生物鹼

生物鹼(英語:alkaloid)是一種天然存在的含氮鹼性化合物。一些人工合成但結構类似的化合物有時也被稱作生物鹼。除了碳、氫、氮,生物鹼也可以含有氧、硫、氯、溴、磷等元素。[2] 生物鹼与其它含氮碱性化合物之间的分界并不明确,但氨基酸、核苷酸及胺类物质通常不被称作生物碱。
生物鹼在人或動物体内产生藥理反應。雖然大部份的生物鹼對人體有毒,但有些也能入药,起鎮痛或麻醉的作用,以嗎啡及可待因尤为重要。生物鹼大都是氨基酸衍生物,嚐起來有苦澀味,是植物(如馬鈴薯、蕃茄、罂粟)、動物(如貝类)、细菌和真菌等多种生命体的次生代谢物。通过对生物体的粗提取物进行酸碱萃取纯化,可以获得大部份的生物鹼。
发现史
[编辑]自古以来,人类就将含有生物碱的植物用于治疗和娱乐。
例如,早在公元前 2000 年左右,美索不达米亚就已发现药用植物[3]。[30] 荷马(作家)的奥德赛提到了埃及女王送给海伦的礼物,一种带来遗忘的药物。
据信,这份礼物是一种含有鸦片的药物[4]。一本写于公元前一至三世纪的中国室内植物书籍提到了麻黄和罂粟的医疗用途[5]。此外,古柯叶自古以来就被南美原住民使用。

含有有毒生物碱(例如乌头碱和管箭毒碱)的植物提取物自古以来就被用于毒箭[3]。
生物碱的研究始于 19 世纪。
1804年,德国化学家弗里德里希·瑟图纳从鸦片中分离出一种“催眠原理”(拉丁语:principium somniferum),他将其称为“吗啡”,指的是希腊梦神墨菲斯; 在德语和其他一些中欧语言中,这仍然是该药物的名称。 “吗啡”一词在英语和法语中使用,由法国物理学家盖-吕萨克提出。
法国研究人员皮埃尔-佩尔提埃和约瑟夫·卡旺图在生物碱发展的早期对化学做出了重大贡献,他们发现了奎宁 (1820年) 和士的宁 (1818年)。
大约在同一时期还发现了其他几种生物碱,包括黄嘌呤(1817年)、阿托品(1819年)、咖啡因(1820年)、可尼碱(1827年)、尼古丁(1828年)、秋水仙碱(1833年)、金雀花碱(1851年)和可卡因(1860年)。
20 世纪光谱和色谱方法的出现加速了生物碱化学的发展,到 2008 年已鉴定出超过 12,000 种生物碱[6]。
1886 年,德国化学家阿尔伯特·拉登堡首次完成了生物碱的合成。 他通过2-甲基吡啶与乙醛反应并用钠还原生成的2-丙烯基吡啶来生产乌头碱[7]。
生物鹼的分類
[编辑]与大多数其他天然化合物相比,生物碱的特征在于其丰富的结构多样性,没有统一分类。
| 類別 | 主要結構 | 主要合成路線 | 例子 |
|---|---|---|---|
| 與含氮雜環生物鹼(真正生物鹼) | |||
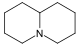
吡咯烷 類衍生物[8]
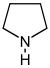 |
鳥氨酸 或 精氨酸 → 腐胺 → N-甲基腐胺 → N-methyl-Δ1-pyrroline [9] | Cuscohygrine, hygrine, hygroline, stachydrine[8][10] | |
托烷 類衍生物[11]
 |
阿托品類 原子取代於位置 3, 6 或 7 |
鳥氨酸或精氨酸→腐胺→N-甲基腐胺→N-甲基-Δ1-吡咯烷酮 [9] | Atropine, scopolamine, hyoscyamine[8][11][12] |
| 可卡因類 原子取代於位置 2, 3 |
Cocaine, ecgonine [11][13] | ||
吡咯里西啶 類衍生物[14]
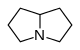 |
非酯類 | 鳥氨酸,精氨酸→腐→類精脒→惹卓裂鹼 [9] | Retronecine, heliotridine, laburnine [14][15] |
| 一元羧酸的複合酯 | Indicine, lindelophin, sarracine [14] | ||
| 大環內二酯 | Platyphylline, trichodesmine[14] | ||
| 1-氨基吡咯烷類 (lolines) | L-脯氨酸+ L-高絲氨酸→N-(3-氨基-3-羧丙基)脯氨酸→降黑麥草堿 [16][17] | Loline, N-formylloline, N-acetylloline[18] | |
哌啶 類衍生物[19]
 |
賴氨酸 → 屍胺 → Δ1-piperideine [20] | Sedamine, lobeline, anaferine, piperine [21][22] | |
| 辛酸 → coniceine → 毒芹碱 [23] | Coniine, coniceine [23] | ||
| 喹 類衍生物[24][25]
|
羽扇豆碱 類 | 賴氨酸 → 屍胺 → Δ1-piperideine [26] | Lupinine, nupharidin [24] |
| 野靛碱 類 | 野靛碱 [24] | ||
| 司巴丁 類 | 司巴丁, lupanine, anahygrine[24] | ||
| 苦參鹼 類 | Matrine, oxymatrine, allomatridine[24][27][28] | ||
| 苦豆鹼 類 | Ormosanine, piptantine[24][29] | ||
吲哚聯啶 類衍生物[30]
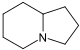 |
賴氨酸 → δ-semialdehyde of Α-氨基己二酸 → pipecolic acid → 1 indolizidinone [31] | Swainsonine, castanospermine [32] | |
吡啶 類衍生物[33][34]
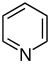 |
吡啶簡單衍生物 | 烟酸 → dihydronicotinic acid → 1,2-dihydropyridine [35] | Trigonelline, ricinine, arecoline [33][36] |
| 多環芳烴非冷凝吡啶衍生物 | Nicotine, nornicotine, anabasine, anatabine [33][36] | ||
| 多環芳烴冷凝吡啶衍生物 | Actinidine, gentianine, pediculinine [37] | ||
