阿昔洛韦:修订间差异
通用名不大写、 增加或調整參考來源 |
ThomasYehYeh(留言 | 贡献) |
||
| 第1行: | 第1行: | ||
{{Drugbox |
|||
{{medical}} |
|||
| Watchedfields = changed |
|||
{{drugbox | |
|||
| verifiedrevid = 477240648 |
|||
|image = Aciclovir structure.svg |
|||
| drug_name = |
|||
|image2 = Aciclovir.jpg |
|||
| |
| USAN = acyclovir |
||
| BAN = acyclovir |
|||
|IUPAC_name = 9-((2-羟乙氧基)-甲基)鸟嘌呤 |
|||
| image = Aciclovir 2D structure.svg |
|||
| synonyms = acycloguanosine |
|||
| image2 = Aciclovir ball-and-stick 2KI5.png |
|||
<!--Clinical data--> |
|||
| pronounce = {{IPAc-en|eɪ|ˈ|s|aɪ|k|l|oʊ-|v|ɪər}} |
|||
| tradename = Zovirax及其他<ref name=Names/> |
|||
| Drugs.com = {{drugs.com|monograph|acyclovir}} |
|||
| MedlinePlus = a681045 |
|||
| DailyMedID = Acyclovir |
|||
| licence_US = Acyclovir |
|||
| pregnancy_AU = B3 |
|||
| routes_of_administration = [[靜脈注射]], [[口服給藥]], [[外用藥物]], {{le|眼部給藥|Ophthalmic drug administration}} |
|||
| ATC_prefix = J05 |
|||
| ATC_suffix = AB01 |
|||
| ATC_supplemental = {{ATC|D06|BB03}}, {{ATC|S01|AD03}}, {{ATC|D06|BB53}} |
|||
| legal_AU = S4 |
|||
| legal_AU_comment = |
|||
| legal_CA = Rx-only |
|||
| legal_UK = POM |
|||
| legal_UK_comment = |
|||
| legal_US = Rx-only |
|||
| legal_status = |
|||
<!--Pharmacokinetic data--> |
|||
| bioavailability = 15–20% (口服)<ref name=MSR>{{cite web|title=Zovirax (acyclovir) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|access-date= 2014-02-05|url=http://reference.medscape.com/drug/zovirax-acyclovir-342601#showall|url-status=live|archive-url=https://web.archive.org/web/20140219005955/http://reference.medscape.com/drug/zovirax-acyclovir-342601#showall|archive-date=2014-02-19}}</ref> |
|||
| protein_bound = 9–33%<ref name = MSR/> |
|||
| metabolism = [[肝臟]] |
|||
| elimination_half-life = 2–4小時 |
|||
| excretion = [[腎]]臟 (62–90%保持原形式) |
|||
<!--Identifiers--> |
|||
| IUPHAR_ligand = 4829 |
| IUPHAR_ligand = 4829 |
||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number = 59277-89-3 |
| CAS_number = 59277-89-3 |
||
| ATC_prefix = J05 |
|||
| ATC_suffix = AB01 |
|||
| ATC_supplemental = {{ATC|D06|BB03}} {{ATC|S01|AD03}} |
|||
| PubChem = 2022 |
| PubChem = 2022 |
||
| PubChemSubstance = 46506002 |
|||
| DrugBank_Ref = {{drugbankcite|changed|??}} |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = APRD00567 |
|||
| DrugBank = DB00787 |
|||
| DrugBank2_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank2 = DB00787 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 1945 |
| ChemSpiderID = 1945 |
||
| 第23行: | 第50行: | ||
| KEGG_Ref = {{keggcite|correct|kegg}} |
| KEGG_Ref = {{keggcite|correct|kegg}} |
||
| KEGG = D00222 |
| KEGG = D00222 |
||
| KEGG2_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG2 = C06810 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| ChEBI = 2453 |
| ChEBI = 2453 |
||
| 第28行: | 第57行: | ||
| ChEMBL = 184 |
| ChEMBL = 184 |
||
| PDB_ligand = AC2 |
| PDB_ligand = AC2 |
||
<!--Chemical data--> |
|||
| IUPAC_name = 2-Amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-3''H''-purin-6-one |
|||
| C=8 | H=11 | N=5 | O=3 |
| C=8 | H=11 | N=5 | O=3 |
||
| SMILES = O=C2/N=C(\Nc1n(cnc12)COCCO)N |
|||
| molecular_weight = 225.21 g/mol |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15) |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = MKUXAQIIEYXACX-UHFFFAOYSA-N |
|||
| synonyms = Acycloguanosine |
|||
| melting_point = 256.5 |
| melting_point = 256.5 |
||
| bioavailability = 10–20% (口服) |
|||
| protein_bound = 9-33% |
|||
| metabolism = 病毒性胸腺嘧啶激酶 |
|||
| elimination_half-life = 2.2–20 hours |
|||
| excretion = [[肾]] |
|||
| pregnancy_category = B3 <small>([[Australia|Au]])</small>, B <small>([[United States|U.S.]])</small> |
|||
| legal_status = unscheduled/S4 <small>(Au)</small>, POM <small>([[United Kingdom|UK]])</small> |
|||
| routes_of_administration = [[intravenous|IV]], oral, topical |
|||
}} |
}} |
||
