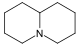
生物鹼

生物鹼(英語:alkaloid)是一種包含氮原子,天然存在的鹼性化合物。一些化學合成但結構與生物鹼相似的化合物有時也被稱作生物鹼。除了碳、氫、氮,生物鹼也可以含有氧、硫或其他元素,如氯、溴、磷等。[2]
很多的生物鹼都對人或動物有藥理反應。生物鹼大都是氨基酸的衍生物,嚐起來有苦澀味。它們常以次生代謝物的形式出現於植物(例如:馬鈴薯、蕃茄)、動物(例如:貝殼類)及蕈類。大部份的生物鹼皆能由它們的植物提取液中以酸-鹼萃取獲得。生物鹼的英文為「alkaloid」,這個字由「alkaline」一字衍生而成。原本「alkaloid」泛指一切含有氮的鹼基。
雖然大部份的生物鹼對於人體有毒,但也有些能入藥。主要有鎮痛或麻醉的作用,以嗎啡及可待因的作用尤其顯著。
生物碱是被大量的各种生物体产生,包括细菌、真菌、植物和动物。它们可以从通过酸碱萃取这些生物体的粗提物进行纯化。
古代的毒藥大多為強烈的有毒生物鹼,据传说犀牛角只要碰到生物鹼就會整個支離破碎[3],因此古代的國王或是皇帝都會利用犀牛角來做酒杯,以避免被毒殺。
生物鹼的分類
与大多数其他类天然化合物相比,生物碱的特征在于很大的结构多样性,和生物碱没有统一分类。
| 類別 | 主要結構 | 主要合成路線 | 例子 |
|---|---|---|---|
| 與含氮雜環生物鹼(真正生物鹼) | |||
吡咯烷 類衍生物[4]
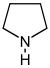 |
鳥氨酸 或 精氨酸 → 腐胺 → N-甲基腐胺 → N-methyl-Δ1-pyrroline [5] | Cuscohygrine, hygrine, hygroline, stachydrine[4][6] | |
托烷 類衍生物[7]
 |
阿托品類 原子取代於位置 3, 6 或 7 |
鳥氨酸或精氨酸→腐胺→N-甲基腐胺→N-甲基-Δ1-吡咯烷酮 [5] | Atropine, scopolamine, hyoscyamine[4][7][8] |
| 可卡因類 原子取代於位置 2, 3 |
Cocaine, ecgonine [7][9] | ||
吡咯里西啶 類衍生物[10]
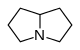 |
非酯類 | 鳥氨酸,精氨酸→腐→類精脒→惹卓裂鹼 [5] | Retronecine, heliotridine, laburnine [10][11] |
| 一元羧酸的複合酯 | Indicine, lindelophin, sarracine [10] | ||
| 大環內二酯 | Platyphylline, trichodesmine[10] | ||
| 1-氨基吡咯烷類 (lolines) | L-脯氨酸+ L-高絲氨酸→N-(3-氨基-3-羧丙基)脯氨酸→降黑麥草堿 [12][13] | Loline, N-formylloline, N-acetylloline[14] | |
哌啶 類衍生物[15]
 |
賴氨酸 → 屍胺 → Δ1-piperideine [16] | Sedamine, lobeline, anaferine, piperine [17][18] | |
| 辛酸 → coniceine → 毒芹碱 [19] | Coniine, coniceine [19] | ||
| 喹 類衍生物[20][21]
|
羽扇豆寧 類 | 賴氨酸 → 屍胺 → Δ1-piperideine [22] | Lupinine, nupharidin [20] |
| 金雀花鹼 類 | Cytisine [20] | ||
| 鷹爪豆鹼 類 | Sparteine, lupanine, anahygrine[20] | ||
| 苦參鹼 類 | Matrine, oxymatrine, allomatridine[20][23][24] | ||
| 苦豆鹼 類 | Ormosanine, piptantine[20][25] | ||
吲哚聯啶 類衍生物[26]
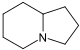 |
賴氨酸 → δ-semialdehyde of Α-氨基己二酸 → pipecolic acid → 1 indolizidinone [27] | Swainsonine, castanospermine [28] | |
吡啶 類衍生物[29][30]
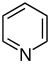 |
吡啶簡單衍生物 | 烟酸 → dihydronicotinic acid → 1,2-dihydropyridine [31] | Trigonelline, ricinine, arecoline [29][32] |
| 多環芳烴非冷凝吡啶衍生物 | Nicotine, nornicotine, anabasine, anatabine [29][32] | ||
| 多環芳烴冷凝吡啶衍生物 | Actinidine, gentianine, pediculinine [33] | ||
| Sesquiterpene pyridine derivatives | 烟酸, 異亮氨酸 [34] | Evonine, hippocrateine, triptonine [30][31] | |
異喹啉 類衍生物及相關生物鹼[35]
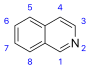 |
Simple derivatives of isoquinoline [36] | Tyrosine or 苯丙氨酸 → 多巴胺 or 酪胺 (for alkaloids Amarillis) [37][38] | Salsoline, lophocerine [35][36] |
| Derivatives of 1- and 3-isoquinolines [39] | N-methylcoridaldine, noroxyhydrastinine [39] | ||
