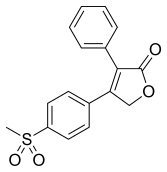
罗非昔布
外观
 | |
 | |
| 临床资料 | |
|---|---|
| 怀孕分级 |
|
| 给药途径 | 口服 |
| ATC码 | |
| 法律规范状态 | |
| 法律规范 |
|
| 药物动力学数据 | |
| 生物利用度 | 93% |
| 血浆蛋白结合率 | 87% |
| 药物代谢 | hepatic |
| 生物半衰期 | 17 hours |
| 排泄途径 | biliary/renal |
| 识别信息 | |
| |
| CAS号 | 162011-90-7 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.077 |
| 化学信息 | |
| 化学式 | C17H14O4S |
| 摩尔质量 | 314.36 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
罗非昔布或罗非考昔(英语:Rofecoxib,/ˌrɒfɪˈkɒksɪb/)商品名Vioxx、Ceoxx和Ceeoxx。是一种非甾体类抗炎药(NSAID),由默克公司制造、出售。美国食品药品监督管理局(FDA)于1999年5月20日批准上市,用以治疗骨关节炎、急性疼痛和痛经。
由于发现到长期且高剂量用药有增加心脏病的风险[1][2],默克公司于2004年9月30日起从市场撤回此药。
合成
[编辑]
注脚
[编辑]- ^ 偉克適致死 藥廠賠10億. [2017-02-26]. (原始内容存档于2017-09-12).
- ^ 食品藥物釣愚:《釣愚:操縱與欺騙的經濟學》選摘(3)-食安|藥物|釣愚:操縱與欺騙的經濟學-風傳媒-喬治.艾克羅夫, 羅伯.席勒. [2017-02-26]. (原始内容存档于2017-04-23).
- ^ Vioxxlawyer.org. Vioxxlawyer.org. [4 January 2015]. (原始内容存档于2015年1月5日).
参考文献
[编辑]- FDA (2005). "Summary minutes for the February 16, 17 and 18, 2005, Joint meeting of the Arthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee." Published on the internet, March 2005. Link (页面存档备份,存于互联网档案馆)
- Fitzgerald GA, Coxibs and Cardiovascular Disease, N Engl J Med 2004;351(17): 1709–1711. PMID 15470192.
- Grassley CE (15 Oct 2004). Grassley questions Merck about communication with the FDA on Vioxx. Press Release.
- Jüni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M (2004). Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet (published online; see also Merck response below)
- Karha J and Topol EJ. The sad story of Vioxx, and what we should learn from it (页面存档备份,存于互联网档案馆) Cleve Clin J Med 2004; 71(12):933-939. PMID 15641522
- Michaels, D. (June 2005) DOUBT Is Their Product Archive.is的存档,存档日期2006-03-21, Scientific American, 292 (6).
- Merck & Co., (5 Nov 2004). Response to Article by Juni et al. Published in The Lancet on Nov. 5. Press Release.
- Merck & Co (30 Sep 2004) Merck Announces Voluntary Worldwide Withdrawal of VIOXX. Press release [1].
- D. M. Mukherjee, S. E. Nissen, and E. J. Topol, "Risk of Cardiovascular Events Associated with Selective COX-2 Inhibitors," Journal of the American Medical Association 186 (2001): 954–959.
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, et al. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 2005;352(11):1081-91. PMID 15713945
- Okie, S (2005) "Raising the safety bar--the FDA's coxib meeting." N Engl J Med. 2005 Mar 31;352(13):1283-5. PMID 15800221.
- Leleti Rajender Reddy, Corey EJ. Facile air oxidation of the conjugate base of rofecoxib (Vioxx), a possible contributor to chronic human toxicity Tetrahedron Lett 2005, 46: 927. doi:10.1016/j.tetlet.2004.12.055
- Swan SK et al., Effect of Cyclooxygenase-2 Inhibition on Renal Function in Elderly Persons Receiving a Low-Salt Diet. Annals of Int Med 2000; 133:1–9
- Targum, SL. (1 Feb. 2001) Review of cardiovascular safety database. FDA memorandum. [2] (页面存档备份,存于互联网档案馆)
- Wolfe, MM et al., Gastrointestinal Toxicity of Nonsteroidal Anti-anflamattory Drugs, New England Journal of Medicine. 1999; 340; 1888-98.
外部链接
[编辑]- National Public Radio 2004 Q&A on the case, following withdrawal announcement (页面存档备份,存于互联网档案馆)
- Court TV's full coverage of the Vioxx civil trials
- Merck website on Vioxx litigation
- FDA Public Health Advisory on Vioxx (页面存档备份,存于互联网档案馆)
- David Michaels. Doubt is Their Product Scientific American, June 2004, p. 96-101
- JURIST, Much Pain, Much Gain: Skeptical Ruminations on the Vioxx Litigation
- Ted Frank, American Enterprise Institute, The Vioxx Litigation, Part I and Part II, December 2005
- briandeer.com - Vioxx: the UK connection (页面存档备份,存于互联网档案馆)
- Campaign for compensation for Vioxx victims outside the US