'''阿昔洛韋''' ({{lang-en|Ac<u>i</u>clovir }},簡稱ACV,也可拼寫為ac<u>y</u>clovir),<ref>{{cite web | vauthors = Kevin ES |title=The Aciclovir |url=https://deutschemedz.de/aciclovir/ |publisher=Kevin |language=DE |access-date= 2017-05-25}}</ref>是一種[[抗病毒藥物]]。<ref name=deClercq2005>{{cite news| vauthors = de Clercq E, Field HJ | publication-date = January 2006 | date = 2005-10-05 | title = Antiviral prodrugs – the development of successful prodrug strategies for antiviral chemotherapy| periodical = [[British Journal of Pharmacology]] | publisher = Wiley-Blackwell| volume = 147 | issue = 1| pages = 1–11| pmid= 16284630 | pmc= 1615839 | doi = 10.1038/sj.bjp.0706446}}</ref>主要用於治療[[單純皰疹病毒]](HSV)、[[水痘]]和[[帶狀皰疹]]的[[感染]]。<ref name=AHFS2015/>其他用途包括預防[[器官移植]]後[[巨細胞病毒屬]]感染,和預防[[人類疱疹病毒第四型]](EBV)感染之後的嚴重併發症。<ref name=AHFS2015/><ref name="pmid20739216">{{cite journal | vauthors = Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME | title = Antiviral treatment for severe EBV infections in apparently immunocompetent patients | journal = Journal of Clinical Virology | volume = 49 | issue = 3 | pages = 151–157 | date = November 2010 | pmid = 20739216 | doi = 10.1016/j.jcv.2010.07.008 }}</ref>它有[[口服給藥]]、塗抹用[[乳霜]]或[[靜脈注射]]的形式。<ref name=AHFS2015>{{cite web|title=Acyclovir|url=https://www.drugs.com/monograph/acyclovir.html|publisher=The American Society of Health-System Pharmacists|access-date=2015-01-01|url-status=live|archive-url=https://web.archive.org/web/20150105113809/http://www.drugs.com/monograph/acyclovir.html|archive-date=2015-01-05}}</ref> |
|||
'''阿昔洛韋'''<ref>https://www.termonline.cn/search?searchText=aciclovir</ref>(aciclovir,acyclovir,ACV)又称'''无环鸟苷'''<ref>https://terms.naer.edu.tw/detail/fc21ff8b73b4166391ff19e0002396a1/?seq=1</ref>(acycloguanosine),是一种化学合成的[[鸟嘌呤]][[核苷类似物]],用作[[抗病毒药物]]<ref>{{Cite journal|title=Antiviral prodrugs - the development of successful prodrug strategies for antiviral chemotherapy|url=https://www.ncbi.nlm.nih.gov/pubmed/16284630|last=De Clercq|first=Erik|last2=Field|first2=Hugh J.|date=2006-01-01|journal=British Journal of Pharmacology|issue=1|doi=10.1038/sj.bjp.0706446|volume=147|pages=1–11|issn=0007-1188|pmc=1615839|pmid=16284630|access-date=2016-12-16|archive-date=2016-12-20|archive-url=https://web.archive.org/web/20161220174627/https://www.ncbi.nlm.nih.gov/pubmed/16284630|dead-url=no}}</ref>。其抗病毒作用具有高度选择性,主要对[[单纯疱疹病毒]]1型和2型具有强烈抑制作用,对水痘-带状疱疹病毒也有一定的抑制作用。 |
|||
使用後常見的副作用有[[噁心]]和[[腹瀉]]。<ref name=AHFS2015/>可能的的嚴重副作用有[[腎]]臟問題和{{le|血小板減少症|thrombocytopenia|血小板減少}}。<ref name=AHFS2015/>[[肝臟]]或腎臟功能較差的人使用時,應更為謹慎。<ref name=AHFS2015/>一般認為個體在[[妊娠|懷孕]]期間使用對胎兒屬於安全,尚未觀察到任何危害案例。<ref name=AHFS2015/><ref>{{cite web|title=Prescribing medicines in pregnancy database|url=http://www.tga.gov.au/hp/medicines-pregnancy.htm|work=Australian Government|access-date=2014-04-22|date=2014-03-03|url-status=live|archive-url=https://web.archive.org/web/20140408040902/http://www.tga.gov.au/hp/medicines-pregnancy.htm|archive-date=2014-04-08}}</ref>使用個體進行[[母乳哺育]],對於嬰兒似乎屬於安全。<ref name=Ric2015>{{cite book| vauthors = Hamilton R |title=Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition|date=2015|publisher=Jones & Bartlett Learning|isbn=9781284057560|page=59}}</ref><ref>{{cite web|title=Acyclovir use while Breastfeeding|url=https://www.drugs.com/breastfeeding/acyclovir.html|access-date=2016-03-08|date=2015-03-10|quote=Even with the highest maternal dosages, the dosage of acyclovir in milk is only about 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants|url-status=live|archive-url=https://web.archive.org/web/20160305200238/http://www.drugs.com/breastfeeding/acyclovir.html|archive-date= 2016-03-05}}</ref>阿昔洛韋是一種模仿[[鳥苷]]的{{le|核苷類似物|nucleoside analogue}},<ref name=AHFS2015/>其作用是抑制[[病毒]]生產[[去氧核醣核酸|DNA]]的能力。<ref name=AHFS2015/> |
|||
阿昔洛韋主要用來治療[[单纯疱疹病毒]]感染、[[水痘]]、[[带状疱疹]]。另外也應用在移植手術後,預防[[人類疱疹病毒第四型]]等[[巨細胞病毒]]感染的預防性投藥。同時具有口服劑型與[[靜脈注射]]劑型。<ref name=":0">{{Cite web|url=http://www.drugs.com/monograph/acyclovir.html|title="Acyclovir|accessdate=2015-01-01|author=|date=|publisher=The American Society of Health-System Pharmacists.|archive-date=2015-01-05|archive-url=https://web.archive.org/web/20150105113809/http://www.drugs.com/monograph/acyclovir.html|dead-url=no}}</ref>阿昔洛韦是最常用的抗病毒药物之一。 |
|||
