TGA)与日本[[医药品医疗机器综合机构|独立行政法人医药品医疗器械综合机构]](PDMA)在15mg(用于老年人)与20mg(用于年轻人)剂量下治疗[[失眠症]]。 |
|||
=== 可用剂型 === |
=== 可用剂型 === |
||
2023年10月18日 (三) 11:41的版本
| 此條目疑似为广告或包含宣传性内容。 (2023年7月26日) |
 | |
 | |
| 臨床資料 | |
|---|---|
| 其他名稱 | MK-4305; MK4305 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a614046 |
| 核准狀況 | |
| 依賴性 | 低 |
| 成癮性 | 低 |
| 给药途径 | 口服 |
| 藥物類別 | 食欲素受体激动剂; 催眠药; 镇定剂 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 | |
| 识别信息 | |
| |
| CAS号 | 1030377-33-3 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.546 |
| 化学信息 | |
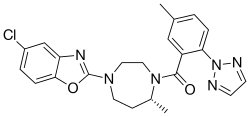
| 化学式 | C23H23ClN6O2 |
| 摩尔质量 | 450.93 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
苏沃雷生(Suvorexant),品牌名Belsomra,是一种食欲素受体拮抗剂,被用于治疗失眠症,主要用于入睡或睡眠维持困难的成年患者的治疗。[1][2][3]苏沃雷生具有加速入睡、延长睡眠时间、减少半夜醒来次数以及提高睡眠质量的作用。[1][2][4]其药效中等,[5]与其他食欲素受体拮抗剂类似,但低于苯二氮卓类药物与非苯二氮卓类药物。[6]苏沃雷生的给药方式为口服给药。[1][2][3][7]
苏沃雷生的副作用包括嗜睡、头晕、白天嗜睡和镇静、头痛、头晕、梦境异常、口干和次日驾驶能力受损。[1][4][8]偶见睡眠麻痹,做梦异常与梦游等复杂睡眠行为与出现自杀意念。[1][2][8]服用这种药物似乎不会产生明显的药物耐受性、药物依赖、戒断症状和反弹现象。[1][9][10]苏沃雷生是一种双重食欲素受体激动剂(DORA),选择性拮抗食欲素1型受体(Ox1R)和食欲素2型受体(Ox2R)。[2]该药物的达峰时间为2至3小时,清除半衰期为12小时。[1][2]与苯二氮卓类药物与非苯二氮卓类药物不同的是,苏沃雷生并不会与 GABA 受体发生相互作用,而是通过其特殊的药理学机制发挥作用。[2][11]
苏沃雷生的药物研发始于2006年,[12]并于2014年上市。[1]由于其潜在的滥用可能性,[1][13]该药物在中国大陆被列为第二类精神药品,[14]在美国则被列为附表 IV 管控物质。[15]在其他地方,如澳大利亚,苏沃雷生仅为处方药物而不受到管制。苏沃雷生暂无仿制药可用。[16][17][18]除苏沃雷生外,其他食欲素受体拮抗剂如达利雷生与莱博雷生亦已上市。[19][20]
医疗用途
苏沃雷生的适应症为成人失眠症,主要用于入睡与睡眠维持困难的治疗。[1][2][3]在15-20mg的剂量下,可以观察到服用苏沃雷生的试验组与安慰剂组相比,平均入睡时间减少达10分钟,睡眠维持时长增加约15至30分钟,总入睡时长增加约10至20分钟。[1]2017年对苏沃雷生治疗失眠的随机对照试验进行的系统回顾和荟萃分析同样发现,在为期1至3月的治疗期间,该药物改善了主观入睡时间、主观总睡眠时间和主观睡眠质量。[4]据报道,在批准剂量下(≤20mg),苏沃雷生用于治疗失眠症的效力为中等水平。[8][21][22][23]
网络荟萃分析评估了苏沃雷生的促眠效果,并将其与其他食欲素受体拮抗剂如达利雷生与莱博雷生,以及包括苯二氮卓类药物、非苯二氮卓类药物、抗组胺药、镇静类抗抑郁药物(如多虑平、米氮平、阿米替林、曲唑酮)和褪黑素受体激动剂在内的其他助眠类药物的效果进行了比较。[24][25][26][27]一项于2022年发表的大规模系统回顾和网络荟萃分析研究发现,苏沃雷生用于治疗失眠症时,与对照组相比其标准化效应值(SMD)为0.31(95% CI为0.01至0.62)。[6]在治疗失眠症时间为4周时,苏沃雷生的疗效似乎和莱博雷生(SMD 0.36,95% CI 0.08至0.63)与达利雷生(SMD 0.23,95% CI -0.01至0.48)相当,而苯二氮卓类药物与非苯二氮卓类药物则显示出了更为强大的作用效果(SMD为0.45至0.83),与抗组胺类药物(如多虑平、多西拉敏与曲米帕明)相似(SMD为0.30至0.55)。[6]