| Sesquiterpene pyridine derivatives | 烟酸, 異亮氨酸 [38] | Evonine, hippocrateine, triptonine [34][35] | |
異喹啉 類衍生物及相關生物鹼[39]
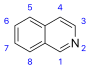 |
Simple derivatives of isoquinoline [40] | Tyrosine or 苯丙氨酸 → 多巴胺 or 酪胺 (for alkaloids Amarillis) [41][42] | Salsoline, lophocerine [39][40] |
| Derivatives of 1- and 3-isoquinolines [43] | N-methylcoridaldine, noroxyhydrastinine [43] | ||
| Derivatives of 1- and 4-phenyltetrahydroisoquinolines [40] | Cryptostilin [40][44] | ||
| Derivatives of 5-naftil-isoquinoline [45] | Ancistrocladine [45] | ||
| Derivatives of 1- and 2-benzyl-izoquinolines [46] | Papaverine, laudanosine, sendaverine | ||
| Cularine group[47] | Cularine, yagonine [47] | ||
| Pavines and isopavines [48] | Argemonine, amurensine [48] | ||
| Benzopyrrocolines [49] | Cryptaustoline [40] | ||
| Protoberberines [40] | Berberine, canadine, ophiocarpine, mecambridine, corydaline [50] | ||
| Phthalidisoquinolines [40] | Hydrastine, narcotine (Noscapine) [51] | ||
| Spirobenzylisoquinolines [40] | Fumaricine [48] | ||
| Ipecacuanha alkaloids[52] | Emetine, protoemetine, ipecoside [52] | ||
| Benzophenanthridines [40] | Sanguinarine, oxynitidine, corynoloxine [53] | ||
| Aporphines [40] | Glaucine, coridine, liriodenine [54] | ||
| Proaporphines [40] | Pronuciferine, glaziovine [40][49] | ||
| Homoaporphines [55] | Kreysiginine, multifloramine [55] | ||
| Homoproaporphines [55] | Bulbocodine [47] | ||
| 嗎啡s[56] | 嗎啡, 可待因, 蒂巴因, 青藤碱 [57] | ||
| Homomorphines [58] | Kreysiginine, androcymbine [56] | ||
| Tropoloisoquinolines [40] | Imerubrine [40] | ||
| Azofluoranthenes [40] | Rufescine, imeluteine [59] | ||
| Amaryllis alkaloids[60] | Lycorine, ambelline, tazettine, galantamine, montanine [61] | ||
| Erythrina alkaloids[44] | Erysodine, erythroidine [44] | ||
| Phenanthrene derivatives [40] | Atherosperminine [40][50] | ||
| Protopines [40] | Protopine, oxomuramine, corycavidine [53] | ||
| Aristolactam [40] | Doriflavin [40] | ||
噁唑 類衍生物[[62]
 |
Tyrosine → tyramine [63] | Annuloline, halfordinol, texaline, texamine[64] | |
異噁唑 類衍生物
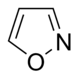 |
Ibotenic acid → Muscimol | Ibotenic acid, Muscimol | |
噻唑 類衍生物[65]
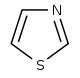 |
1-Deoxy-D-xylulose 5-phosphate (DOXP), tyrosine, cysteine [66] | Nostocyclamide, thiostreptone [65][67] | |
喹唑啉 類衍生物[68]
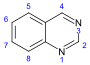 |
3,4-Dihydro-4-quinazolone derivatives | Anthranilic acid or phenylalanine or ornithine [69] | Febrifugine[70] |
| 1,4-Dihydro-4-quinazolone derivatives | Glycorine, arborine, glycosminine[70] | ||
| Pyrrolidine and piperidine quinazoline derivatives | Vazicine (peganine) [62] | ||
| 吖啶 類衍生物[62]
|
Anthranilic acid [71] | Rutacridone, acronicine[72][73] | |
喹啉 類衍生物[74][75]
 |
