2,5-二甲氧基苯乙胺衍生物
外觀

2,5-二甲氧基苯乙胺衍生物,也稱為2C類化合物(英語:2C-x)或2C家族(英語:2C family)是苯乙胺衍生物中的一類,苯基上2號位與5號位的氫被甲氧基取代,即以2,5-二甲氧基苯乙胺(也稱為2C-H)為母結構,其中絕大多數的取代基位於4號位,少數位於3號位,6號位基本上沒有取代基[1]。許多這一類化合物由亞歷山大·舒爾金在1970年代到1980年代首次合成[2]。
衍生物
[編輯]| 名稱 | R3基團 | R4基團 | R6基團 | 分子式 | 2D結構 | CAS號 |
|---|---|---|---|---|---|---|
| 2C-B | H | Br | H | C 10H 14BrNO 2 |

|
66142-81-2 |
| 2C-Bn | H | CH2C6H5 | H | C17H21NO2 | 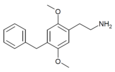
|
2888537-43-5 |
| 2C-Bu | H | CH2CH2CH2CH3 | H | C14H23NO2 | 
|
2888537-44-6 |
| 2C-C | H | Cl | H | C10H14ClNO2 | 
|
88441-14-9 |
| 2C-C-3 [3] | Cl | Cl | Cl | C10H12Cl3NO2 | 
|
1112937-89-9 |
| 2C-CN | H | C≡N | H | C11H14N2O2 | 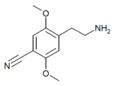
|
88441-07-0 |
| 2C-cP | H | C3H5 | H | C13H19NO2 | 
|
2888537-46-8 |
| 2C-D | H | CH3 | H | C11H17NO2 | 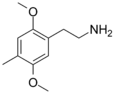
|
24333-19-5 |
| 2C-E | H | CH2CH3 | H | C12H19NO2 | 
|
71539-34-9 |
| 2C-EF | H | CH2CH2F | H | C12H18FNO2 | 
|
1222814-77-8 |
| 2C-F | H | F | H | C10H14FNO2 | 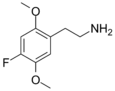
|
207740-15-6 |
| 2C-G | CH3 | CH3 | H | C12H19NO2 | 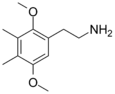
|
207740-18-9 |
| 2C-G-1 | CH2 | H | C11H15NO2 | 
|
2888537-47-9 | |
| 2C-G-2 | (CH2)2 | H | C12H17NO2 | 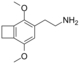
|
2888537-48-0 | |
| 2C-G-3 | (CH2)3 | H | C13H19NO2 | 
|
207740-19-0 | |
| 2C-G-4 | (CH2)4 | H | C14H21NO2 | 
|
952006-59-6 | |
| 2C-G-5 | (CH2)5 | H | C15H21NO2 | 
|
207740-20-3 | |
| 2C-G-6 | (CH2)6 | H | C16H23NO2 | 
|
2888537-49-1 | |
| 2C-G-N | (CH)4 | H | C14H17NO2 | 
|
207740-21-4 | |
| 2C-H | H | H | H | C10H15NO2 | 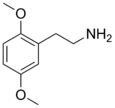
|
3600-86-0 |
| 2C-I | H | I | H | C10H14INO2 | 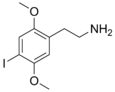
|
69587-11-7 |
| 2C-iP | H | CH(CH3)2 | H | C13H21NO2 | 
|
1498978-47-4 |
| 2C-N | H | NO2 | H | C10H14N2O4 | 
|
261789-00-8 |
| 2C-NH2 | H | NH2 | H | C10H16N2O2 | 
|
168699-66-9 |
| 2C-PYR | H | 吡咯烷基 | H | C14H22N2O2 | 
|
910381-23-6 |
| 2C-PIP | H | 哌啶基 | H | C15H24N2O2 | 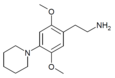
|
1898118-63-2 |
| 2C-O | H | OCH3 | H | C11H17NO3 | 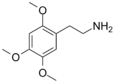
|
15394-83-9 |
| 2C-O-4 | H | OCH(CH3)2 | H | C13H21NO3 | 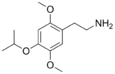
|
952006-65-4 |
| 2C-MOM [4] | H | CH2OCH3 | H | C12H19NO3 | 
|
1898203-98-9 |
| 2C-P | H | CH2CH2CH3 | H | C13H21NO2 | 
|
207740-22-5 |
| 2C-Ph | H | C6H5 | H | C16H19NO2 | 
|
1217170-12-1 |
| 2C-Se | H | SeCH3 | H | C11H17NO2Se | 
|
1189246-68-1 |
| 2C-T | H | SCH3 | H | C11H17NO2S | 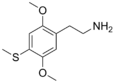