食欲素受体拮抗剂(如苏沃雷生)主要通过增加快速动眼期睡眠(REM)来增加总睡眠时间,而其对非快速动眼期睡眠(NREM)没有影响甚至可能减少其持续时间。而这与大多数其他安眠药不同,后者要么不影响REM睡眠,要么减少其持续时间。这些差异造成的影响尚未完全阐明[19]。与如苯二氮䓬类药物和非苯二氮卓类药物等其他安眠药不同的是,食欲素受体拮抗剂不会干扰睡眠结构,这可能可以为患者提供更为宁静的睡眠。
由于在临床试验阶段就被排除在外,目前尚不清楚苏沃雷生在有物质滥用与酗酒史的人群中的使用是否安全。一项Cochrane回顾发现,苏沃雷生在用于痴呆症人群失眠问题的短期治疗时有效且副作用较少。[28]因未在儿童与亲少年人群中进行研究,目前尚不清楚苏沃雷生对其睡眠问题是否有效且安全。
苏沃雷生已被美国食品药品监督管理局在5-20mg剂量下和澳大利亚政府药物管理(TGA)与日本独立行政法人医药品医疗器械综合机构(PDMA)在15mg(用于老年人)与20mg(用于年轻人)剂量下治疗失眠症。
可用剂型
苏沃雷生的可用剂型为5,10,15和20mg的薄膜衣片。[1][8][13]
参考资料
- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 DailyMed - BELSOMRA- suvorexant tablet, film coated. dailymed.nlm.nih.gov. [2023-07-23]. (原始内容存档于2019-07-02).
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Jacobson, Laura H.; Callander, Gabrielle E.; Hoyer, Daniel. Suvorexant for the treatment of insomnia. Expert Review of Clinical Pharmacology. 2014-11, 7 (6) [2023-07-23]. ISSN 1751-2441. PMID 25318834. doi:10.1586/17512433.2014.966813. (原始内容存档于2023-07-23).
- ^ 3.0 3.1 3.2 苏沃雷生[精二]_其他镇静催眠药_镇静药与催眠药_作用于神经系统的药物_湖南药事服务网. www.hnysfww.com. [2023-07-23]. (原始内容存档于2023-07-23).
- ^ 4.0 4.1 4.2 Kuriyama, Akira; Tabata, Hiromitsu. Suvorexant for the treatment of primary insomnia: A systematic review and meta-analysis. Sleep Medicine Reviews. 2017-10, 35 [2023-07-23]. ISSN 1532-2955. PMID 28365447. doi:10.1016/j.smrv.2016.09.004. (原始内容存档于2023-03-13).
- ^ Table 1: The Single Nucleotide Polymorphisms in cathepsin B protein mined from literature (PMID: 16492714).. dx.doi.org. [2023-07-23]. (原始内容存档于2019-10-12).
- ^ 6.0 6.1 6.2 De Crescenzo, Franco; D'Alò, Gian Loreto; Ostinelli, Edoardo G; Ciabattini, Marco; Di Franco, Valeria; Watanabe, Norio; Kurtulmus, Ayse; Tomlinson, Anneka; Mitrova, Zuzana; Foti, Francesca; Del Giovane, Cinzia. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. The Lancet. 2022-07, 400 (10347) [2023-07-23]. doi:10.1016/S0140-6736(22)00878-9. (原始内容存档于2023-06-30) (英语).