Simple derivatives of quinoline derivatives of 2 – quinolones and 4-quinolone | Anthranilic acid → 3-carboxyquinoline [76] | Cusparine, echinopsine, evocarpine[75][77][78] |
| Tricyclic terpenoids | Flindersine[75][79] | ||
| Furanoquinoline derivatives | Dictamnine, fagarine, skimmianine[75][80][81] | ||
| Quinines | Tryptophan → tryptamine → strictosidine (with secologanin) → korinanteal → cinhoninon [42][76] | Quinine, quinidine, cinchonine, cinhonidine [79] | |
吲哚 類衍生物[57]
 |
非異戊二烯吲哚生物鹼 | ||
| Simple indole derivatives [82] | Tryptophan → tryptamine or 5-hydroxitriptofan [83] | Serotonin, psilocybin, dimethyltryptamine (DMT), bufotenin [84][85] | |
| Simple derivatives of β-carboline [86] | Harman, harmine, harmaline, eleagnine [82] | ||
| Pyrroloindole alkaloids [87] | Physostigmine (eserine), etheramine, physovenine, eptastigmine[87] | ||
| 半萜類吲哚生物鹼' | |||
| Ergot alkaloids[57] | Tryptophan → chanoclavine → agroclavine → elimoclavine → paspalic acid → lysergic acid [87] | Ergotamine, ergobasine, ergosine[88] | |
| 單萜吲哚生物鹼 | |||
| Corynanthe type alkaloids[83] | Tryptophan → tryptamine → strictosidine (with secologanin) [83] | Ajmalicine, sarpagine, vobasine, ajmaline, yohimbine, reserpine, mitragynine,[89][90] group strychnine and (Strychnine brucine, aquamicine, vomicine [91]) | |
| Iboga-type alkaloids[83] | Ibogamine, ibogaine, voacangine[83] | ||
| Aspidosperma-type alkaloids[83] | Vincamine, vinca alkaloids, vincotine, aspidospermine[92][93] | ||
咪唑 類衍生物[62]
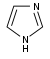 |
Directly from histidine[94] | Histamine, pilocarpine, pilosine, stevensine[62][94] | |
嘌呤 類衍生物[95]
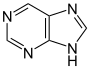 |
Xanthosine (formed in purine biosynthesis) → 7 methylxantosine → 7-methyl xanthine → theobromine → caffeine [42] | Caffeine, theobromine, theophylline, saxitoxin [96][97] | |
| 側鏈上含氮原子的生物側鏈鹼 | |||
β-苯乙胺 類衍生物[49]
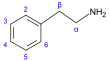 |
Tyrosine or phenylalanine → dioxyphenilalanine → dopamine → adrenaline and mescaline tyrosine → tyramine phenylalanine → 1-phenylpropane-1,2-dione → cathinone → ephedrine and pseudoephedrine [38][98][99] | Tyramine, ephedrine, pseudoephedrine, mescaline, cathinone, catecholamines (adrenaline, noradrenaline, dopamine)[38][100] | |
秋水仙素 類衍生物 [101]
 |
Tyrosine or phenylalanine → dopamine → autumnaline → colchicine [102] | Colchicine, colchamine[101] | |
毒蕈鹼 [103]
 |
Glutamic acid → 3-ketoglutamic acid → muscarine (with pyruvic acid)[104] | Muscarine, allomuscarine, epimuscarine, epiallomuscarine[103] | |
芐胺[105]
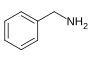 |
Phenylalanine with valine, leucine or isoleucine[106] | Capsaicin, dihydrocapsaicin, nordihydrocapsaicin, vanillylamine[105][107] | |
| 多胺生物鹼 | |||
| 腐胺 衍生物[108]
|
ornithine → putrescine → spermidine → spermine[109] | Paucine [108] | |
| 亞精胺 衍生物[108]
|
Lunarine, codonocarpine[108] | ||
| 精胺 衍生物[108]
|