阿昔洛韋於1974年由[[葛蘭素史克]]藥業取得[[美國]]專利,並於1981年於美國獲准用於醫療用途。<ref name=Fis2006>{{cite book | vauthors = Fischer J, Ganellin CR |title=Analogue-based Drug Discovery |date=2006 |publisher=John Wiley & Sons |isbn=9783527607495 |page=504 |url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA504 }}</ref>此藥物已列入[[世界衛生組織基本藥物標準清單]]中。<ref name="WHO21st">{{cite book | vauthors = ((World Health Organization)) | title = World Health Organization model list of essential medicines: 21st list 2019 | year = 2019 | hdl = 10665/325771 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO | hdl-access=free }}</ref><ref name="WHO22nd">{{cite book | vauthors = ((World Health Organization)) | title = World Health Organization model list of essential medicines: 22nd list (2021) | year = 2021 | hdl = 10665/345533 | author-link = World Health Organization | publisher = World Health Organization | location = Geneva | id = WHO/MHP/HPS/EML/2021.02 | hdl-access=free }}</ref>它有[[通用名藥物]],且以許多品牌在全球銷售。<ref name=Names>{{cite web|title=Aciclovir|url=https://www.drugs.com/international/aciclovir.html|website=Drugs.com|access-date= 2015-09-06|url-status=live|archive-url=https://web.archive.org/web/20150923225253/http://www.drugs.com/international/aciclovir.html|archive-date= 2015-09-23}}</ref>它是美國於2020年排名第162最常使用的[[處方藥]],開立的處方箋數量超過300萬張。<ref>{{cite web | title = The Top 300 of 2020 | url = https://clincalc.com/DrugStats/Top300Drugs.aspx | website = ClinCalc | access-date = 2022-10-07}}</ref><ref>{{cite web | title = Acyclovir - Drug Usage Statistics | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/Acyclovir | access-date = 2022-10-07 }}</ref> |
|||
阿昔洛韋最常見的副作用是噁心、嘔吐。有時會造成腎臟受損或血小板低下等較嚴重的副作用。 由於藥物代謝的關係,肝腎功能較差者使用藥物時的劑量要多加注意。<ref name=":0" /> 阿昔洛韋是相對安全的婦女用藥,並未觀察到特殊的副作用。<ref name=":0" /><ref>{{Cite web|url=http://www.tga.gov.au/hp/medicines-pregnancy.htm#.U1Yw8Bc3tqw|title=Prescribing medicines in pregnancy database|accessdate=2014-04-22|author=|date=2014-03-03|publisher=Australian Government.|archive-date=2014-04-08|archive-url=https://web.archive.org/web/20140408040902/http://www.tga.gov.au/hp/medicines-pregnancy.htm#.U1Yw8Bc3tqw|dead-url=no}}</ref> 即便在[[母乳餵養]]時使用也相對安全。<ref name="#1">{{Cite book|url=https://www.worldcat.org/oclc/922644939|date=2014-01-01|publisher=Jones & Bartlett Learning|chapter=Tarascon pocket pharmacopoeia 2015 deluxe lab coat edition.|oclc=922644939|title=|access-date=2016-12-16|archive-date=2019-07-13|archive-url=https://web.archive.org/web/20190713084658/https://www.worldcat.org/title/tarascon-pocket-pharmacopoeia-2015-deluxe-lab-coat-edition/oclc/922644939|dead-url=no}}</ref>阿昔洛韋是一種從[[鳥苷]]而來的核酸衍生物,其作用原理為阻止病毒複製[[脱氧核糖核酸]]。<ref name=":0" /> |
|||
==醫療用途== |
|||
阿昔洛韋於1977年被發現。<ref>{{Cite book|url=https://www.worldcat.org/oclc/854681015|last=Erik,|first=De Clercq,|date=2013-01-01|publisher=Academic Press|chapter=Anti-viral agents|oclc=854681015|title=|access-date=2016-12-16|archive-date=2019-07-13|archive-url=https://web.archive.org/web/20190713124826/https://www.worldcat.org/title/anti-viral-agents/oclc/854681015|dead-url=no}}</ref>阿昔洛韦曾因其极高的选择性和低细胞毒性而被视为是抗病毒治疗的新时代的开始,其发明者美国药理学家[[格特鲁德·B·埃利恩]](Gertrude Belle Elion)也部分因此而获得1988年[[诺贝尔生理学或医学奖]]。阿昔洛韋也被列入[[世界卫生组织基本药物标准清单]],是建立基礎照護系統時,最重要的必備基礎藥物之一。<ref>{{Cite web|url=http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1|title=WHO Model List of EssentialMedicines|accessdate=2014-04-22|author=World Health Organization.|date=October 2013|publisher=|archive-date=2014-04-23|archive-url=https://web.archive.org/web/20140423005004/http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1|dead-url=no}}</ref> 目前阿昔洛韋已經是[[通用名药物]],在全球有許多不同的商品名。<ref>{{Cite web|url=http://www.drugs.com/international/aciclovir.html|title=Aciclovir|accessdate=6 September 20|author=Drugs.com|date=|publisher=|archive-date=2015-09-23|archive-url=https://web.archive.org/web/20150923225253/http://www.drugs.com/international/aciclovir.html|dead-url=no}}</ref>阿昔洛韋每一劑的批發價約為0.03 到0.12美金。<ref>{{Cite web|url=http://erc.msh.org/dmpguide/resultsdetail.cfm?language=english&code=ACI400T&s_year=2014&year=2014&str=400%20mg&desc=Aciclovir&pack=new&frm=TAB-CAP&rte=PO&class_code2=06%2E4%2E1%2E&supplement=&class_name=%2806%2E4%2E1%2E%29Antiherpes%20medicines%3Cbr%3E|title=Aciclovir|accessdate=2015-09-06|author=International Drug Price Indicator Guide.|date=|publisher=|deadurl=yes|archiveurl=https://web.archive.org/web/20170305062222/http://erc.msh.org/dmpguide/resultsdetail.cfm?language=english&code=ACI400T&s_year=2014&year=2014&str=400%20mg&desc=Aciclovir&pack=new&frm=TAB-CAP&rte=PO&class_code2=06%2E4%2E1%2E&supplement=&class_name=%2806%2E4%2E1%2E%29Antiherpes%20medicines%3Cbr%3E|archivedate=2017年3月5日}}</ref>在美國,每劑阿昔洛韋大約0.5美金。<ref name=":0" /><ref name="#1"/> |