| Derivatives of 1- and 4-phenyltetrahydroisoquinolines [36] | Cryptostilin [36][40] | ||
| Derivatives of 5-naftil-isoquinoline [41] | Ancistrocladine [41] | ||
| Derivatives of 1- and 2-benzyl-izoquinolines [42] | Papaverine, laudanosine, sendaverine | ||
| Cularine group[43] | Cularine, yagonine [43] | ||
| Pavines and isopavines [44] | Argemonine, amurensine [44] | ||
| Benzopyrrocolines [45] | Cryptaustoline [36] | ||
| Protoberberines [36] | Berberine, canadine, ophiocarpine, mecambridine, corydaline [46] | ||
| Phthalidisoquinolines [36] | Hydrastine, narcotine (Noscapine) [47] | ||
| Spirobenzylisoquinolines [36] | Fumaricine [44] | ||
| Ipecacuanha alkaloids[48] | Emetine, protoemetine, ipecoside [48] | ||
| Benzophenanthridines [36] | Sanguinarine, oxynitidine, corynoloxine [49] | ||
| Aporphines [36] | Glaucine, coridine, liriodenine [50] | ||
| Proaporphines [36] | Pronuciferine, glaziovine [36][45] | ||
| Homoaporphines [51] | Kreysiginine, multifloramine [51] | ||
| Homoproaporphines [51] | Bulbocodine [43] | ||
| 嗎啡s[52] | 嗎啡, 可待因, 蒂巴因, 青藤碱 [53] | ||
| Homomorphines [54] | Kreysiginine, androcymbine [52] | ||
| Tropoloisoquinolines [36] | Imerubrine [36] | ||
| Azofluoranthenes [36] | Rufescine, imeluteine [55] | ||
| Amaryllis alkaloids[56] | Lycorine, ambelline, tazettine, galantamine, montanine [57] | ||
| Erythrina alkaloids[40] | Erysodine, erythroidine [40] | ||
| Phenanthrene derivatives [36] | Atherosperminine [36][46] | ||
| Protopines [36] | Protopine, oxomuramine, corycavidine [49] | ||
| Aristolactam [36] | Doriflavin [36] | ||
噁唑 類衍生物[[58]
 |
Tyrosine → tyramine [59] | Annuloline, halfordinol, texaline, texamine[60] | |
異噁唑 類衍生物
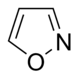 |
Ibotenic acid → Muscimol | Ibotenic acid, Muscimol | |
噻唑 類衍生物[61]
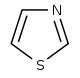 |
1-Deoxy-D-xylulose 5-phosphate (DOXP), tyrosine, cysteine [62] | Nostocyclamide, thiostreptone [61][63] | |
喹唑啉 類衍生物[64]
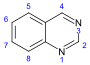 |
3,4-Dihydro-4-quinazolone derivatives | Anthranilic acid or phenylalanine or ornithine [65] | Febrifugine[66] |
| 1,4-Dihydro-4-quinazolone derivatives | Glycorine, arborine, glycosminine[66] | ||
| Pyrrolidine and piperidine quinazoline derivatives | Vazicine (peganine) [58] | ||
| 吖啶 類衍生物[58]
|
Anthranilic acid [67] | Rutacridone, acronicine[68][69] | |
喹啉 類衍生物[70][71]
 |
Simple derivatives of quinoline derivatives of 2 – quinolones and 4-quinolone | Anthranilic acid → 3-carboxyquinoline [72] | Cusparine, echinopsine, evocarpine[71][73][74] |
| Tricyclic terpenoids | Flindersine[71][75] | ||
