膽管癌
| 膽管癌 | |
|---|---|
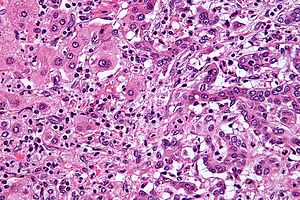 | |
| 肝內膽管癌(右側)和正常肝細胞(左側)的組織切片,HE染色。 | |
| 症状 | 腹痛、黄疸、體重下降、全身搔癢、發燒[1] |
| 起病年龄 | 約70歲[2] |
| 类型 | 肝內型(Intrahepatic)、肝門型(perihilar)、遠端型(distal)[2] |
| 风险因素 | 原發性硬化性膽管炎、溃疡性结肠炎、特定肝吸蟲感染、先天肝發育異常[1] |
| 診斷方法 | 顯微病理切片[3] |
| 治療 | 手術切除、化学疗法、放射線療法、膽道支架、肝臟移植[1] |
| 预后 | 通常很差[4] |
| 患病率 | 約每年每10萬人1–2例(西方國家)[5] |
| 分类和外部资源 | |
| 醫學專科 | 肿瘤学 |
| ICD-11 | XH7M15 |
| OMIM | 615619 |
| DiseasesDB | 2505 |
| MedlinePlus | 000291 |
| eMedicine | 277393、365065 |
膽管癌(Cholangiocarcinoma),又稱膽道癌,是一種由膽道上皮細胞(或呈現上皮細胞分化特征的細胞)癌变所造成的癌症[6]。膽管癌主要的症狀為肝功能異常、腹痛、黃疸、全身搔癢、發燒和體重減輕[1];此外患者的糞便顏色可能變淺,尿液顏色變深[3]。同屬膽道系統癌症的疾病還包括膽囊癌和十二指腸乳頭癌[7]。膽管癌是一種罕見的腺癌[2]。
膽管癌的風險因子包含原發性硬化性膽管炎、溃疡性结肠炎、肝硬化、丙型肝炎、乙型肝炎、特定肝吸蟲感染,以及先天肝臟結構異常等等[1][2][8]。但大多數膽管癌患者缺乏明確的風險因子背景可供辨識[2]。疾病的診斷須結合血液检查、醫學影像,和內視鏡检查,有時需手術取出檢體進行病理診斷[3]。確診須經由顯微鏡檢進行[3]。
多數患者在診斷出膽管癌時,疾病已經進展至晚期,無法治癒[1]。在這些無法治癒的病人可進行和缓医疗,包含手術切除、化学疗法、放射線療法,以及置放膽道支架等等[1]。完全手術切除是唯一的治癒希望,但有約三分之一的病人腫瘤會進犯總膽管,而此類腫瘤無法手術切除,因此僅有少數腫瘤可以進行完全切除[1]。完全切除後仍然建議繼續進行化療及放療[1]。有些符合特定條件的病人可以進行肝臟移植[2],但術後的五年存活率仍不到五成[5]。
膽管癌在西方世界相當罕見,大約每年每10萬人僅 0.5–2 例[1][5]。东南亚等肝吸蟲流行的地區發生率較高[4],如泰国約每年每10萬人60例[4]。韓國、上海的發生率甚至高於罕見癌症的標準[9]。膽管癌一般發生於70歲左右,但患有原發性硬化性膽管炎者常在40歲左右即發病[2]。現今西方國家的肝內型膽管癌比例比起過去較高[5]。
症狀和症候群

膽管癌最常見的生理變化為肝功能異常、黃疸(膽管阻塞後,膽汁導致眼睛和皮膚變黃)、腹痛(30%–50%)、全身搔癢(66%)、體重減輕(30%–50%)、發燒(小於20%)、糞便和尿液顏色改變[10][11]。症狀的類別取決於腫瘤在膽管中的位置:位於肝外膽管者較可能發生黃疸;位在肝內膽者則較常發生腹痛,但不常伴隨黃疸[12]。
膽管癌患者的肝功能血液檢驗報告往往會呈現出各種「阻塞性」特徵:意即膽色素、鹼性磷酸酶、丙麩胺酸轉移酶濃度上升,但转氨酶濃度正常,此類檢驗結果排除了發炎或肝實質組織感染的可能,明確指出黃疸的病灶來自膽管阻塞[13]。多數的膽管癌患者CA19-9濃度也會上升[14]。
風險因子

雖然多數膽管癌患者形成腫瘤的原因並不明確,但目前已发现相当多的風險因子。西方國家最常見的風險因子為原發性硬化性膽管炎(PSC),這是一種和潰瘍性結腸炎高度相關的膽道發炎疾病[15],流行病學統計指出PSC患者一生中罹患膽管癌的機率為10%–15%[16],法醫病理的一系列研究則顯示此比率可能高達30%[17]。PSC增加膽管癌罹患風險的機制目前尚未明瞭。
特定的肝吸蟲疾病也是膽管癌的風險因子,香貓肝吸蟲(分布於泰國、寮國、越南)[18][19][20]或中華肝吸蟲(分布於中國大陸、台灣、東西伯利亞、韓國、越南)[21][22]感染已證實和膽管癌有關。病毒感染(如B型肝炎或C型肝炎)[23][24][25]、酒精性肝炎、肝硬化或其他形式的慢性肝臟疾病,都會大幅提升患者罹患膽管癌的風險[26][27]。一份研究中指出愛滋病也可能是膽管癌的風險因子,但目前還不清楚此現象是愛滋病毒本身還是相關的干擾因子(如C型肝炎感染)所造成的[26]。膽型螺旋桿菌和肝型螺旋桿菌等細菌感染也可能造成膽道癌症[28]。
先天性肝內膽道囊腫(五種膽道囊腫中的一種)患者一生罹患膽管癌的概率为15%[29][30];罕見的遺傳性非息肉結腸癌和膽道乳突瘤也可能和膽管癌有關[31][32]。膽結石和膽管癌沒有明顯的關聯;肝內膽管結石和膽管癌卻強烈相關,後者在西方國家並不常見,但在亞洲部分地區(如臺灣)卻非常普遍[33][34][35]。二氧化釷以前常用作放射造影的對比劑,但人體受暴露後的30至40年內可能會產生膽管癌,美國為此已於1950年代禁用此藥品[36][37]。
病理生理學

膽管癌可能影響膽管中的任何位置──包括肝臟內和肝臟外:發生在肝臟內者稱肝內膽管癌;在肝臟外者稱肝外膽管癌;在肝外膽管與肝臟相接處者稱肝門膽管癌。膽管癌若發生在左右兩肝管匯流形成總肝管處(即膽囊三角內),則稱為克拉茨金瘤(Klatskin tumor)[38]。
雖然膽管癌在膽道上皮有腺癌的組織和分子特徵,但細胞真正的來源仍屬未知,有證據指出造成腫瘤的初始轉型細胞可能來自多能性肝幹細胞[39][40][41]。膽管癌的發展可分為以下幾個階段:早期的增生和化生、接著異生、最後發展成腫瘤,這個過程和結腸癌的發展相似[42]。慢性發炎和膽管阻塞會造成膽汁流動受阻,這可能也會促進膽管癌發展[42][43][44]。
在組織學上,膽管癌的細胞可能以未分化型、分化型、或過渡型出現,它們通常由活化的纖維結締組織或促結締組織包圍,纖維化組織的存在使得分化型癌細胞和正常上皮細胞不易分辨。角蛋白、癌胚抗原和粘蛋白染色或許能幫助診斷,但目前沒有專門的免疫組織化學染色能區分惡性和良性的膽道組織[45]。
診斷

膽管癌的確切病程必須由病理檢查來判定,意即必須進行組織切片或檢驗手術以取得腫瘤組織[46]。阻塞性黃疸的病人可能疑似患有膽管癌,其中原發性硬化性膽管炎是造成膽管癌的高風險因子,但它本身的症狀和膽管癌難以分辨,在這種情形下,造影上顯示的團狀物或膽管擴張等診斷線索不具有代表性,因此要有效診斷出膽管癌相當困難[47] 。