Verbascenine, aphelandrine [108] | ||
| Peptide (cyclopeptide) alkaloids | |||
| Peptide alkaloids with a 13-membered cycle [110][111] | Nummularine C type | From different amino acids [110] | Nummularine C, Nummularine S [110] |
| Ziziphine type | Ziziphine A, sativanine H [110] | ||
| Peptide alkaloids with a 14-membered cycle [110][111] | Frangulanine type | Frangulanine, scutianine J [111] | |
| Scutianine A type | Scutianine A [110] | ||
| Integerrine type | Integerrine, discarine D [111] | ||
| Amphibine F type | Amphibine F, spinanine A [110] | ||
| Amfibine B type | Amphibine B, lotusine C [110] | ||
| Peptide alkaloids with a 15-membered cycle [111] | Mucronine A type | Mucronine A [112][111] | |
| Pseudoalkaloids (terpenes and steroids) | |||
Diterpenes [112]
 |
Lycoctonine type | Mevalonic acid → izopentenilpyrophosfate → geranyl pyrophosphate [113][114] | Aconitine, delphinine [112][115] |
Steroids[116]
 |
Cholesterol, arginine[117] | Solasodine, solanidine, veralkamine, batrachotoxin[118] | |
参考文献
[编辑]引用
[编辑]- ^ Andreas Luch. Molecular, clinical and environmental toxicology. Springer. 2009: 20. ISBN 3-7643-8335-6.
- ^ Chemical Encyclopedia: alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ 3.0 3.1 Aniszewski, Tadeusz. Preface. Alkaloids. Elsevier. 2015: xvii–xviii.
- ^ Hesse, Stefan. Industrieroboterperipherie. Industrieroboterpraxis. Wiesbaden: Vieweg+Teubner Verlag. 1998: 327–338. ISBN 978-3-322-88982-9.
- ^ Backmatter. Frankfurt – Hesse-Darmstadt. Walter de Gruyter – K. G. Saur. 2007-06-16: 291–304.
- ^ Begley, Tadhg P. Overview and Introduction. Comprehensive Natural Products II. Elsevier. 2010: 1–2.
- ^ Lieberstein, Samuel. Great Soviet Encyclopedia: A Translation of the Third Edition. Vol. 1. Russian Review. 1975-04, 34 (2). ISSN 0036-0341. doi:10.2307/127750.
- ^ 8.0 8.1 8.2 Plemenkov, p. 224
- ^ 9.0 9.1 9.2 Aniszewski, p. 75
- ^ Orekhov, p. 33
- ^ 11.0 11.1 11.2 Chemical Encyclopedia: Tropan alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Hesse, p. 34
- ^ Aniszewski, p. 27
- ^ 14.0 14.1 14.2 14.3 Chemical Encyclopedia: Pyrrolizidine alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Plemenkov, p. 229
- ^ Blankenship JD, Houseknecht JB, Pal S, Bush LP, Grossman RB, Schardl CL. Biosynthetic precursors of fungal pyrrolizidines, the loline alkaloids. Chembiochem. 2005, 6 (6): 1016–1022. PMID 15861432. doi:10.1002/cbic.200400327.
- ^ Faulkner JR, Hussaini SR, Blankenship JD, Pal S, Branan BM, Grossman RB, Schardl CL. On the sequence of bond formation in loline alkaloid biosynthesis. Chembiochem. 2006, 7 (7): 1078–1088. PMID 16755627. doi:10.1002/cbic.200600066.
- ^ Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP. Loline alkaloids: currencies of mutualism. Phytochemistry. 2007, 68 (7): 980–996. PMID 17346759. doi:10.1016/j.phytochem.2007.01.010.