|
61638-09-3 |
| 2C-DFM [5]:770 | H | CHF2 | H | C11H15F2NO2 | 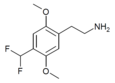
|
1891474-10-4 |
| 2C-TFM | H | CF3 | H | C11H14F3NO2 | 
|
159277-08-4 |
| 2C-TFE | H | CH2CF3 | H | C12H16F3NO2 | 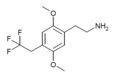
|
2888537-56-0 |
| 2C-PFE | H | CF2CF3 | H | C12H14F5NO2 | 
|
暫未註冊 |
| 2C-PFS | H | SF5 | H | C10H14F5NO2S | 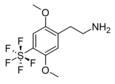
|
暫未註冊 |
| 2C-YN | H | C≡CH | H | C12H15NO2 | 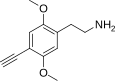
|
752982-24-4 |
| 2C-V | H | CH=CH2 | H | C12H17NO2 | 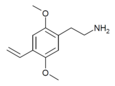
|
2888537-57-1 |
| 2C-AL[6] | H | CH2CH=CH2 | H | C13H19NO2 | 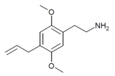
|
2756686-02-7 |
含硫衍生物
[編輯]| 名稱 | R3基團 | R4基團 | R6基團 | 分子式 | 2D結構 | CAS號 |
|---|---|---|---|---|---|---|
| 2C-T | H | SCH3 | H | C11H17NO2S | 
|
61638-09-3 |
| 2C-T-2 | H | SCH2CH3 | H | C12H19NO2S | 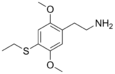
|
207740-24-7 |
| 2C-T-3[7] | H | SCH2C(=CH2)CH3 | H | C14H19NO2S | 
|
648957-40-8 |
| 2C-T-4 | H | SCH(CH3)2 | H | C13H19NO2S | 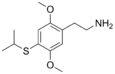
|
207740-25-8 |
| 2C-T-5[7] | H | H | 
|
1187859-38-6 | ||
| 2C-T-6[7] | H | H | 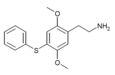
|
2888537-50-4 | ||
| 2C-T-7 | H | S(CH2)2CH3 | H | C13H19NO2S | 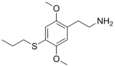
|
207740-26-9 |
| 2C-T-8 | H | SCH2CH(CH2)2 | H | C14H19NO2S | 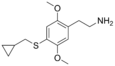
|
207740-27-0 |
| 2C-T-9[7] | H | H | C14H21NO2S | 
|
207740-28-1 | |
| 2C-T-10[7] | H | H | 
|
2888537-51-5 | ||
| 2C-T-11[7] | H | H | 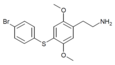
|
1798390-41-6 | ||
| 2C-T-12[7] | H | H | 
|
2888537-52-6 | ||
| 2C-T-13 | H | S(CH2)2OCH3 | H | 
|
207740-30-5 | |
| 2C-T-14[7] | H | H | 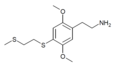
|
暫未註冊 | ||
| 2C-T-15 | H | SCH(CH2)2 | H | C13H17NO2S | 
|
952006-95-0 |
| 2C-T-16[8] | H | SCH2CH=CH2 | H | 
|
648957-42-0 | |
| 2C-T-17 | H | SCH(CH3)CH2CH3 | H | 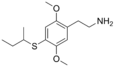
|
207740-32-7 | |
| 2C-T-18[7] | H | H | 
|
2888537-53-7 | ||
| 2C-T-19 | H | SCH2CH2CH2CH3 | H | C14H21NO2S | 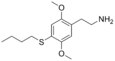
|
732244-33-6 |
| 2C-T-21 | H | S(CH2)2F | H | 
|
207740-33-8 | |
| 2C-T-21.5[7] | H | H | 
|
648957-46-4 | ||
| 2C-T-22[7] | H | H | 
|
648957-48-6 | ||
| 2C-T-23[7] | H | H | 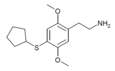
|
2888537-54-8 | ||
| 2C-T-24[7] | H | H | 
|
暫未註冊 | ||
| 2C-T-25[7] | H | H | C14H21NO2S | 
|
740797-11-9 | |
| 2C-T-27[7] | H | H | 
|
648957-52-2 | ||
| 2C-T-28[7] | H | H | 
|
648957-54-4 | ||