血液檢查
目前沒有特定的血液檢查方法能直接診斷出膽管癌。膽管癌患者血漿中的癌胚抗原和CA19-9濃度通常會提高,但其專一度和敏感度都不足以成為常規的檢查標準。血液檢查常配合造影方法,在疑似膽管癌的案例中作為有用的判斷依據[48]。
腹部造影

針對可疑的阻塞性黃疸患者,肝臟和膽道超音波常是首选的检查方法[49][50],超音波可以辨識出膽管阻塞和擴張,在某些案例中也足夠診斷出膽管癌[51]。X射線電腦斷層掃描(CT)在膽管癌的診斷中也充当重要角色[52][53][54]。
膽管造影

雖然腹部造影在膽管癌的診斷上很有用,但针对性的膽管造影也是必須的。最廣泛使用的膽管造影術為內視鏡逆行性膽胰管攝影術(ERCP),此種內視鏡必須由腸胃專科醫師或受過訓練的外科醫師操作,雖然這是一種有風險的侵入性方法,但它同時能取得檢體、放置支架、或進行其他能排除膽管阻塞的措施[13]。內視鏡超音波可以與ERCP同時進行,如此將能更準確地取得檢體、得知淋巴結的入侵情形、並評估手術切除的可行性[55]。除了ERCP外,經皮肝穿刺膽道造影(PTC)也是可行的選擇;核磁共振膽胰管造影(MRCP)則是一種非侵入性的替代方法[56][57][58],有些研究者建議以MRCP取代ERCP,因為MRCP能更精準地確認腫瘤並避免ERCP操作的風險[59][60][61]。
手術
外科手術是取得組織切片和準確得知膽管癌病程的唯一方法,分期腹腔鏡手術在特定情形下可以替代更具侵入性的剖腹手術 [46][62];以手術切除腫瘤是目前唯一能治癒膽管癌的方法,但只適用於尚未遠端轉移的病患[63]。
病理
膽管癌在組織學上歸類為中度到高度分化的腺癌。免疫組織化學方法在診斷上很有用,能協助醫師區別膽管癌、肝細胞癌與其他腸胃道癌的後期轉移[64]。細胞刮削通常對膽管癌診斷的精確度很低[65],這和癌細胞周圍促纖維細胞產生的基質有關[66]。
分期
目前至少有三種癌症分期系統用於膽管癌(Bismuth系統、Blumgart系統和美國癌症聯合委員會的系統),但沒有一種能有效預測患者存活率[67]。癌症分期最重要的問題在於腫瘤能否成功地以手術移除,但對膽管癌而言,答案通常要到手術進行當下才能判定。
治療
膽管癌是個難以醫治且快速致死的疾病,只有在腫瘤能以手術根除的情況下才有機會治癒。除非已有明確證據指出患者無法進行手術,多數案例要到手術進行當下才能評估手術成功率[69],因此患者多會先進行一次試探性手術[13]。馬約診所使用標準化的肝臟移植手術和嚴格的手術篩選條件,在早期膽管癌治療中取得顯著的成功[70];肝臟移植後的輔助性療法在無法切除腫瘤的特定個案中有明显的作用[71]。
輔助性化療和放療
由於有高達85%的膽管癌患者在手術後三年內復發,患者術後常會使用輔助性化療或放療以期增加治癒機會[72]。若術後腫瘤組織邊緣呈陰性反應(例如腫瘤已完全清除),輔助療法不一定能帶來好處,報告指出輔助性放療可能產生正面[73][74]或負面[12][75][76]的結果;對於腫瘤已成功清除的病人而言,輔助性化療似乎也沒有意义[77]。結合化療和放療的效果目前並不清楚[78]。若術後腫瘤組織邊緣呈陽性反應,代表腫瘤沒有根除,一般會建議進行輔助性放療和化療[79]。目前的研究結果顯示化療的效果似乎有優於放療的趨勢[72],針對化療藥物吉西他濱/順鉑的第三期隨機對照試驗已經在2014年註冊[80]。
後期治療
膽管癌絕大多數的病例都無法以手術根除[81],患者通常會接受緩和性化療,也可能配合放射治療。隨機對照試驗的結果顯示化療在這些患者中能增進生活品質並延長壽命[82]。目前沒有單一的化療用藥常規,若狀況允許,也常會建議患者申請臨床試驗[79]。治療膽管癌的化療藥物包括5-氟尿嘧啶/亞葉酸 [83]、單用吉西他濱 [84]或吉西他濱加上順鉑 [85]、愛萊諾迪肯[86]或卡培他濱[87]。一個小型的可行性研究指出酪氨酸激酶抑制劑厄洛替尼在後期膽管癌患者中可能是有益的[88]。
病程
以手術移除腫瘤是目前唯一可能治癒膽管癌的方法。由於癌細胞會透過淋巴結遠端轉移,無法切除腫瘤的患者五年存活率為0%[89];全部膽管癌患者的五年存活率則為5%[90]。若無法切除的腫瘤藉由肝內膽管或肝門靜脈侵犯肝臟,即使患者其餘生理機能正常,其餘命的中位數也小於6個月[91]。
對能進行手術的病例而言,手術成功的機率取決於腫瘤的位置,以及腫瘤是否能加以根除。遠端型膽管癌(腫瘤發生於總膽管)患者通常會進行胰十二指腸切除術,長期存活率為15%–25%,另有一系列報告指出腫瘤未侵犯到淋巴結的患者有54%的五年存活率[92]。肝內膽管癌(腫瘤發生於肝內膽管)患者通常會將部分肝臟移除,不同的系列報告估計術後存活率為22%–66%,存活率差異取決於手術移除的完整度和癌細胞是否侵犯到淋巴結[93]。肝門型膽管癌(腫瘤發生於肝外膽管與肝臟相接處,又稱克拉茨金瘤)通常無法進行手術,只有少數罕見的病例有開刀治療的可能,手術過程通常會包括膽囊切除術或部分肝臟移除,能進行手術的肝門型膽管癌患者五年存活率為20%–50%[94]。
由原發性硬化性膽管炎發展而來的膽管癌患者情況通常更糟,這或許是因為腫瘤在診斷出來前就已經惡化[17][95]。一些臨床證據指出入侵性較高的手術和佐劑治療可能會有較好的效果[96]。
流行病學

膽管癌是一種相對少見的癌症,美國每年大約有2000到3000樁新病例,換算成年發生率約每十萬人1至2例[97],法醫病理研究指出盛行率約0.01% - 0.46%[98][99]。由於區域性流行病的緣故,膽管癌在亞洲有較高的流行率,在上海、韓國和泰國北部的發生率都已超過罕見癌症的標準(大於每年每十萬人6例)[9],尤其是泰國北部的年發生率高達每十萬人14.6例以上,統合分析研究指出中華肝吸蟲和香貓肝吸蟲是最主要的風險因子[100]。台灣的膽管癌年發生率為每十萬人4.7例,屬於罕見癌症,但發生率仍較西方國家高出許多,台灣的中華肝吸蟲感染盛行率雖然已經大幅降低,但另一項西方國家很少見的風險因子——肝內膽管結石和台灣的膽管癌病例則有強烈關聯[100]。
膽管癌的發生率會隨著年齡增加,男性發生的機率稍微較女性高(可能是因為男性有較高比例的原發性硬化性膽管炎患者)[101]。根據法醫病理研究,帶有原發性硬化性膽管炎的患者膽管癌流行率可能高達30%[17]。多份研究紀錄指出截至二十世紀末為止,北美、歐洲、亞洲和澳洲的肝內膽管癌發生率都有上升的趨勢[102],並於2000年後逐漸趨於穩定[103],肝內膽管癌發生率上升的原因目前並不清楚,診斷方法的進步可能有關,但潛在風險因子的盛行率(例如愛滋病感染)在同個時期也有增加,因此可能也扮演一定的角色[26]。然而,近年來肝外膽管癌和肝門膽管癌的發生率卻呈現下降的趨勢[103]。美國可能由於有完善的醫療照護,膽管癌患者的住院人數和院內死亡率也都趨於降低。[104]