|||
[[File:Pile of round aciclovir tablets.jpg|thumb|每片含阿昔洛韋400毫克的丸劑。]] |
|||
此藥物主要用於治療單純皰疹病毒、水痘和帶狀皰疹感染,包括:<ref name =MSR/><ref name=AMH>{{cite book | title = Australian Medicines Handbook | year = 2013 | publisher = The Australian Medicines Handbook Unit Trust | isbn = 978-0-9805790-9-3 | edition = 2013 | place = Adelaide | editor = Rossi, S }}</ref><ref name = BNF>{{cite book | last1 = Joint Formulary Committee | title = British National Formulary (BNF) | year = 2013 | url = https://archive.org/details/bnf65britishnati0000unse | isbn = 978-0-85711-084-8 | edition = 65 | location = London | publisher = Pharmaceutical Press | url-access = registration }}</ref> |
|||
==作用机理== |
|||
*[[生殖器]]單純皰疹病毒(治療和[[預防醫學|預防]]) |
|||
阿昔洛韦与以前的核苷类似物类抗病毒药物不同,它只含有部分的核苷结构,其[[糖环]]被一条开放链取代。阿昔洛韦可被病毒胸腺嘧啶激酶(Thymidine Kinase, TK)催化磷酸化变成一磷酸无环鸟苷(acyclo-GMP),此过程比细胞胸腺嘧啶激酶快3000多倍。在细胞酶的催化下转变成二磷酸无环鸟苷(acyclo-GDP)及三磷酸无环鸟苷(acyclo-GTP)。acyclo-GMP是一种十分有效的DNA聚合酶抑制剂,它与病毒的亲和力是细胞聚合酶的100倍。作为酶作用物,acyclo-GTP与病毒的DNA结合,导致DNA链终止。同时,病毒酶不能将acyclo-GTP从DNA链上除去,从而导致DNA聚合酶的作用被进一步的抑制。可能是因为细胞磷酸酶的作用,acyclo-GTP在细胞中的代谢相当迅速。 |
|||
*[[新生兒單純疱疹病毒感染]] |
|||
*[[唇瘡]] |
|||
*帶狀泡疹 |
|||
*[[免疫缺陷]]者的急性水痘感染 |
|||
*[[單純皰疹病毒腦炎]] |
|||
*免疫缺陷者的急性{{le|皮膚黏膜交界處|mucocutaneous junction}}單純皰疹病毒 |
|||
*[[單純疱疹性角膜炎]]和單純皰疹病毒{{le|瞼緣炎|blepharitis}} |
|||
*為免疫能力低下族群(如接受[[癌症]][[化學治療]]者)提供皰疹病毒預防<ref name="Cancer">{{cite journal | vauthors = Elad S, Zadik Y, Hewson I, Hovan A, Correa ME, Logan R, Elting LS, Spijkervet FK, Brennan MT | title = A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea | journal = Supportive Care in Cancer | volume = 18 | issue = 8 | pages = 993–1006 | date = August 2010 | pmid = 20544224 | doi = 10.1007/s00520-010-0900-3 | s2cid = 2969472 }}</ref> |
|||
其治療EBV病毒感染的效力尚不清楚。<ref name=AHFS2015/>尚未發現它對治療EBV引起的[[傳染性單核細胞增多症]]有效。<ref>{{cite journal | vauthors = Gershburg E, Pagano JS | title = Epstein-Barr virus infections: prospects for treatment | journal = The Journal of Antimicrobial Chemotherapy | volume = 56 | issue = 2 | pages = 277–281 | date = August 2005 | pmid = 16006448 | doi = 10.1093/jac/dki240 | citeseerx = 10.1.1.320.6721 }}</ref>[[伐昔洛韋]]和阿昔洛韋透過抑制病毒的DNA複製而發揮作用,但截至2016年,幾乎沒有證據顯示其對治療EBV病毒有效。此藥物價格昂貴,有引起抗病毒藥物抗藥性的風險,並且(在1%至10%的病例中)可能會導致患者發生不舒服的[[副作用]]。<ref name="pmid27933614">{{cite journal | vauthors = De Paor M, O'Brien K, Fahey T, Smith SM | title = Antiviral agents for infectious mononucleosis (glandular fever) | journal = The Cochrane Database of Systematic Reviews | volume = 2016 | issue = 12 | pages = CD011487 | date = December 2016 | pmid = 27933614 | pmc = 6463965 | doi = 10.1002/14651858.CD011487.pub2 }}</ref> |
|||
==副作用== |
|||
一般[[耐受性]]良好。局部用药有其轻度刺激症状,静脉滴注外渗可引起局部炎症和[[静脉炎]],还有厌食、恶心、头痛和皮症。 |
|||
口服形式的阿昔洛韋似乎不會將罹患帶狀皰疹個體的疼痛風險降低。<ref>{{cite journal | vauthors = Chen N, Li Q, Yang J, Zhou M, Zhou D, He L | title = Antiviral treatment for preventing postherpetic neuralgia | journal = The Cochrane Database of Systematic Reviews | volume = 2014 | issue = 2 | pages = CD006866 | date = February 2014 | pmid = 24500927 | doi = 10.1002/14651858.CD006866.pub3 | pmc = 10583132 }}</ref>使用阿昔洛韋治療眼部皰疹患者,可能比使用[[碘苷]]更為有效及安全。<ref name=Wilhelmus2015>{{cite journal | vauthors = Wilhelmus KR | title = Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis | journal = The Cochrane Database of Systematic Reviews | volume = 1 | pages = CD002898 | date = January 2015 | issue = 1 | pmid = 25879115 | pmc = 4443501 | doi = 10.1002/14651858.CD002898.pub5 }}</ref>目前尚不清楚含阿昔洛韋眼藥水是否比含{{le|溴夫定|brivudine}}眼藥水更為有效。<ref name=Wilhelmus2015 /> |
|||
== 临床应用 == |
|||
阿昔洛韦为[[单纯疱疹病毒|HSV]]感染的首选药物。局部应用治疗疱疹性角膜炎、单纯疱疹和带状疱疹,口服或静脉注射可有效治疗单纯疱疹脑炎、生殖器疱疹、免疫缺陷病人单纯疱疹感染等。 |
|||