- ^ Plemenkov, p. 225
- ^ Aniszewski, p. 95
- ^ Hesse, p. 31
- ^ Orekhov, p. 80
- ^ 23.0 23.1 Dewick, p. 381
- ^ 24.0 24.1 24.2 24.3 24.4 24.5 Chemical Encyclopedia: Quinolizidine alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Saxton, Vol. 1, p. 93
- ^ Aniszewski, p. 98
- ^ Saxton, Vol. 1, p. 91
- ^ Joseph P. Michael. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2002, 19: 458–475. doi:10.1039/b208137g.
- ^ Saxton, Vol. 1, p. 92
- ^ Dewick, p. 310
- ^ Aniszewski, p. 96
- ^ Aniszewski, p. 97
- ^ 33.0 33.1 33.2 Plemenkov, p. 227
- ^ 34.0 34.1 Chemical Encyclopedia: pyridine alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ 35.0 35.1 Aniszewski, p. 107
- ^ 36.0 36.1 Aniszewski, p. 85
- ^ Plemenkov, p. 228
- ^ 38.0 38.1 38.2 Aniszewski, p. 110
- ^ 39.0 39.1 Hesse, p. 36
- ^ 40.00 40.01 40.02 40.03 40.04 40.05 40.06 40.07 40.08 40.09 40.10 40.11 40.12 40.13 40.14 40.15 40.16 40.17 40.18 40.19 Chemical Encyclopedia: isoquinoline alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Aniszewski, pp. 77–78
- ^ 42.0 42.1 42.2 Begley, Alkaloid Biosynthesis
- ^ 43.0 43.1 Saxton, Vol. 3, p. 122
- ^ 44.0 44.1 44.2 Hesse, p. 54
- ^ 45.0 45.1 Hesse, p. 37
- ^ Hesse, p. 38
- ^ 47.0 47.1 47.2 Hesse, p. 46
- ^ 48.0 48.1 48.2 Hesse, p. 50
- ^ 49.0 49.1 49.2 Kenneth W. Bentley. β-Phenylethylamines and the isoquinoline alkaloids (PDF). Nat. Prod. Rep. 1997, 14 (4): 387–411 [2016-04-23]. PMID 9281839. doi:10.1039/NP9971400387. (原始内容存档 (PDF)于2014-04-13).
- ^ 50.0 50.1 Hesse, p. 47
- ^ Hesse, p. 39
- ^ 52.0 52.1 Hesse, p. 41
- ^ 53.0 53.1 Hesse, p. 49
- ^ Hesse, p. 44
- ^ 55.0 55.1 55.2 Saxton, Vol. 3, p. 164
- ^ 56.0 56.1 Hesse, p. 51
- ^ 57.0 57.1 57.2 Plemenkov, p. 236
- ^ Saxton, Vol. 3, p. 163
- ^ Saxton, Vol. 3, p. 168
- ^ Hesse, p. 52
- ^ Hesse, p. 53
- ^ 62.0 62.1 62.2 62.3 62.4 Plemenkov, p. 241
- ^ Brossi, Vol. 35, p. 261
- ^ Brossi, Vol. 35, pp. 260–263
- ^ 65.0 65.1 Plemenkov, p. 242
- ^ Begley, Cofactor Biosynthesis
- ^ John R. Lewis. Amaryllidaceae, muscarine, imidazole, oxazole, thiazole and peptide alkaloids, and other miscellaneous alkaloids. Nat. Prod. Rep. 2000, 17 (1): 57–84. PMID 10714899. doi:10.1039/a809403i.
- ^ Chemical Encyclopedia: Quinazoline alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Aniszewski, p. 106
- ^ 70.0 70.1 Aniszewski, p. 105
- ^ Richard B. Herbert; Herbert, Richard B.; Herbert, Richard B. The biosynthesis of plant alkaloids and nitrogenous microbial metabolites. Nat. Prod. Rep. 1999, 16: 199–208. doi:10.1039/a705734b.