| 國家 | 肝內膽管癌 (男/女) | 肝外膽管癌 (男/女) |
|---|---|---|
| 美國 | 0.60 / 0.43 | 0.70 / 0.87 |
| 日本 | 0.23 / 0.10 | 5.87 / 5.20 |
| 澳洲 | 0.70 / 0.53 | 0.90 / 1.23 |
| 英格蘭/威爾斯 | 0.83 / 0.63 | 0.43 / 0.60 |
| 蘇格蘭 | 1.17 / 1.00 | 0.60 / 0.73 |
| 法國 | 0.27 / 0.20 | 1.20 / 1.37 |
| 義大利 | 0.13 / 0.13 | 2.10 / 2.60 |
研究
近期研究顯示不同患者的癌細胞具有高度的基因變異,這和腫瘤發生位置、造成疾病的風險因子等都有關聯[106],研究者正嘗試由手術切除的組織篩選患者腫瘤細胞的基因型,以施予患者特定的標靶藥物[107]。隨著膽管癌細胞生成和腫瘤微環境的可能分子路徑正式提出,阻斷這些路徑的抑制劑也成為治療膽管癌的候選藥物[108]。光動力治療則是使用對特定光波長敏感的藥物的新型療法,隨機對照試驗的結果顯示此療法對無法手術切除腫瘤的患者而言能有效提高存活率[109][110]。另外,偵測腫瘤基質細胞副產物在血液中濃度的技術也正在發展,此方法可用於協助癌症診斷[111]。
參考資料
- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 Bile Duct Cancer (Cholangiocarcinoma) Treatment (PDQ®)–Health Professional Version. National Cancer Institute. 14 March 2018 [21 January 2019]. (原始内容存档于2019-07-09) (英语).
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Razumilava, N; Gores, GJ. Cholangiocarcinoma.. Lancet. 21 June 2014, 383 (9935): 2168–79. PMID 24581682. doi:10.1016/S0140-6736(13)61903-0.
- ^ 3.0 3.1 3.2 3.3 Bile Duct Cancer (Cholangiocarcinoma) Symptoms, Tests, Prognosis, and Stages. National Cancer Institute. 5 July 2018 [21 January 2019]. (原始内容存档于2019-07-08) (英语).
- ^ 4.0 4.1 4.2 Bosman, Frank T. Chapter Chapter 5.6: Liver cancer. Stewart, Bernard W.; Wild, Christopher P (编). World Cancer Report. the International Agency for Research on Cancer, World Health Organization. 2014: Chapter 5.6 [2019-02-06]. ISBN 978-92-832-0443-5. (原始内容存档于2016-09-19).

- ^ 5.0 5.1 5.2 5.3 Bridgewater, JA; Goodman, KA; Kalyan, A; Mulcahy, MF. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling.. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting. 2016, 35: e194–203. PMID 27249723. doi:10.1200/EDBK_160831.
- ^ NCI Dictionary of Cancer Terms. National Cancer Institute. 2 February 2011 [21 January 2019]. (原始内容存档于2019-07-09) (英语).
- ^ Benavides, M; Antón, A; Gallego, J; Gómez, MA; Jiménez-Gordo, A; La Casta, A; Laquente, B; Macarulla, T; Rodríguez-Mowbray, JR; Maurel, J. Biliary tract cancers: SEOM clinical guidelines.. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. December 2015, 17 (12): 982–7. PMID 26607930. doi:10.1007/s12094-015-1436-2.
- ^ Steele, JA; Richter, CH; Echaubard, P; Saenna, P; Stout, V; Sithithaworn, P; Wilcox, BA. Thinking beyond Opisthorchis viverrini for risk of cholangiocarcinoma in the lower Mekong region: a systematic review and meta-analysis.. Infectious diseases of poverty. 17 May 2018, 7 (1): 44. PMID 29769113. doi:10.1186/s40249-018-0434-3.
- ^ 9.0 9.1 9.2 Bragazzi MC, Cardinale V, Carpino G, Venere R, Semeraro R, Gentile R, Gaudio E, Alvaro D. Cholangiocarcinoma: epidemiology and risk factors. Translational Gastrointestinal Cancer. 2011, 1 (1): 21–32. doi:10.3978/j.issn.2224-4778.2011.11.04.