| Furanoquinoline derivatives | Dictamnine, fagarine, skimmianine[71][76][77] | ||
| Quinines | Tryptophan → tryptamine → strictosidine (with secologanin) → korinanteal → cinhoninon [38][72] | Quinine, quinidine, cinchonine, cinhonidine [75] | |
吲哚 類衍生物[53]
 |
非異戊二烯吲哚生物鹼 | ||
| Simple indole derivatives [78] | Tryptophan → tryptamine or 5-hydroxitriptofan [79] | Serotonin, psilocybin, dimethyltryptamine (DMT), bufotenin [80][81] | |
| Simple derivatives of β-carboline [82] | Harman, harmine, harmaline, eleagnine [78] | ||
| Pyrroloindole alkaloids [83] | Physostigmine (eserine), etheramine, physovenine, eptastigmine[83] | ||
| 半萜類吲哚生物鹼' | |||
| Ergot alkaloids[53] | Tryptophan → chanoclavine → agroclavine → elimoclavine → paspalic acid → lysergic acid [83] | Ergotamine, ergobasine, ergosine[84] | |
| 單萜吲哚生物鹼 | |||
| Corynanthe type alkaloids[79] | Tryptophan → tryptamine → strictosidine (with secologanin) [79] | Ajmalicine, sarpagine, vobasine, ajmaline, yohimbine, reserpine, mitragynine,[85][86] group strychnine and (Strychnine brucine, aquamicine, vomicine [87]) | |
| Iboga-type alkaloids[79] | Ibogamine, ibogaine, voacangine[79] | ||
| Aspidosperma-type alkaloids[79] | Vincamine, vinca alkaloids, vincotine, aspidospermine[88][89] | ||
咪唑 類衍生物[58]
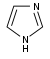 |
Directly from histidine[90] | Histamine, pilocarpine, pilosine, stevensine[58][90] | |
嘌呤 類衍生物[91]
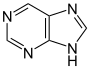 |
Xanthosine (formed in purine biosynthesis) → 7 methylxantosine → 7-methyl xanthine → theobromine → caffeine [38] | Caffeine, theobromine, theophylline, saxitoxin [92][93] | |
| 側鏈上含氮原子的生物側鏈鹼 | |||
β-苯乙胺 類衍生物[45]
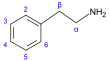 |
Tyrosine or phenylalanine → dioxyphenilalanine → dopamine → adrenaline and mescaline tyrosine → tyramine phenylalanine → 1-phenylpropane-1,2-dione → cathinone → ephedrine and pseudoephedrine [34][94][95] | Tyramine, ephedrine, pseudoephedrine, mescaline, cathinone, catecholamines (adrenaline, noradrenaline, dopamine)[34][96] | |
秋水仙素 類衍生物 [97]
 |
Tyrosine or phenylalanine → dopamine → autumnaline → colchicine [98] | Colchicine, colchamine[97] | |
毒蕈鹼 [99]
 |
Glutamic acid → 3-ketoglutamic acid → muscarine (with pyruvic acid)[100] | Muscarine, allomuscarine, epimuscarine, epiallomuscarine[99] | |
芐胺[101]
 |
Phenylalanine with valine, leucine or isoleucine[102] | Capsaicin, dihydrocapsaicin, nordihydrocapsaicin, vanillylamine[101][103] | |
| 多胺生物鹼 | |||
| 腐胺 衍生物[104]
|
ornithine → putrescine → spermidine → spermine[105] | Paucine [104] | |
| 亞精胺 衍生物[104]
|