靜脈注射形式的阿昔洛韋可有效治療不同種類皰疹病毒引起的嚴重狀況,包括嚴重局部感染、嚴重生殖器感染、水痘和皰疹病毒腦炎。它對全身性或創傷性皰疹感染、皰疹性濕疹和單純皰疹病毒腦炎的治療也有效。對20世紀80年代發表的研究報告審查,顯示如果於疫情爆發初期使用阿昔洛韋,可在減少發病數量和持續時間方面發揮一定作用。<ref>{{cite journal | vauthors = Worrall G | title = Acyclovir in recurrent herpes labialis | journal = BMJ | volume = 312 | issue = 7022 | pages = 6 | date = January 1996 | pmid = 8555890 | pmc = 2349724 | doi = 10.1136/bmj.312.7022.6 | url = http://www.bmj.com/cgi/content/full/312/7022/6 | url-status = live | archive-url = https://web.archive.org/web/20070515183925/http://www.bmj.com/cgi/content/full/312/7022/6 | archive-date = 2007-05-15 }} – Editorial</ref>研究顯示外用阿昔洛韋在疫情爆發初期和晚期使用均有效,這項審查採用更嚴格的方法和更大的樣本量,因此具有更高的可信度。<ref>{{cite journal | vauthors = Spruance SL, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T | title = Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials | journal = Antimicrobial Agents and Chemotherapy | volume = 46 | issue = 7 | pages = 2238–2243 | date = July 2002 | pmid = 12069980 | pmc = 127288 | doi = 10.1128/aac.46.7.2238-2243.2002 }}</ref>在試驗中顯示藥物對預防[[人類免疫缺乏病毒|HIV]]傳播並無作用,但可幫助未接受[[抗反轉錄病毒藥物]](ART)治療的人群減緩HIV疾病的進展。這項發現強調對HIV感染者中測試簡單、廉價的非ART處理策略(例如使用阿昔洛韋和[[複方新諾明]])的重要性。<ref>{{cite journal | vauthors = Mascolinli M, Kort R | title = 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention: summary of key research and implications for policy and practice - biomedical prevention | journal = Journal of the International AIDS Society | volume = 13 | issue = Suppl 1 | pages = S4 | date = June 2010 | pmid = 20519025 | pmc = 2880255 | doi = 10.1186/1758-2652-13-S1-S4 | doi-access = free }}</ref> |
|||
===懷孕=== |
|||
阿昔洛韋被[[美國食品藥物管理局]](FDA)歸類為B類藥物(藥物在動物研究中並未顯示出對胎兒的風險,但尚無足够的懷孕婦女研究来確定其安全性),<ref name=Label>{{cite web | publisher = FDA | work = GSK | url =http://www.accessdata.fda.gov/drugsatfda_docs/label/2001/18828s26s27lbl.pdf | title = Acyclovir label | archive-url = https://web.archive.org/web/20170908162359/https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/18828s26s27lbl.pdf | archive-date=2017-09-08 | date = 2005 }}</ref>[[美國疾病管制與預防中心]](CDC)和其他機構已於2005年宣布在生殖器皰疹嚴重復發或首次發作期間,可使用阿昔洛韋作治療。<ref>{{cite journal | vauthors = | title = Drugs for non-HIV viral infections | journal = Treatment Guidelines from the Medical Letter | volume = 3 | issue = 32 | pages = 23–32 | date = April 2005 | pmid = 15767977 }}</ref>對於嚴重的HSV感染(尤其是傳播性HSV),也可使用靜脈注射形式進行治療。<ref name="pmid19357635">{{cite journal | vauthors = Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H | title = Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America | journal = MMWR. Recommendations and Reports| volume = 58 | issue = RR-4 | pages = 1–207; quiz CE1–4 | date = April 2009 | pmid = 19357635 | doi = | url = https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5804a1.htm }}</ref> |
|||
對{{le|小鼠|mouse}}、[[兔]]子和{{le|大鼠|rat}}(使用超過相當於人類使用劑量的10倍以上)在{{le|器官形成|organogenesis}}過程中所做的研究未證明會導致[[先天性障礙|出生缺陷]]。<ref name =TGA/>在對大鼠進行的研究中,於懷孕第10天給予相當於人類標準穩態濃度63倍的藥物,<ref group = Note>Subject to the same conditions as before</ref>會出現頭部和尾部發生異常的結果。<ref name = TGA/> |
|||
CDC於2006年推薦使用阿昔洛韋於治療個體於懷孕期間的水痘感染,特別是在中期和晚期。<ref>{{cite book | author = Centers for Disease Control and Prevention. | title = Epidemiology and prevention of vaccine-preventable diseases. | edition = 9th | publisher = Public Health Foundation | date = January 2006 | pages = 171–192 }}</ref> |
|||
阿昔洛韋會在乳汁中出現,因此建議進行[[母乳哺育]]的婦女應予謹慎。有限的試驗研究顯示母親口服阿昔洛韋後,接受哺乳的嬰兒每天會接觸到大約0.3毫克/公斤的劑量。若哺乳期母親乳房附近或乳房有皰疹病症,應避免親自哺乳。<ref name=Label/><ref name="Gartner_2005">{{cite journal | vauthors = Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI | title = Breastfeeding and the use of human milk | journal = Pediatrics | volume = 115 | issue = 2 | pages = 496–506 | date = February 2005 | pmid = 15687461 | doi = 10.1542/peds.2004-2491 | s2cid = 5791615 | doi-access = free }}</ref> |
|||
==不良影響== |
|||
===全身治療=== |
|||
使用阿昔洛韋作全身性治療(口服或靜脈注射)常見的藥物不良反應(≥1%的患者)包括噁心、[[嘔吐]]、[[腹瀉]]、[[腦病變]](僅發生於靜脈注射形式)、注射部位反應和[[頭痛]]。有報導在高劑量使用時會出現[[幻覺]]。不常見的不良反應(0.1-1%的患者)有煩躁、[[眩暈]]、[[困惑]]、[[頭暈]]、[[水腫]]、[[關節痛]]、喉嚨痛、[[便秘]]、腹痛、脫髮、皮[[疹]]和虛弱。罕見的不良反應(<0.1%的患者)有[[昏迷]]、[[癲癇發作]]、[[嗜中性白血球低下]]、[[白细胞减少症|白血球減少症]]、{{le|尿結晶|crystalluria}}、[[食慾不振|厭食]]、疲倦、[[肝炎]]、[[史蒂芬斯-強森症候群]]、{{le|中毒性表皮壞死鬆解症|toxic epidermal necrolysis}}、[[血栓性血小板減少性紫癜]]、[[過敏性休克]]<ref name="AMH" />和[[科塔爾症候群]]。 |