- ^ https://www.facebook.com/Drugscom. Generic Belsomra Availability. Drugs.com. [2023-07-23]. (原始内容存档于2023-04-11) (英语).
- ^ 8.0 8.1 8.2 8.3 Sutton, Eliza. Profile of suvorexant in the management of insomnia. Drug Design, Development and Therapy. 2015-11 [2023-07-23]. ISSN 1177-8881. PMC 4651361
 . PMID 26648692. doi:10.2147/DDDT.S73224. (原始内容存档于2018-06-02) (英语).
. PMID 26648692. doi:10.2147/DDDT.S73224. (原始内容存档于2018-06-02) (英语).
- ^ Keks, Nicholas A.; Hope, Judy; Keogh, Simone. Suvorexant: scientifically interesting, utility uncertain. Australasian Psychiatry: Bulletin of Royal Australian and New Zealand College of Psychiatrists. 2017-12, 25 (6) [2023-07-23]. ISSN 1440-1665. PMID 28994603. doi:10.1177/1039856217734677. (原始内容存档于2023-07-23).
- ^ Muehlan, Clemens; Vaillant, Cedric; Zenklusen, Isabelle; Kraehenbuehl, Stephan; Dingemanse, Jasper. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opinion on Drug Metabolism & Toxicology. 2020-11-01, 16 (11) [2023-07-23]. ISSN 1742-5255. doi:10.1080/17425255.2020.1817380. (原始内容存档于2023-05-28) (英语).
- ^ Atkin, Tobias; Comai, Stefano; Gobbi, Gabriella. Drugs for Insomnia beyond Benzodiazepines: Pharmacology, Clinical Applications, and Discovery. Pharmacological Reviews. 2018-04, 70 (2) [2023-07-23]. ISSN 1521-0081. PMID 29487083. doi:10.1124/pr.117.014381. (原始内容存档于2023-07-23).
- ^ Preskorn, Sheldon H. CNS Drug Development: Lessons from the Development of Ondansetron, Aprepitant, Ramelteon, Varenicline, Lorcaserin, and Suvorexant. Part I. Journal of Psychiatric Practice®. 2014-11, 20 (6) [2023-07-23]. ISSN 1527-4160. doi:10.1097/01.pra.0000456594.66363.6f. (原始内容存档于2023-07-23) (美国英语).
- ^ 13.0 13.1 Citrome, L. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed?. International Journal of Clinical Practice. 2014-12, 68 (12) [2023-07-23]. doi:10.1111/ijcp.12568. (原始内容存档于2023-07-23) (英语).
- ^ 郝瑀然. 国家药监局 公安部 国家卫生健康委关于调整麻醉药品和精神药品目录的公告_国务院部门文件_中国政府网. www.gov.cn. [2023-07-23]. (原始内容存档于2023-07-23).
- ^ Federal Register :: Request Access. unblock.federalregister.gov. [2023-07-23]. (原始内容存档于2023-06-05).
- ^ https://www.facebook.com/Drugscom. Generic Belsomra Availability. Drugs.com. [2023-07-23]. (原始内容存档于2023-04-11) (英语).
- ^ Janto, Kayla; Prichard, J. Roxanne; Pusalavidyasagar, Snigdha. An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics. Journal of Clinical Sleep Medicine. 2018-08-15, 14 (08) [2023-07-23]. ISSN 1550-9389. PMC 6086961
 . PMID 30092886. doi:10.5664/jcsm.7282. (原始内容存档于2023-02-18) (英语).
. PMID 30092886. doi:10.5664/jcsm.7282. (原始内容存档于2023-02-18) (英语).
- ^ Janto, Kayla; Prichard, J. Roxanne; Pusalavidyasagar, Snigdha. An Update on Dual Orexin Receptor Antagonists and Their Potential Role in Insomnia Therapeutics. Journal of Clinical Sleep Medicine. 2018-08-15, 14 (08) [2023-07-23]. ISSN 1550-9389. PMC 6086961
 . PMID 30092886. doi:10.5664/jcsm.7282. (原始内容存档于2023-02-18) (英语).