- ^ Nagorney D, Donohue J, Farnell M, Schleck C, Ilstrup D. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993, 128 (8): 871–7; discussion 877–9. PMID 8393652. doi:10.1001/archsurg.1993.01420200045008.
- ^ Bile duct cancer: cause and treatment. [2016-06-04]. (原始内容存档于2018-04-23).
- ^ 12.0 12.1 Nakeeb A, Pitt H, Sohn T, Coleman J, Abrams R, Piantadosi S, Hruban R, Lillemoe K, Yeo C, Cameron J. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996, 224 (4): 463–73; discussion 473–5. PMC 1235406
 . PMID 8857851. doi:10.1097/00000658-199610000-00005.
. PMID 8857851. doi:10.1097/00000658-199610000-00005.
- ^ 13.0 13.1 13.2 Mark Feldman; Lawrence S. Friedman; Lawrence J. Brandt (编). Sleisenger and Fordtran's Gastrointestinal and Liver Disease 8th. Saunders. 21 July 2006: 1493–6. ISBN 978-1-4160-0245-1.
- ^ Levy, Cynthia; Lymp, James; Angulo, Paul; Gores, Gregory J.; Larusso, Nicholas; Lindor, Keith D. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Digestive Diseases and Sciences. 2005-09-01, 50 (9): 1734–1740. ISSN 0163-2116. PMID 16133981. doi:10.1007/s10620-005-2927-8.
- ^ Chapman R. Risk factors for biliary tract carcinogenesis. Ann Oncol. 1999, 10 (Suppl 4): 308–11. PMID 10436847. doi:10.1023/A:1008313809752.
- ^ 下列為有關原發性硬化膽管炎及膽管癌的相關性研究:
- Bergquist A, Ekbom A, Olsson R, Kornfeldt D, Lööf L, Danielsson A, Hultcrantz R, Lindgren S, Prytz H, Sandberg-Gertzén H, Almer S, Granath F, Broomé U. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002, 36 (3): 321–7. PMID 11867174. doi:10.1016/S0168-8278(01)00288-4.
- Bergquist A, Glaumann H, Persson B, Broomé U. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology. 1998, 27 (2): 311–6. PMID 9462625. doi:10.1002/hep.510270201.
- Burak K, Angulo P, Pasha T, Egan K, Petz J, Lindor K. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004, 99 (3): 523–6. PMID 15056096. doi:10.1111/j.1572-0241.2004.04067.x.
- ^ 17.0 17.1 17.2 Rosen C, Nagorney D, Wiesner R, Coffey R, LaRusso N. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann Surg. 1991, 213 (1): 21–5. PMC 1358305
 . PMID 1845927. doi:10.1097/00000658-199101000-00004.
. PMID 1845927. doi:10.1097/00000658-199101000-00004.
- ^ Watanapa P, Watanapa W. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002, 89 (8): 962–70. PMID 12153620. doi:10.1046/j.1365-2168.2002.02143.x.
- ^ Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Medicine. 2007, 4 (7): 1148–1155. PMC 1913093
 . PMID 17622191. doi:10.1371/journal.pmed.0040201.
. PMID 17622191. doi:10.1371/journal.pmed.0040201.
- ^ Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010, 72: 305–350. PMID 20624536. doi:10.1016/S0065-308X(10)72011-X.
- ^ Rustagi T, Dasanu CA. Risk Factors for Gallbladder Cancer and Cholangiocarcinoma: Similarities, Differences and Updates. J Gastrointest Cancer. 2012, 43 (2): 137–147. PMID 21597894. doi:10.1007/s12029-011-9284-y.
- ^ Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012, 61 (1): 17–24. PMID 21741496. doi:10.1016/j.parint.2011.06.007.
- ^ Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000, 88 (11): 2471–7. PMID 10861422. doi:10.1002/1097-0142(20000601)88:11<2471::AID-CNCR7>3.0.CO;2-T.
- ^ Yamamoto S, Kubo S, Hai S, Uenishi T, Yamamoto T, Shuto T, Takemura S, Tanaka H, Yamazaki O, Hirohashi K, Tanaka T. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci. 2004, 95 (7): 592–5. PMID 15245596. doi:10.1111/j.1349-7006.2004.tb02492.x.
- ^ Lu H, Ye M, Thung S, Dash S, Gerber M. Detection of hepatitis C virus RNA sequences in cholangiocarcinomas in Chinese and American patients. Chin Med J (Engl). 2000, 113 (12): 1138–41. PMID 11776153.
- ^ 26.0 26.1 26.2 Shaib Y, El-Serag H, Davila J, Morgan R, McGlynn K. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005, 128 (3): 620–6. PMID 15765398. doi:10.1053/j.gastro.2004.12.048.
- ^ Sorensen H, Friis S, Olsen J, Thulstrup A, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998, 28 (4): 921–5. PMID 9755226. doi:10.1002/hep.510280404.
- ^ Chang, A. H.; Parsonnet, J. Role of Bacteria in Oncogenesis. Clinical Microbiology Reviews. 2010, 23 (4): 837–857. ISSN 0893-8512. PMC 2952975
 . PMID 20930075. doi:10.1128/CMR.00012-10.
. PMID 20930075. doi:10.1128/CMR.00012-10.
- ^ Lipsett P, Pitt H, Colombani P, Boitnott J, Cameron J. Choledochal cyst disease. A changing pattern of presentation. Ann Surg. 1994, 220 (5): 644–52. PMC 1234452
 . PMID 7979612. doi:10.1097/00000658-199411000-00007.
. PMID 7979612. doi:10.1097/00000658-199411000-00007.
- ^ Dayton M, Longmire W, Tompkins R. Caroli's Disease: a premalignant condition?. Am J Surg. 1983, 145 (1): 41–8. PMID 6295196. doi:10.1016/0002-9610(83)90164-2.
- ^ Mecklin J, Järvinen H, Virolainen M. The association between cholangiocarcinoma and hereditary nonpolyposis colorectal carcinoma. Cancer. 1992, 69 (5): 1112–4. PMID 1310886. doi:10.1002/cncr.2820690508.
- ^ Lee S, Kim M, Lee S, Jang S, Song M, Kim K, Kim H, Seo D, Song D, Yu E, Lee S, Min Y. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004, 100 (4): 783–93. PMID 14770435. doi:10.1002/cncr.20031.
- ^ Lee C, Wu C, Chen G. What is the impact of coexistence of hepatolithiasis on cholangiocarcinoma?. J Gastroenterol Hepatol. 2002, 17 (9): 1015–20. PMID 12167124. doi:10.1046/j.1440-1746.2002.02779.x.
- ^ Su C, Shyr Y, Lui W, P'Eng F. Hepatolithiasis associated with cholangiocarcinoma. Br J Surg. 1997, 84 (7): 969–73. PMID 9240138. doi:10.1002/bjs.1800840717.
- ^ Donato F, Gelatti U, Tagger A, Favret M, Ribero M, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001, 12 (10): 959–64. PMID 11808716. doi:10.1023/A:1013747228572.