|||
由於靜脈注射阿昔洛韋會造成腎臟中尿結晶,會對5%至10%的使用者造成可逆性{{le|腎毒性|nephrotoxicity}}。對於脫水和已存在腎功能不全的患者進行快速輸注阿昔洛韋有較高尿結晶腎病變風險。充足攝取水分、減緩輸注速度以及根據患者腎功能而調整劑量將可將這類風險降低。<ref name=razonable>{{cite journal | vauthors = Razonable RR | title = Antiviral drugs for viruses other than human immunodeficiency virus | journal = Mayo Clinic Proceedings | volume = 86 | issue = 10 | pages = 1009–1026 | date = October 2011 | pmid = 21964179 | pmc = 3184032 | doi = 10.4065/mcp.2011.0309 }}</ref><ref name="pmid6285711">{{cite journal | vauthors = Brigden D, Rosling AE, Woods NC | title = Renal function after acyclovir intravenous injection | journal = The American Journal of Medicine | volume = 73 | issue = 1A | pages = 182–185 | date = July 1982 | pmid = 6285711 | doi = 10.1016/0002-9343(82)90087-0 }}</ref><ref name="pmid3376977">{{cite journal | vauthors = Sawyer MH, Webb DE, Balow JE, Straus SE | title = Acyclovir-induced renal failure. Clinical course and histology | journal = The American Journal of Medicine | volume = 84 | issue = 6 | pages = 1067–1071 | date = June 1988 | pmid = 3376977 | doi = 10.1016/0002-9343(88)90313-0 | url = https://zenodo.org/record/1253778 }}</ref> |
|||
阿昔洛韋[[代謝產物]][[9-羧基甲氧基甲基鳥嘌呤]](9-CMMG) 已被證明會產生神經系統不良事件,特別是對老年人和腎功能不全的人群。<ref>{{cite journal | vauthors = Helldén A, Odar-Cederlöf I, Diener P, Barkholt L, Medin C, Svensson JO, Säwe J, Ståhle L | title = High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study | journal = Nephrology, Dialysis, Transplantation | volume = 18 | issue = 6 | pages = 1135–1141 | date = June 2003 | pmid = 12748346 | doi = 10.1093/ndt/gfg119 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Berry L, Venkatesan P | title = Aciclovir-induced neurotoxicity: Utility of CSF and serum CMMG levels in diagnosis | journal = Journal of Clinical Virology | volume = 61 | issue = 4 | pages = 608–610 | date = December 2014 | pmid = 25440915 | doi = 10.1016/j.jcv.2014.09.001 }}</ref><ref>{{cite journal | vauthors = Chowdhury MA, Derar N, Hasan S, Hinch B, Ratnam S, Assaly R | title = Acyclovir-Induced Neurotoxicity: A Case Report and Review of Literature | journal = American Journal of Therapeutics | volume = 23 | issue = 3 | pages = e941–e943 | date = 2016 | pmid = 24942005 | doi = 10.1097/MJT.0000000000000093 | s2cid = 32983100 }}</ref> |
|||
===外用療法=== |
|||
含阿昔洛韋的外用乳霜通常會導致(≥1%) 的個體皮膚乾燥、剝落或短暫刺痛/灼熱感。罕見的不良反應有[[紅斑]]或發[[癢]]。<ref name="AMH" />當施用於眼睛時,通常會導致(≥1%)的個體發生短暫的輕微刺痛。在眼部使用阿昔洛韋,有少數(0.1-1%的患者)會發生{{le|角膜點狀上皮擦傷|punctate keratitis}}或是過敏反應。<ref name="AMH" /> |
|||
==藥物交互作用== |
|||
在體外進行複製研究發現[[酮康唑]]與阿昔洛韋併用時,對HSV-1和HSV-2的感染具有協同、劑量相關性抗病毒活性。然而這種效應尚未在[[臨床試驗]]上得到證實,因此需進行更多研究來評估協同作用的實際潛力。<ref>{{cite journal | vauthors = Pottage JC, Kessler HA, Goodrich JM, Chase R, Benson CA, Kapell K, Levin S | title = In vitro activity of ketoconazole against herpes simplex virus | journal = Antimicrobial Agents and Chemotherapy | volume = 30 | issue = 2 | pages = 215–219 | date = August 1986 | pmid = 3021048 | pmc = 180521 | doi = 10.1128/aac.30.2.215 }}</ref> |
|||
針對[[丙磺舒]]與阿昔洛韋同時給藥的研究顯示阿昔洛韋的[[生物半衰期]]可延長,受到尿液和腎清除的速率被降低。<ref name=Label/> |
|||
[[干擾素]]與阿昔洛韋合併使用時會產生協同作用,對接受靜脈注射干擾素的患者於施用阿昔洛韋時應謹慎。<ref>GlaxoSmithKline. Zovirax® (acyclovir sodium) for injection prescribing information. Research Triangle Park, NC; 2003 Nov</ref> |
|||
雖然HIV感染者經常會將[[齊多夫定]]與阿昔洛韋一起使用,但至少一名患者在接受靜脈注射阿昔洛韋30-60天後出現極度嗜睡和昏睡的[[神經毒性]],停用阿昔洛韋後症狀得到舒緩。<ref>{{cite journal | vauthors = Bach MC | title = Possible drug interaction during therapy with azidothymidine and acyclovir for AIDS | journal = The New England Journal of Medicine | volume = 316 | issue = 9 | pages = 547 | date = February 1987 | pmid = 3468354 | doi = 10.1056/NEJM198702263160912 }}</ref> |
|||
==體液中檢測== |
|||
可對血漿或血清中的阿昔洛韋進行數量檢測,以確認腎功能不全患者的藥物蓄積,或是診斷急性用藥過量中毒狀況。<ref name="DT8">{{cite book| vauthors = Baselt RC |title=Disposition of toxic drugs and chemicals in man|year=2008|publisher=Biomedical Publications|location=Foster City, CA|isbn=9780962652370|pages=29–31|edition=8th}}</ref> |