. PMID 30092886. doi:10.5664/jcsm.7282. (原始内容存档于2023-02-18) (英语).
- ^ 19.0 19.1 Jacobson, Laura H.; Hoyer, Daniel; Lecea, Luis. Hypocretins (orexins): The ultimate translational neuropeptides. Journal of Internal Medicine. 2022-05, 291 (5) [2023-07-23]. ISSN 0954-6820. doi:10.1111/joim.13406. (原始内容存档于2023-06-21) (英语).
- ^ Markham, Anthony. Daridorexant: First Approval. Drugs. 2022-04, 82 (5). ISSN 0012-6667. PMC 9042981
 . PMID 35298826. doi:10.1007/s40265-022-01699-y (英语).
. PMID 35298826. doi:10.1007/s40265-022-01699-y (英语).
- ^ Keks, Nicholas A; Hope, Judy; Keogh, Simone. Suvorexant: scientifically interesting, utility uncertain. Australasian Psychiatry. 2017-12, 25 (6) [2023-07-24]. ISSN 1039-8562. doi:10.1177/1039856217734677. (原始内容存档于2023-02-18) (英语).
- ^ Kripke, Daniel F. Is suvorexant a better choice than alternative hypnotics?. F1000Research. 2015, 4. ISSN 2046-1402. PMC 4648222
 . PMID 26594338. doi:10.12688/f1000research.6845.1.
. PMID 26594338. doi:10.12688/f1000research.6845.1.
- ^ Letter to the FDA Opposing Approval of the Sleep Medicine Suvorexant. Public Citizen. 2013-06-14 [2023-07-24]. (原始内容存档于2023-04-15) (英语).
- ^ McElroy, Heather; O’Leary, Beth; Adena, Michael; Campbell, Renee; Monfared, Amir Abbas Tahami; Meier, Genevieve. Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis. Journal of Managed Care & Specialty Pharmacy. 2021-09, 27 (9) [2023-07-24]. ISSN 2376-0540. doi:10.18553/jmcp.2021.21011. (原始内容存档于2023-05-28).
- ^ Wang, Lu; Pan, Yundan; Ye, Chunyan; Guo, Lizhe; Luo, Sumei; Dai, Sisi; Chen, Na; Wang, E. A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults. Neuroscience & Biobehavioral Reviews. 2021-12-01, 131. ISSN 0149-7634. doi:10.1016/j.neubiorev.2021.09.035 (英语).
- ^ Wang, Lu; Pan, Yundan; Ye, Chunyan; Guo, Lizhe; Luo, Sumei; Dai, Sisi; Chen, Na; Wang, E. A network meta-analysis of the long- and short-term efficacy of sleep medicines in adults and older adults. Neuroscience & Biobehavioral Reviews. 2021-12-01, 131 [2023-07-24]. ISSN 0149-7634. doi:10.1016/j.neubiorev.2021.09.035. (原始内容存档于2023-07-24) (英语).
- ^ Xue, Tao; Wu, Xin; Chen, Shujun; Yang, Yanbo; Yan, Zeya; Song, Zhaoming; Zhang, Wei; Zhang, Jianguo; Chen, Zhouqing; Wang, Zhong. The efficacy and safety of dual orexin receptor antagonists in primary insomnia: A systematic review and network meta-analysis. Sleep Medicine Reviews. 2022-02-01, 61. ISSN 1087-0792. doi:10.1016/j.smrv.2021.101573 (英语).
- ^ McCleery, Jenny; Sharpley, Ann L. Cochrane Dementia and Cognitive Improvement Group , 编. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database of Systematic Reviews. 2020-11-15, 2020 (11). PMC 8094738
 . PMID 33189083. doi:10.1002/14651858.CD009178.pub4 (英语).
. PMID 33189083. doi:10.1002/14651858.CD009178.pub4 (英语).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