| 2C-T-30[7] | H | H | 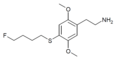
|
775578-10-4 | ||
| 2C-T-31[7] | H | H | 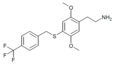
|
765269-48-5 | ||
| 2C-T-32[7] | H | H | 
|
737754-27-7 | ||
| 2C-T-33[7] | H | H | 
|
暫未註冊 |
苯並雜環衍生物
[編輯]| 名稱 | R3基團 | R4基團 | R6基團 | 分子式 | 2D結構 | CAS號 |
|---|---|---|---|---|---|---|
| 2C-B-FLY | 苯並二氫呋喃 | Br | 苯並二氫呋喃 | C12H14BrNO2 | 
|
733720-95-1 |
| 2C-B-BFLY | 苯並二氫吡喃 | Br | 苯並二氫吡喃 | C14H18BrNO2 | 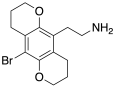
|
502659-24-7 |
| 2C-B-DFLY | 苯並呋喃 | Br | 苯並呋喃 | C12H10BrNO2 | 
|
260809-98-1 |
| 2C-C-FLY | 苯並二氫呋喃 | Cl | 苯並二氫呋喃 | C12H14ClNO2 | 
|
1354633-83-2 |
| 2C-D-FLY | 苯並二氫呋喃 | CH3 | 苯並二氫呋喃 | C13H17NO2 | 
|
1354634-07-3 |
| 2C-E-FLY | 苯並二氫呋喃 | CH2CH3 | 苯並二氫呋喃 | C14H19NO2 | 
|
2697190-39-7 |
| 2C-EF-FLY | 苯並二氫呋喃 | CH2CH2F | 苯並二氫呋喃 | C14H18FNO2 | 
|
|
| 2C-I-FLY | 苯並二氫呋喃 | I | 苯並二氫呋喃 | C12H14INO2 | 
|
1354633-88-7 |
| 2C-T-7-FLY | 苯並二氫呋喃 | SCH2CH3 | 苯並二氫呋喃 | C15H21NO2S | 
|
1354633-05-8 |
參考文獻
[編輯]- ^ Alexander Shulgin, Tania Manning and Paul F Daley. The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press, 2011. ISBN 978-0-9630096-3-0
- ^ Daniel Trachsel, David Lehmann and Christoph Enzensperger. Phenethylamine Von der Struktur zur Funktion, pp 762-810. Nachtschatten Verlag AG, 2013. ISBN 978-3-03788-700-4
- ^ Takahashi M, Nagashima M, Suzuki J, Seto T, Yasuda I, Yoshida T. Creation and application of psychoactive designer drugs data library using liquid chromatography with photodiode array spectrophotometry detector and gas chromatography–mass spectrometry. Talanta, 15 Feb 2009, 77(4): 1245–1272. doi:10.1016/j.talanta.2008.07.062
- ^ Leth-Petersen S, Petersen IN, Jensen AA, Bundgaard C, Bæk M, Kehler J, Kristensen JL. 5-HT2A/5-HT2C receptor pharmacology and intrinsic clearance of N-benzylphenethylamines modified at the primary site of metabolism. ACS Chem. Neurosci., 16 Nov 2016, 7 (11), 1614–1619. doi:10.1021/acschemneuro.6b00265
- ^ Daniel Trachsel; David Lehmann & Christoph Enzensperger. Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. 2013. ISBN 978-3-03788-700-4.
- ^ Kruegel AC. Phenalkylamines and Methods of Treating Mood Disorders. Patent WO 2022/006186 (PDF). [2023-11-19]. (原始內容存檔 (PDF)於2022-12-20).
- ^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 Shulgin's Sulfur Symphony – Part I. countyourculture. 15 January 2011 [22 October 2017]. (原始內容存檔於19 September 2019).
- ^ Daniel Trachsel. Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines). Helvetica Chimica Acta. 2003, 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