|||
==作用機制== |
|||
[[Image:Guanosine aciclovir comparison.svg|200px|thumb|[[鳥苷]](上)與阿昔洛韋(下)分子結構對照。]] |
|||
[[File:Acyclovir.svg|thumb|370x370px|阿昔洛韋作用機制 (抑制HSV DNA聚合酶和掺入HSV DNA)。]] |
|||
阿昔洛韋由病毒的[[胸苷激酶]]轉化為阿昔洛韋單磷酸,然後由宿主細胞[[激酶]]轉化為阿昔洛韋三磷酸(ACV-TP,也稱為阿昔洛-GTP)。<ref name = TGA/>ACV-TP是種非常有效的病毒DNA複製抑制劑。 ACV-TP會競爭性抑制並將病毒DNA聚合酶滅活。<ref>{{cite web|url=https://gsksource.com/pharma/content/gsk/source/us/en/brands/valtrex.html|title=VALTREX (valacyclovir hydrochloride) Caplets -GSKSource|website=gsksource.com|access-date=2019-08-02}}</ref>其單磷酸鹽也會摻入病毒DNA之中,導致[[蛋白質生物合成]]終止。<ref name=TGA>{{cite web|title=PRODUCT INFORMATION NAME OF THE DRUG OZVIR TABLETS|work=TGA eBusiness Services|publisher=Ranbaxy Australia Pty Ltd|date=2011-08-26|access-date=2014-02-06|url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2009-PI-00595-3|format=PDF|url-status=live|archive-url=https://web.archive.org/web/20160820205438/https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2009-PI-00595-3|archive-date=2016-08-20}}</ref><ref name=DM>{{cite web|title=Acyclovir (acyclovir) Capsule Acyclovir (acyclovir) Tablet [Genpharm Inc.]|work=DailyMed|publisher=Genpharm Inc.|date=November 2006|access-date=2014-02-05|url=http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=12ea5042-8ea6-4ee9-a734-684a73edb373|url-status=live|archive-url=https://web.archive.org/web/20140221224423/http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=12ea5042-8ea6-4ee9-a734-684a73edb373|archive-date=2014-02-21}}</ref><ref name=EMC>{{cite web|title=Aciclovir Tablets BP 400mg - Summary of Product Characteristics (SPC)|work=electronic Medicines Compendium|publisher=Actavis UK Ltd|date= 2012-08-20|access-date=2014-02-05|url=http://www.medicines.org.uk/emc/medicine/23721/SPC/Aciclovir+Tablets+BP+400mg/|url-status=live|archive-url=https://web.archive.org/web/20140222201708/http://www.medicines.org.uk/EMC/medicine/23721/SPC/Aciclovir+Tablets+BP+400mg|archive-date=2014-02-22}}</ref> |
|||
===抗藥性=== |
|||
免疫系統健康的人發生阿昔洛韋抗藥性的案例很少見,但在長期接受抗病毒預防的免疫缺陷人群(接受器官移植者、因HIV感染而患有後天免疫缺陷症候群者)中較為常見(高達10%)。 對HSV的抗藥性機制有病毒胸苷激酶缺陷,以及病毒胸苷激酶或DNA聚合酶的突變,而將基質敏感性改變。<ref name="Martindale">{{cite book|title=Martindale: The Complete Drug Reference|chapter=Aciclovir|date=7 August 2013|publisher=Pharmaceutical Press|editor=Sweetman, S|access-date=6 February 2014|chapter-url=http://www.medicinescomplete.com/mc/martindale/current/ms-1682-a.htm|location=London, UK}}</ref><ref>{{cite journal | vauthors = Piret J, Boivin G | title = Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management | journal = Antimicrobial Agents and Chemotherapy | volume = 55 | issue = 2 | pages = 459–472 | date = February 2011 | pmid = 21078929 | pmc = 3028810 | doi = 10.1128/AAC.00615-10 }}</ref> |
|||
===微生物學=== |
|||
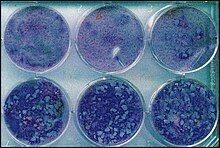
[[File:VZV plaques.jpg|thumb|right|水痘帶狀疱疹病毒在含有不同劑量阿昔洛韋的培養皿中單層[[MRC-5細胞]]上形成斑塊。通計算斑塊數量,可估算出阿昔洛韋對病毒的效力。]] |
|||
阿昔洛韋對[[皰疹病毒科]]中的大多數都有活性,如下(依活性降冪方式排列):<ref name="OBrien1989">{{cite journal | vauthors = O'Brien JJ, Campoli-Richards DM | title = Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy | journal = Drugs | volume = 37 | issue = 3 | pages = 233–309 | date = March 1989 | pmid = 2653790 | doi = 10.2165/00003495-198937030-00002 | s2cid = 240858022 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Wagstaff AJ, Faulds D, Goa KL | title = Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy | journal = Drugs | volume = 47 | issue = 1 | pages = 153–205 | date = January 1994 | pmid = 7510619 | doi = 10.2165/00003495-199447010-00009 | doi-access = free }}</ref> |
|||
*單純皰疹病毒 I 型 (HSV-1) |
|||
*單純皰疹病毒 II 型 (HSV-2) |
|||
*[[水痘帶狀皰疹病毒]](VZV) |
|||
*EBV病毒 |
|||
*巨細胞病毒屬(HCMV) – 活性最低 |
|||
==藥物動力學== |
|||