- ^ Plemenkov, pp. 231, 246
- ^ Hesse, p. 58
- ^ Plemenkov, p. 231
- ^ 75.0 75.1 75.2 75.3 Chemical Encyclopedia: Quinoline alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ 76.0 76.1 Aniszewski, p. 114
- ^ Orekhov, p. 205
- ^ Hesse, p. 55
- ^ 79.0 79.1 Plemenkov, p. 232
- ^ Orekhov, p. 212
- ^ Aniszewski, p. 118
- ^ 82.0 82.1 Aniszewski, p. 112
- ^ 83.0 83.1 83.2 83.3 83.4 83.5 Aniszewski, p. 113
- ^ Hesse, p. 15
- ^ Saxton, Vol. 1, p. 467
- ^ Dewick, pp. 349–350
- ^ 87.0 87.1 87.2 Aniszewski, p. 119
- ^ Hesse, p. 29
- ^ Hesse, pp. 23–26
- ^ Saxton, Vol. 1, p. 169
- ^ Saxton, Vol. 5, p. 210
- ^ Hesse, pp. 17–18
- ^ Dewick, p. 357
- ^ 94.0 94.1 Aniszewski, p. 104
- ^ Hesse, p. 72
- ^ Hesse, p. 73
- ^ Dewick, p. 396
- ^ Dewick, p. 382
- ^ PlantCyc Pathway: ephedrine biosynthesis 互联网档案馆的存檔,存档日期2011-12-10.
- ^ Hesse, p. 76
- ^ 101.0 101.1 Chemical Encyclopedia: colchicine alkaloids (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Aniszewski, p. 77
- ^ 103.0 103.1 Hesse, p. 81
- ^ Brossi, Vol. 23, p. 376
- ^ 105.0 105.1 Hesse, p. 77
- ^ Brossi, Vol. 23, p. 268
- ^ Brossi, Vol. 23, p. 231
- ^ 108.0 108.1 108.2 108.3 108.4 108.5 Hesse, p. 82
- ^ Spermine Biosynthesis. [2016-04-23]. (原始内容存档于2016-12-04).
- ^ 110.0 110.1 110.2 110.3 110.4 110.5 110.6 110.7 Dimitris C. Gournelif; Gregory G. Laskarisb; Robert Verpoorte. Cyclopeptide alkaloids. Nat. Prod. Rep. 1997, 14 (1): 75–82. PMID 9121730. doi:10.1039/NP9971400075.
- ^ 111.0 111.1 111.2 111.3 111.4 111.5 Plemenkov, p. 243
- ^ 112.0 112.1 112.2 Hesse, p. 84
- ^ Chemical Encyclopedia: Terpenes (页面存档备份,存于互联网档案馆). xumuk.ru
- ^ Begley, Natural Products: An Overview
- ^ Atta-ur-Rahman and M. Iqbal Choudhary. Diterpenoid and steroidal alkaloids. Nat. Prod. Rep. 1997, 14 (2): 191–203. PMID 9149410. doi:10.1039/np9971400191.
- ^ Hesse, p. 88
- ^ Dewick, p. 388
- ^ Plemenkov, p. 247
来源
[编辑]- Aniszewski, Tadeusz. Alkaloids – secrets of life. Amsterdam: Elsevier. 2007. ISBN 978-0-444-52736-3.
- Begley, Tadhg P. Encyclopedia of Chemical Biology. Wiley. 2009. ISBN 978-0-471-75477-0. doi:10.1002/cbic.200900262.
- Brossi, Arnold. The Alkaloids: Chemistry and Pharmacology. Academic Press. 1989.
- Dewick, Paul M. Medicinal Natural Products. A Biosynthetic Approach 2nd. Wiley. 2002. ISBN 0-471-49640-5.
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley-VCH. 2008. ISBN 978-3-527-31521-5.
- Grinkevich, N. I.; Safronich, L. N. The chemical analysis of medicinal plants: Proc. allowance for pharmaceutical universities. M. 1983.
- Hesse, Manfred. Alkaloids: Nature's Curse or Blessing?. Wiley-VCH. 2002. ISBN 978-3-906390-24-6.
- Knunyants, I. L. Chemical Encyclopedia. Soviet Encyclopedia. 1988 [2014-08-23]. (原始内容存档于2021-03-07).
- Orekhov, A. P. Chemistry alkaloids Acad. 2. M.: USSR. 1955.
- Plemenkov, V. V. Introduction to the Chemistry of Natural Compounds. Kazan. 2001.
- Saxton, J. E. The Alkaloids. A Specialist Periodical Report. London: The Chemical Society. 1971.
- Veselovskaya, N. B.; Kovalenko, A. E. Drugs. Moscow: Triada-X. 2000.
- Wink, M. Mode of action and toxicology of plant toxins and poisonous plants. Mitt. Julius Kühn-Inst. 2009, 421: 93–112 [18 March 2014]. (原始内容存档于2014-03-18).