- ^ Sahani D, Prasad S, Tannabe K, Hahn P, Mueller P, Saini S. Thorotrast-induced cholangiocarcinoma: case report. Abdom Imaging. 2003, 28 (1): 72–4. PMID 12483389. doi:10.1007/s00261-001-0148-y.
- ^ Zhu A, Lauwers G, Tanabe K. Cholangiocarcinoma in association with Thorotrast exposure. J Hepatobiliary Pancreat Surg. 2004, 11 (6): 430–3. PMID 15619021. doi:10.1007/s00534-004-0924-5.
- ^ Klatskin G. Adenocarcinoma Of The Hepatic Duct At Its Bifurcation Within The Porta Hepatis. An Unusual Tumor With Distinctive Clinical And Pathological Features. Am J Med. 1965, 38 (2): 241–56. PMID 14256720. doi:10.1016/0002-9343(65)90178-6.
- ^ Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006, 25 (27): 3818–22. PMID 16799623. doi:10.1038/sj.onc.1209558.
- ^ Liu C, Wang J, Ou Q. Possible stem cell origin of human cholangiocarcinoma. World J Gastroenterol. 2004, 10 (22): 3374–6. PMID 15484322.
- ^ Sell S, Dunsford H. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989, 134 (6): 1347–63. PMC 1879951
 . PMID 2474256.
. PMID 2474256.
- ^ 42.0 42.1 Sirica A. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005, 41 (1): 5–15. PMID 15690474. doi:10.1002/hep.20537.
- ^ Holzinger F, Z'graggen K, Büchler M. Mechanisms of biliary carcinogenesis: a pathogenetic multi-stage cascade towards cholangiocarcinoma. Ann Oncol. 1999, 10 (Suppl 4): 122–6. PMID 10436802. doi:10.1023/A:1008321710719.
- ^ Gores G. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003, 37 (5): 961–9. PMID 12717374. doi:10.1053/jhep.2003.50200.
- ^ de Groen P, Gores G, LaRusso N, Gunderson L, Nagorney D. Biliary tract cancers. N Engl J Med. 1999, 341 (18): 1368–78. PMID 10536130. doi:10.1056/NEJM199910283411807.
- ^ 46.0 46.1 Weber S, DeMatteo R, Fong Y, Blumgart L, Jarnagin W. Staging Laparoscopy in Patients With Extrahepatic Biliary Carcinoma: Analysis of 100 Patients. Ann Surg. 2002, 235 (3): 392–9. PMC 1422445
 . PMID 11882761. doi:10.1097/00000658-200203000-00011.
. PMID 11882761. doi:10.1097/00000658-200203000-00011.
- ^ Razumilava, Nataliya; Gores, Gregory J.; Lindor, Keith D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011, 54 (5): 1842–1852. ISSN 0270-9139. doi:10.1002/hep.24570.
- ^ 以下研究顯示了非原發性硬化性膽管炎引發的膽管癌有關的血液指標(如CA19-9)和其表現量:
- Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004, 24 (2): 139–54. PMID 15192787. doi:10.1055/s-2004-828891.
- Siqueira E, Schoen R, Silverman W, Martin J, Rabinovitz M, Weissfeld J, Abu-Elmaagd K, Madariaga J, Slivka A, Martini J. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc. 2002, 56 (1): 40–7. PMID 12085033. doi:10.1067/mge.2002.125105.
- Levy C, Lymp J, Angulo P, Gores G, Larusso N, Lindor K. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005, 50 (9): 1734–40. PMID 16133981. doi:10.1007/s10620-005-2927-8.
- Patel A, Harnois D, Klee G, LaRusso N, Gores G. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000, 95 (1): 204–7. PMID 10638584. doi:10.1111/j.1572-0241.2000.01685.x.
- ^ Saini S. Imaging of the hepatobiliary tract. N Engl J Med. 1997, 336 (26): 1889–94. PMID 9197218. doi:10.1056/NEJM199706263362607.
- ^ Sharma M, Ahuja V. Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective. Trop Gastroenterol. 1999, 20 (4): 167–9. PMID 10769604.
- ^ Bloom C, Langer B, Wilson S. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics. 1999, 19 (5): 1199–218. PMID 10489176. doi:10.1148/radiographics.19.5.g99se081199.
- ^ Valls C, Gumà A, Puig I, Sanchez A, Andía E, Serrano T, Figueras J. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000, 25 (5): 490–6. PMID 10931983. doi:10.1007/s002610000079.
- ^ Tillich M, Mischinger H, Preisegger K, Rabl H, Szolar D. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998, 171 (3): 651–8. PMID 9725291. doi:10.2214/ajr.171.3.9725291.
- ^ Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr. 1999, 23 (5): 670–7. PMID 10524843. doi:10.1097/00004728-199909000-00004.
- ^ Sugiyama M, Hagi H, Atomi Y, Saito M. Diagnosis of portal venous invasion by pancreatobiliary carcinoma: value of endoscopic ultrasonography. Abdom Imaging. 1997, 22 (4): 434–8. PMID 9157867. doi:10.1007/s002619900227.
- ^ Schwartz L, Coakley F, Sun Y, Blumgart L, Fong Y, Panicek D. Neoplastic pancreaticobiliary duct obstruction: evaluation with breath-hold MR cholangiopancreatography. AJR Am J Roentgenol. 1998, 170 (6): 1491–5. PMID 9609160. doi:10.2214/ajr.170.6.9609160.
- ^ Zidi S, Prat F, Le Guen O, Rondeau Y, Pelletier G. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut. 2000, 46 (1): 103–6. PMC 1727781
 . PMID 10601064. doi:10.1136/gut.46.1.103.
. PMID 10601064. doi:10.1136/gut.46.1.103.
- ^ Lee M, Park K, Shin Y, Yoon H, Sung K, Kim M, Lee S, Kang E. Preoperative evaluation of hilar cholangiocarcinoma with contrast-enhanced three-dimensional fast imaging with steady-state precession magnetic resonance angiography: comparison with intraarterial digital subtraction angiography. World J Surg. 2003, 27 (3): 278–83. PMID 12607051. doi:10.1007/s00268-002-6701-1.
- ^ Yeh T, Jan Y, Tseng J, Chiu C, Chen T, Hwang T, Chen M. Malignant perihilar biliary obstruction: magnetic resonance cholangiopancreatographic findings. Am J Gastroenterol. 2000, 95 (2): 432–40. PMID 10685746. doi:10.1111/j.1572-0241.2000.01763.x.
- ^ Freeman M, Sielaff T. A modern approach to malignant hilar biliary obstruction. Rev Gastroenterol Disord. 2003, 3 (4): 187–201. PMID 14668691.