Lunarine, codonocarpine[104] | ||
| 精胺 衍生物[104]
|
Verbascenine, aphelandrine [104] | ||
| Peptide (cyclopeptide) alkaloids | |||
| Peptide alkaloids with a 13-membered cycle [106][107] | Nummularine C type | From different amino acids [106] | Nummularine C, Nummularine S [106] |
| Ziziphine type | Ziziphine A, sativanine H [106] | ||
| Peptide alkaloids with a 14-membered cycle [106][107] | Frangulanine type | Frangulanine, scutianine J [107] | |
| Scutianine A type | Scutianine A [106] | ||
| Integerrine type | Integerrine, discarine D [107] | ||
| Amphibine F type | Amphibine F, spinanine A [106] | ||
| Amfibine B type | Amphibine B, lotusine C [106] | ||
| Peptide alkaloids with a 15-membered cycle [107] | Mucronine A type | Mucronine A [108][107] | |
| Pseudoalkaloids (terpenes and steroids) | |||
Diterpenes [108]
 |
Lycoctonine type | Mevalonic acid → izopentenilpyrophosfate → geranyl pyrophosphate [109][110] | Aconitine, delphinine [108][111] |
Steroids[112]
 |
Cholesterol, arginine[113] | Solasodine, solanidine, veralkamine, batrachotoxin[114] | |
参考文献
引用
- ^ Andreas Luch. Molecular, clinical and environmental toxicology. Springer. 2009: 20. ISBN 3-7643-8335-6.
- ^ Chemical Encyclopedia: alkaloids. xumuk.ru
- ^ 蔡振兴. 犀角九龙杯. 2014.
|newspaper=被忽略 (帮助) - ^ 4.0 4.1 4.2 Plemenkov, p. 224
- ^ 5.0 5.1 5.2 Aniszewski, p. 75
- ^ Orekhov, p. 33
- ^ 7.0 7.1 7.2 Chemical Encyclopedia: Tropan alkaloids. xumuk.ru
- ^ Hesse, p. 34
- ^ Aniszewski, p. 27
- ^ 10.0 10.1 10.2 10.3 Chemical Encyclopedia: Pyrrolizidine alkaloids. xumuk.ru
- ^ Plemenkov, p. 229
- ^ Blankenship JD, Houseknecht JB, Pal S, Bush LP, Grossman RB, Schardl CL. Biosynthetic precursors of fungal pyrrolizidines, the loline alkaloids. Chembiochem. 2005, 6 (6): 1016–1022. PMID 15861432. doi:10.1002/cbic.200400327.
- ^ Faulkner JR, Hussaini SR, Blankenship JD, Pal S, Branan BM, Grossman RB, Schardl CL. On the sequence of bond formation in loline alkaloid biosynthesis. Chembiochem. 2006, 7 (7): 1078–1088. PMID 16755627. doi:10.1002/cbic.200600066.
- ^ Schardl CL, Grossman RB, Nagabhyru P, Faulkner JR, Mallik UP. Loline alkaloids: currencies of mutualism. Phytochemistry. 2007, 68 (7): 980–996. PMID 17346759. doi:10.1016/j.phytochem.2007.01.010.
- ^ Plemenkov, p. 225
- ^ Aniszewski, p. 95
- ^ Hesse, p. 31
- ^ Orekhov, p. 80
- ^ 19.0 19.1 Dewick, p. 381
- ^ 20.0 20.1 20.2 20.3 20.4 20.5 Chemical Encyclopedia: Quinolizidine alkaloids. xumuk.ru
- ^ Saxton, Vol. 1, p. 93
- ^ Aniszewski, p. 98
- ^ Saxton, Vol. 1, p. 91
- ^ Joseph P. Michael. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 2002, 19: 458–475. doi:10.1039/b208137g.