- ^ Szklaruk J, Tamm E, Charnsangavej C. Preoperative imaging of biliary tract cancers. Surg Oncol Clin N Am. 2002, 11 (4): 865–76. PMID 12607576. doi:10.1016/S1055-3207(02)00032-7.
- ^ Callery M, Strasberg S, Doherty G, Soper N, Norton J. Staging laparoscopy with laparoscopic ultrasonography: optimizing resectability in hepatobiliary and pancreatic malignancy. J Am Coll Surg. 1997, 185 (1): 33–9. PMID 9208958. doi:10.1016/s1072-7515(97)00003-3.
- ^ 63.0 63.1 Tsao J, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, Miyachi M, Kanai M, Uesaka K, Oda K, Rossi R, Braasch J, Dugan J. Management of Hilar Cholangiocarcinoma: Comparison of an American and a Japanese Experience. Ann Surg. 2000, 232 (2): 166–74. PMC 1421125
 . PMID 10903592. doi:10.1097/00000658-200008000-00003.
. PMID 10903592. doi:10.1097/00000658-200008000-00003.
- ^ Länger F, von Wasielewski R, Kreipe HH. The importance of immunohistochemistry for the diagnosis of cholangiocarcinomas. Pathologe. 2006, 27 (4): 244–50. PMID 16758167. doi:10.1007/s00292-006-0836-z (德语).
- ^ Darwin PE, Kennedy A. Cholangiocarcinoma 於 eMedicine
- ^ Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointestinal endoscopy. 2014, 81 (1): 168–176. doi:10.1016/j.gie.2014.09.017.
- ^ Zervos E, Osborne D, Goldin S, Villadolid D, Thometz D, Durkin A, Carey L, Rosemurgy A. Stage does not predict survival after resection of hilar cholangiocarcinomas promoting an aggressive operative approach. Am J Surg. 2005, 190 (5): 810–5. PMID 16226963. doi:10.1016/j.amjsurg.2005.07.025.
- ^ Rajagopalan V, Daines W, Grossbard M, Kozuch P. Gallbladder and biliary tract carcinoma: A comprehensive update, Part 1. Oncology (Williston Park). 2004, 18 (7): 889–96. PMID 15255172.
- ^ Su C, Tsay S, Wu C, Shyr Y, King K, Lee C, Lui W, Liu T, P'eng F. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996, 223 (4): 384–94. PMC 1235134
 . PMID 8633917. doi:10.1097/00000658-199604000-00007.
. PMID 8633917. doi:10.1097/00000658-199604000-00007.
- ^ C. B. Rosen; J. K. Heimbach; G. J. Gores. Surgery for cholangiocarcinoma: the role of liver transplantation. The Official Journal of the International Hepato Pancreato Biliary Association. 2008, 10 (3): 186–9. PMC 2504373
 . PMID 18773052. doi:10.1080/13651820801992542.
. PMID 18773052. doi:10.1080/13651820801992542.
- ^ Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. December 2006, 82 (12): 1703–7 [2016-07-21]. PMID 17198263. doi:10.1097/01.tp.0000253551.43583.d1. (原始内容存档于2013-05-22).
- ^ 72.0 72.1 Vogel A, Wege H, Caca K, Nashan B, Neumann U. The Diagnosis and Treatment of Cholangiocarcinoma. Dtsch Arztebl Int. 2014, 111 (44): 748–754. PMC 4239580
 . doi:10.3238/arztebl.2014.0748.
. doi:10.3238/arztebl.2014.0748.
- ^ Todoroki T, Ohara K, Kawamoto T, Koike N, Yoshida S, Kashiwagi H, Otsuka M, Fukao K. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys. 2000, 46 (3): 581–7. PMID 10701737. doi:10.1016/S0360-3016(99)00472-1.
- ^ Alden M, Mohiuddin M. The impact of radiation dose in combined external beam and intraluminal Ir-192 brachytherapy for bile duct cancer. Int J Radiat Oncol Biol Phys. 1994, 28 (4): 945–51. PMID 8138448. doi:10.1016/0360-3016(94)90115-5.
- ^ González González D, Gouma D, Rauws E, van Gulik T, Bosma A, Koedooder C. Role of radiotherapy, in particular intraluminal brachytherapy, in the treatment of proximal bile duct carcinoma. Ann Oncol. 1999, 10 (Suppl 4): 215–20. PMID 10436826. doi:10.1023/A:1008339709327.
- ^ Pitt H, Nakeeb A, Abrams R, Coleman J, Piantadosi S, Yeo C, Lillemore K, Cameron J. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995, 221 (6): 788–97; discussion 797–8. PMC 1234714
 . PMID 7794082. doi:10.1097/00000658-199506000-00017.
. PMID 7794082. doi:10.1097/00000658-199506000-00017.
- ^ Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002, 95 (8): 1685–95. PMID 12365016. doi:10.1002/cncr.10831.
- ^ Howell M, Valle JW. The role of adjuvant chemotherapy and radiotherapy for cholangiocarcinoma. Best Practice & Research Clinical Gastroenterology. 2015, 29 (2): 333–343. PMID 10701737. doi:10.1016/j.bpg.2015.03.001.
- ^ 79.0 79.1 National Comprehensive Cancer Network (NCCN) guidelines on evaluation and treatment of hepatobiliary malignanciesPDF (216 KB). Accessed 13 March 2007.
- ^ Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klümpen HJ, ..., Shannon J. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial)-a randomized, multidisciplinary, multinational phase III trial. BMC cancer. 2015, 15 (1): 1. doi:10.1186/s12885-015-1498-0.
- ^ Vauthey J, Blumgart L. Recent advances in the management of cholangiocarcinomas. Semin. Liver Dis. 1994, 14 (2): 109–14. PMID 8047893. doi:10.1055/s-2007-1007302.
- ^ Glimelius B, Hoffman K, Sjödén P, Jacobsson G, Sellström H, Enander L, Linné T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996, 7 (6): 593–600. PMID 8879373. doi:10.1093/oxfordjournals.annonc.a010676.
- ^ Choi C, Choi I, Seo J, Kim B, Kim J, Kim C, Um S, Kim J, Kim Y. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J Clin Oncol. 2000, 23 (4): 425–8. PMID 10955877. doi:10.1097/00000421-200008000-00023.
- ^ Park J, Oh S, Kim S, Kwon H, Kim J, Jin-Kim H, Kim Y. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a phase II study. Jpn J Clin Oncol. 2005, 35 (2): 68–73. PMID 15709089. doi:10.1093/jjco/hyi021.
- ^ Giuliani F, Gebbia V, Maiello E, Borsellino N, Bajardi E, Colucci G. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol. 2006, 17 (Suppl 7): vii73–7. PMID 16760299. doi:10.1093/annonc/mdl956.
- ^ Bhargava P, Jani C, Savarese D, O'Donnell J, Stuart K, Rocha Lima C. Gemcitabine and irinotecan in locally advanced or metastatic biliary cancer: preliminary report. Oncology (Williston Park). 2003, 17 (9 Suppl 8): 23–6. PMID 14569844.
- ^ Knox J, Hedley D, Oza A, Feld R, Siu L, Chen E, Nematollahi M, Pond G, Zhang J, Moore M. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005, 23 (10): 2332–8. PMID 15800324. doi:10.1200/JCO.2005.51.008.
- ^ Philip P, Mahoney M, Allmer C, Thomas J, Pitot H, Kim G, Donehower R, Fitch T, Picus J, Erlichman C. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006, 24 (19): 3069–74. PMID 16809731. doi:10.1200/JCO.2005.05.3579.
- ^ Yamamoto M, Takasaki K, Yoshikawa T. Lymph Node Metastasis in Intrahepatic Cholangiocarcinoma. Japanese Journal of Clinical Oncology. 1999, 29 (3): 147–150. PMID 10225697. doi:10.1093/jjco/29.3.147.
- ^ Farley D, Weaver A, Nagorney D. "Natural history" of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995, 70 (5): 425–9. PMID 7537346. doi:10.4065/70.5.425.
- ^ Grove MK, Hermann RE, Vogt DP, Broughan TA. Role of radiation after operative palliation in cancer of the proximal bile ducts. Am J Surg. 1991, 161 (4): 454–458. PMID 1709795. doi:10.1016/0002-9610(91)91111-U.
- ^ 以下為有關遠端膽管癌手術治療結果的研究:
- Nakeeb A, Pitt H, Sohn T, Coleman J, Abrams R, Piantadosi S, Hruban R, Lillemoe K, Yeo C, Cameron J. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996, 224 (4): 463–73; discussion 473–5. PMC 1235406
 . PMID 8857851. doi:10.1097/00000658-199610000-00005.
. PMID 8857851. doi:10.1097/00000658-199610000-00005. - Nagorney D, Donohue J, Farnell M, Schleck C, Ilstrup D. Outcomes after curative resections of cholangiocarcinoma. Arch Surg. 1993, 128 (8): 871–7; discussion 877–9. PMID 8393652. doi:10.1001/archsurg.1993.01420200045008.
- Jang J, Kim S, Park D, Ahn Y, Yoon Y, Choi M, Suh K, Lee K, Park Y. Actual Long-term Outcome of Extrahepatic Bile Duct Cancer After Surgical Resection. Ann Surg. 2005, 241 (1): 77–84. PMC 1356849
 . PMID 15621994. doi:10.1097/01.sla.0000150166.94732.88.
. PMID 15621994. doi:10.1097/01.sla.0000150166.94732.88. - Bortolasi L, Burgart L, Tsiotos G, Luque-De León E, Sarr M. Adenocarcinoma of the distal bile duct. A clinicopathologic outcome analysis after curative resection. Dig Surg. 2000, 17 (1): 36–41. PMID 10720830. doi:10.1159/000018798.
- Fong Y, Blumgart L, Lin E, Fortner J, Brennan M. Outcome of treatment for distal bile duct cancer. Br J Surg. 1996, 83 (12): 1712–5. PMID 9038548. doi:10.1002/bjs.1800831217.
- Nakeeb A, Pitt H, Sohn T, Coleman J, Abrams R, Piantadosi S, Hruban R, Lillemoe K, Yeo C, Cameron J. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996, 224 (4): 463–73; discussion 473–5. PMC 1235406
- ^ 下列研究指出了肝內膽管癌的治療結果:
- Nakeeb A, Pitt H, Sohn T, Coleman J, Abrams R, Piantadosi S, Hruban R, Lillemoe K, Yeo C, Cameron J. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996, 224 (4): 463–73; discussion 473–5. PMC 1235406
 . PMID 8857851. doi:10.1097/00000658-199610000-00005.
. PMID 8857851. doi:10.1097/00000658-199610000-00005. - Lieser M, Barry M, Rowland C, Ilstrup D, Nagorney D. Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg. 1998, 5 (1): 41–7. PMID 9683753. doi:10.1007/PL00009949.
- Valverde A, Bonhomme N, Farges O, Sauvanet A, Flejou J, Belghiti J. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg. 1999, 6 (2): 122–7. PMID 10398898. doi:10.1007/s005340050094.
- Nakagohri T, Asano T, Kinoshita H, Kenmochi T, Urashima T, Miura F, Ochiai T. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg. 2003, 27 (3): 289–93. PMID 12607053. doi:10.1007/s00268-002-6696-7.
- Weber S, Jarnagin W, Klimstra D, DeMatteo R, Fong Y, Blumgart L. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001, 193 (4): 384–91. PMID 11584966. doi:10.1016/S1072-7515(01)01016-X.
- Nakeeb A, Pitt H, Sohn T, Coleman J, Abrams R, Piantadosi S, Hruban R, Lillemoe K, Yeo C, Cameron J. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996, 224 (4): 463–73; discussion 473–5. PMC 1235406
- ^ 以下報告估算了肝門型膽管癌患者的術後存活率:
- Burke E, Jarnagin W, Hochwald S, Pisters P, Fong Y, Blumgart L. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998, 228 (3): 385–94. PMC 1191497
 . PMID 9742921. doi:10.1097/00000658-199809000-00011.
. PMID 9742921. doi:10.1097/00000658-199809000-00011. - Tsao J, Nimura Y, Kamiya J, Hayakawa N, Kondo S, Nagino M, Miyachi M, Kanai M, Uesaka K, Oda K, Rossi R, Braasch J, Dugan J. Management of Hilar Cholangiocarcinoma: Comparison of an American and a Japanese Experience. Ann Surg. 2000, 232 (2): 166–74. PMC 1421125
 . PMID 10903592. doi:10.1097/00000658-200008000-00003.
. PMID 10903592. doi:10.1097/00000658-200008000-00003. - Chamberlain R, Blumgart L. Hilar cholangiocarcinoma: a review and commentary. Ann Surg Oncol. 2000, 7 (1): 55–66. PMID 10674450. doi:10.1007/s10434-000-0055-4.
- Washburn W, Lewis W, Jenkins R. Aggressive surgical resection for cholangiocarcinoma. Arch Surg. 1995, 130 (3): 270–6. PMID 7534059. doi:10.1001/archsurg.1995.01430030040006.
- Nagino M, Nimura Y, Kamiya J, Kanai M, Uesaka K, Hayakawa N, Yamamoto H, Kondo S, Nishio H. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998, 45 (19): 7–13. PMID 9496478.
- Rea D, Munoz-Juarez M, Farnell M, Donohue J, Que F, Crownhart B, Larson D, Nagorney D. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 2004, 139 (5): 514–23; discussion 523–5. PMID 15136352. doi:10.1001/archsurg.139.5.514.
- Launois B, Reding R, Lebeau G, Buard J. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000, 7 (2): 128–34. PMID 10982604. doi:10.1007/s005340050166.