- ^ Saxton, Vol. 1, p. 92
- ^ Dewick, p. 310
- ^ Aniszewski, p. 96
- ^ Aniszewski, p. 97
- ^ 29.0 29.1 29.2 Plemenkov, p. 227
- ^ 30.0 30.1 Chemical Encyclopedia: pyridine alkaloids. xumuk.ru
- ^ 31.0 31.1 Aniszewski, p. 107
- ^ 32.0 32.1 Aniszewski, p. 85
- ^ Plemenkov, p. 228
- ^ 34.0 34.1 34.2 Aniszewski, p. 110
- ^ 35.0 35.1 Hesse, p. 36
- ^ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 36.13 36.14 36.15 36.16 36.17 36.18 36.19 Chemical Encyclopedia: isoquinoline alkaloids. xumuk.ru
- ^ Aniszewski, pp. 77–78
- ^ 38.0 38.1 38.2 Begley, Alkaloid Biosynthesis
- ^ 39.0 39.1 Saxton, Vol. 3, p. 122
- ^ 40.0 40.1 40.2 Hesse, p. 54
- ^ 41.0 41.1 Hesse, p. 37
- ^ Hesse, p. 38
- ^ 43.0 43.1 43.2 Hesse, p. 46
- ^ 44.0 44.1 44.2 Hesse, p. 50
- ^ 45.0 45.1 45.2 Kenneth W. Bentley. β-Phenylethylamines and the isoquinoline alkaloids (PDF). Nat. Prod. Rep. 1997, 14 (4): 387–411. PMID 9281839. doi:10.1039/NP9971400387.
- ^ 46.0 46.1 Hesse, p. 47
- ^ Hesse, p. 39
- ^ 48.0 48.1 Hesse, p. 41
- ^ 49.0 49.1 Hesse, p. 49
- ^ Hesse, p. 44
- ^ 51.0 51.1 51.2 Saxton, Vol. 3, p. 164
- ^ 52.0 52.1 Hesse, p. 51
- ^ 53.0 53.1 53.2 Plemenkov, p. 236
- ^ Saxton, Vol. 3, p. 163
- ^ Saxton, Vol. 3, p. 168
- ^ Hesse, p. 52
- ^ Hesse, p. 53
- ^ 58.0 58.1 58.2 58.3 58.4 Plemenkov, p. 241
- ^ Brossi, Vol. 35, p. 261
- ^ Brossi, Vol. 35, pp. 260–263
- ^ 61.0 61.1 Plemenkov, p. 242
- ^ Begley, Cofactor Biosynthesis
- ^ John R. Lewis. Amaryllidaceae, muscarine, imidazole, oxazole, thiazole and peptide alkaloids, and other miscellaneous alkaloids. Nat. Prod. Rep. 2000, 17 (1): 57–84. PMID 10714899. doi:10.1039/a809403i.
- ^ Chemical Encyclopedia: Quinazoline alkaloids. xumuk.ru
- ^ Aniszewski, p. 106
- ^ 66.0 66.1 Aniszewski, p. 105
- ^ Richard B. Herbert; Herbert, Richard B.; Herbert, Richard B. The biosynthesis of plant alkaloids and nitrogenous microbial metabolites. Nat. Prod. Rep. 1999, 16: 199–208. doi:10.1039/a705734b.
- ^ Plemenkov, pp. 231, 246
- ^ Hesse, p. 58
- ^ Plemenkov, p. 231
- ^ 71.0 71.1 71.2 71.3 Chemical Encyclopedia: Quinoline alkaloids. xumuk.ru
- ^ 72.0 72.1 Aniszewski, p. 114
- ^ Orekhov, p. 205
- ^ Hesse, p. 55
- ^ 75.0 75.1 Plemenkov, p. 232
- ^ Orekhov, p. 212
- ^ Aniszewski, p. 118
- ^ 78.0 78.1 Aniszewski, p. 112
- ^ 79.0 79.1 79.2 79.3 79.4 79.5 Aniszewski, p. 113
- ^ Hesse, p. 15
- ^ Saxton, Vol. 1, p. 467
- ^ Dewick, pp. 349–350
- ^ 83.0 83.1 83.2 Aniszewski, p. 119
- ^ Hesse, p. 29
- ^ Hesse, pp. 23–26
- ^ Saxton, Vol. 1, p. 169
- ^ Saxton, Vol. 5, p. 210
- ^ Hesse, pp. 17–18
- ^ Dewick, p. 357
- ^ 90.0 90.1 Aniszewski, p. 104
- ^ Hesse, p. 72
- ^ Hesse, p. 73
- ^ Dewick, p. 396
- ^ Dewick, p. 382
- ^ PlantCyc Pathway: ephedrine biosynthesis 互联网档案馆的存檔,存档日期2011-12-10.
- ^ Hesse, p. 76
- ^ 97.0 97.1 Chemical Encyclopedia: colchicine alkaloids. xumuk.ru
- ^ Aniszewski, p. 77
- ^ 99.0 99.1 Hesse, p. 81
- ^ Brossi, Vol. 23, p. 376
- ^ 101.0 101.1 Hesse, p. 77
- ^ Brossi, Vol. 23, p. 268
- ^ Brossi, Vol. 23, p. 231
- ^ 104.0 104.1 104.2 104.3 104.4 104.5 Hesse, p. 82
- ^ Spermine Biosynthesis
- ^ 106.0 106.1 106.2 106.3 106.4 106.5 106.6 106.7 Dimitris C. Gournelif; Gregory G. Laskarisb; Robert Verpoorte. Cyclopeptide alkaloids. Nat. Prod. Rep. 1997, 14 (1): 75–82. PMID 9121730. doi:10.1039/NP9971400075.
- ^ 107.0 107.1 107.2 107.3 107.4 107.5 Plemenkov, p. 243
- ^ 108.0 108.1 108.2 Hesse, p. 84
- ^ Chemical Encyclopedia: Terpenes. xumuk.ru
- ^ Begley, Natural Products: An Overview
- ^ Atta-ur-Rahman and M. Iqbal Choudhary. Diterpenoid and steroidal alkaloids. Nat. Prod. Rep. 1997, 14 (2): 191–203. PMID 9149410. doi:10.1039/np9971400191.
- ^ Hesse, p. 88
- ^ Dewick, p. 388
- ^ Plemenkov, p. 247
来源
- Aniszewski, Tadeusz. Alkaloids – secrets of life. Amsterdam: Elsevier. 2007. ISBN 978-0-444-52736-3.
- Begley, Tadhg P. Encyclopedia of Chemical Biology. Wiley. 2009. ISBN 978-0-471-75477-0. doi:10.1002/cbic.200900262.
- Brossi, Arnold. The Alkaloids: Chemistry and Pharmacology. Academic Press. 1989.
- Dewick, Paul M. Medicinal Natural Products. A Biosynthetic Approach 2nd. Wiley. 2002. ISBN 0-471-49640-5.
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Wiley-VCH. 2008. ISBN 978-3-527-31521-5.
- Grinkevich, N. I.; Safronich, L. N. The chemical analysis of medicinal plants: Proc. allowance for pharmaceutical universities. M. 1983.
- Hesse, Manfred. Alkaloids: Nature's Curse or Blessing?. Wiley-VCH. 2002. ISBN 978-3-906390-24-6.
- Knunyants, I. L. Chemical Encyclopedia. Soviet Encyclopedia. 1988.
- Orekhov, A. P. Chemistry alkaloids Acad. 2. M.: USSR. 1955.
- Plemenkov, V. V. Introduction to the Chemistry of Natural Compounds. Kazan. 2001.
- Saxton, J. E. The Alkaloids. A Specialist Periodical Report. London: The Chemical Society. 1971.
- Veselovskaya, N. B.; Kovalenko, A. E. Drugs. Moscow: Triada-X. 2000.
- Wink, M. Mode of action and toxicology of plant toxins and poisonous plants. Mitt. Julius Kühn-Inst. 2009, 421: 93–112 [18 March 2014].
外部連結
参见
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||