- Burke E, Jarnagin W, Hochwald S, Pisters P, Fong Y, Blumgart L. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998, 228 (3): 385–94. PMC 1191497
- ^ Kaya M, de Groen P, Angulo P, Nagorney D, Gunderson L, Gores G, Haddock M, Lindor K. Treatment of cholangiocarcinoma complicating primary sclerosing cholangitis: the Mayo Clinic experience. Am J Gastroenterol. 2001, 96 (4): 1164–9. PMID 11316165. doi:10.1111/j.1572-0241.2001.03696.x.
- ^ Nakeeb A, Tran K, Black M, Erickson B, Ritch P, Quebbeman E, Wilson S, Demeure M, Rilling W, Dua K, Pitt H. Improved survival in resected biliary malignancies. Surgery. 2002, 132 (4): 555–63; discission 563–4. PMID 12407338. doi:10.1067/msy.2002.127555.
- ^ Landis S, Murray T, Bolden S, Wingo P. Cancer statistics, 1998. CA Cancer J Clin. 1998, 48 (1): 6–29. PMID 9449931. doi:10.3322/canjclin.48.1.6.
- ^ Vauthey J, Blumgart L. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994, 14 (2): 109–14. PMID 8047893. doi:10.1055/s-2007-1007302.
- ^ Cancer Statistics Home Page — National Cancer Institute. [2016-07-21]. (原始内容存档于2015-04-29).
- ^ 100.0 100.1 Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Science. 2010, 101 (3): 579–585. PMID 20085587. doi:10.1111/j.1349-7006.2009.01458.x.
- ^ Henson D, Albores-Saavedra J, Corle D. Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates. Cancer. 1992, 70 (6): 1498–501. PMID 1516001. doi:10.1002/1097-0142(19920915)70:6<1498::AID-CNCR2820700609>3.0.CO;2-C.
- ^ 有關二十世紀末膽管癌發生率的論文如下:
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002, 2: 10. PMC 113759
 . PMID 11991810. doi:10.1186/1471-2407-2-10.
. PMID 11991810. doi:10.1186/1471-2407-2-10. - Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001, 33 (6): 1353–7. PMID 11391522. doi:10.1053/jhep.2001.25087.
- Shaib Y, Davila J, McGlynn K, El-Serag H. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?. J Hepatol. 2004, 40 (3): 472–7. PMID 15123362. doi:10.1016/j.jhep.2003.11.030.
- West J, Wood H, Logan R, Quinn M, Aithal G. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006, 94 (11): 1751–8. PMC 2361300
 . PMID 16736026. doi:10.1038/sj.bjc.6603127.
. PMID 16736026. doi:10.1038/sj.bjc.6603127. - Khan S, Taylor-Robinson S, Toledano M, Beck A, Elliott P, Thomas H. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002, 37 (6): 806–13. PMID 12445422. doi:10.1016/S0168-8278(02)00297-0.
- Welzel T, McGlynn K, Hsing A, O'Brien T, Pfeiffer R. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006, 98 (12): 873–5. PMID 16788161. doi:10.1093/jnci/djj234.
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002, 2: 10. PMC 113759
- ^ 103.0 103.1 Banales, Jesus M.; Cardinale, Vincenzo; Carpino, Guido; Marzioni, Marco; Andersen, Jesper B.; Invernizzi, Pietro; Lind, Guro E.; Folseraas, Trine; Forbes, Stuart J.; Fouassier, Laura; Geier, Andreas; Calvisi, Diego F.; Mertens, Joachim C.; Trauner, Michael; Benedetti, Antonio; Maroni, Luca; Vaquero, Javier; Macias, Rocio I. R.; Raggi, Chiara; Perugorria, Maria J.; Gaudio, Eugenio; Boberg, Kirsten M.; Marin, Jose J. G.; Alvaro, Domenico. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nature Reviews Gastroenterology & Hepatology. 2016, 13 (5): 261–280. ISSN 1759-5045. doi:10.1038/nrgastro.2016.51.
- ^ Wadhwa, Vaibhav; Jobanputra, Yash; Thota, Prashanthi N.; Narayanan Menon, K.V.; Parsi, Mansour A.; Sanaka, Madhusudhan R. Healthcare utilization and costs associated with cholangiocarcinoma. Gastroenterology Report. 2016: gow026. ISSN 2052-0034. doi:10.1093/gastro/gow026.
- ^ Khan S, Taylor-Robinson S, Toledano M, Beck A, Elliott P, Thomas H. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002, 37 (6): 806–13. PMID 12445422. doi:10.1016/S0168-8278(02)00297-0.
- ^ Cardinale, Vincenzo. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World Journal of Gastrointestinal Oncology. 2012, 4 (5): 94. ISSN 1948-5204. doi:10.4251/wjgo.v4.i5.94.
- ^ Voss J, Holtegaard L, Kerr S, Fritcher E, Roberts L, Gores G, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Human pathology. 2013, 44 (7): 1216–1222. doi:10.1016/j.humpath.2012.11.006.
- ^ Patel, Tushar; Borad, Mitesh; Rizvi, Sumera; Gores, Gregory. Cholangiocarcinoma: Molecular Pathways and Therapeutic Opportunities. Seminars in Liver Disease. 2014, 34 (04): 456–464. ISSN 0272-8087. doi:10.1055/s-0034-1394144.
- ^ Zoepf, Thomas; Jakobs, Ralf; Arnold, Joachim C.; Apel, Darius; Riemann, Jurgen F. Palliation of Nonresectable Bile Duct Cancer: Improved Survival After Photodynamic Therapy. The American Journal of Gastroenterology. 2005, 100 (11): 2426–2430. ISSN 0002-9270. doi:10.1111/j.1572-0241.2005.00318.x.
- ^ Ortner, Marianne E.J; Caca, Karel; Berr, Frieder; Liebetruth, Jochen; Mansmann, Ulrich; Huster, Dominik; Voderholzer, Winfried; Schachschal, Guido; Mössner, Joachim; Lochs, Herbert. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003, 125 (5): 1355–1363. ISSN 0016-5085. doi:10.1016/j.gastro.2003.07.015.
- ^ Alphonse ES, Gregory JG. Desmoplastic Stroma and Cholangiocarcinoma: Clinical Implications and Therapeutic Targeting. Hepatology. 2014, 59 (6): 2397–2402. doi:10.1002/hep.26762.
外部連結
- 美國癌症協會對膽管癌的詳細指引 (英語)
- 美國國家癌症中心給肝外膽管癌病患的相關資訊 (英語)
- 美國國家癌症中心對膽管癌的介紹 (英語)
- 膽管癌基金會:提供膽管癌病患和其家屬、親友、照護者相關的資源。(英語)
- Alan Morement 紀念基金會:英國唯一致力於膽管癌的慈善團體。(英語)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