阿昔洛韋的水溶性不佳,口服形式的生物利用度不高(15-30%),因此如果需要高濃度的效果,則需透過靜脈注射的形式。口服給藥後1-2小時後出現[[最大血藥濃度]]。根據{{le|美國生物製藥分類系統|Biopharmaceutical Classification System}}(BCS),阿昔洛韋屬於BCS III 類藥物,即具可溶性但腸道滲透性低。<ref name="J.R. Karmokeretal.2019">{{cite journal | vauthors = Karmoker JR, Hasan I, Ahmed N, Saifuddin M, Reza MS |year=2019 |title=Development and Optimization of Acyclovir Loaded Mucoadhesive Microspheres by Box -Behnken Design |journal=Dhaka University Journal of Pharmaceutical Sciences |volume=18|issue=1|pages=1–12 |doi=10.3329/dujps.v18i1.41421|doi-access=free }}</ref>阿昔洛韋分佈率高,報導的蛋白質結合率為9%至33%。<ref name="emc SPC">{{cite web|url=https://www.medicines.org.uk/EMC/medicine/23721/SPC/Aciclovir+Tablets+BP+400mg|title=Aciclovir Tablets BP 400mg - Summary of Product Characteristics (SmPC) - (emc)|website=www.medicines.org.uk}}</ref>阿昔洛韋的生物半衰期(t<sub>½</sub>)取決於年齡:新生兒為4小時、1-12歲兒童為2-3小時,而成人為3小時。<ref name = MSR/> |
|||
==歷史== |
|||
阿昔洛韋被視為是種啟動抗病毒藥物新時代的先發者,它具有極高的選擇性和低[[細胞毒性]]。<ref name=deClercq2005/>它在1970年代中期被發現後,即開始用作有效治療皰疹病毒家族中大多數(包括單純皰疹病毒和水痘帶狀皰疹病毒)引起感染的有效藥物。合成阿昔洛韋的基礎是一種由[[加勒比]][[海綿]]{{le|Cryptotethya crypta|Cryptotethya crypta}}分離出的核苷。<ref>{{cite book| vauthors = Garrison T |title=Oceanography: An Invitation to Marine Science, 3rd ed.|year=1999|publisher=Wadsworth Publishing Company|location=Belmont, CA|pages=471}}</ref><ref name=bioactive>{{cite journal | vauthors = Sepčić K | s2cid = 84041855 | title = Bioactive Alkylpyridinium Compounds from Marine Sponges | journal = Toxin Reviews | volume = 19 | issue = 2 | pages = 139–160 | year = 2000 | doi = 10.1081/TXR-100100318 }}</ref><ref name=Laport2009>{{cite journal | vauthors = Laport MS, Santos OC, Muricy G | title = Marine sponges: potential sources of new antimicrobial drugs | journal = Current Pharmaceutical Biotechnology | volume = 10 | issue = 1 | pages = 86–105 | date = January 2009 | pmid = 19149592 | doi = 10.2174/138920109787048625 }}</ref>它是由Howard Schaeffer與{{le|羅伯·文思|Robert Vince (scientist)}}、S. Bittner和S. Gurwara共同發現的腺苷類似物 - 無環腺苷,具有不錯的抗病毒活性。<ref name="Schaffer JMC">{{cite journal | vauthors = Schaeffer HJ, Gurwara S, Vince R, Bittner S | title = Novel substrate of adenosine deaminase | journal = Journal of Medicinal Chemistry | volume = 14 | issue = 4 | pages = 367–369 | date = April 1971 | pmid = 5553754 | doi = 10.1021/jm00286a024 }}</ref>後來Schaeffer加入葛蘭素史克,與藥理學家 [[格特魯德·B·埃利恩]]繼續開發此藥物。<ref>{{cite journal | vauthors = Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ | title = Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 74 | issue = 12 | pages = 5716–5720 | date = December 1977 | pmid = 202961 | pmc = 431864 | doi = 10.1073/pnas.74.12.5716 | doi-access = free | bibcode = 1977PNAS...74.5716E }}</ref>一項於1979年發佈關於阿昔洛韋的美國專利中將Schaeffer列為發明人。<ref>{{cite patent|country=US|number=4146715 | title = 2-Amido-9-(2-acyloxyethoxymethyl)hypoxanthines | inventor = Schaeffer HJ | assign1 = Burrough Wellcome Co | gdate = 1979-03-27 | postscript = . }}</ref>羅伯·文思後來發明[[阿巴卡韋]]- 一種用於HIV患者的[逆轉錄酶抑制劑]]。<ref>{{cite journal | vauthors = Vince R | year = 2008 | title = A brief history of the development of Ziagen | journal = Chemtracts | volume = 21 | pages = 127–134 }}</ref>埃利恩則因使用開創性[[藥物設計]]於新藥物開發而榮獲1988年[[諾貝爾生理學或醫學獎]]。 |
|||
伐昔洛韋是阿昔洛韋的一種[[前藥]],於1995年核准用於醫療用途,經攝取後在體內轉化為阿昔洛韋。<ref name=AHFS2019>{{cite web |title=Valacyclovir Hydrochloride Monograph for Professionals |url=https://www.drugs.com/monograph/valacyclovir-hydrochloride.html |website=Drugs.com |publisher=American Society of Health-System Pharmacists |access-date= 2019-03-17 }}</ref> |
|||
阿昔洛韋加上[[氫羥腎上腺皮質素]]的乳霜複方藥於2009年在美國獲得核准上市(商品名為Xerese),用於治療早期復發性唇瘡,以減少成人和兒童(6歲以上)發展成瘍性唇瘡的可能性,並將癒合時間縮短。<ref>{{cite web | title=Drug Approval Package: Acyclovir and Hydrocortisone NDA #022436 | website=U.S. [[Food and Drug Administration]] (FDA) | date=2019-12-13 | url=https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022436_acyclovir_hydrocortisone_toc.cfm | archive-url=https://web.archive.org/web/20191213054239/https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022436_acyclovir_hydrocortisone_toc.cfm | archive-date=2019-12-13 | url-status=live | access-date= 2019-12-13}} {{PD-notice}}</ref><ref name="Xerese label">{{cite web | title=Xerese- acyclovir and hydrocortisone cream | website=[[DailyMed]] | date=2019-12-12 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3b6ac164-0f1e-4f36-94a1-1fdb07d710f5 | archive-url=https://web.archive.org/web/20191213054630/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3b6ac164-0f1e-4f36-94a1-1fdb07d710f5 | archive-date=2019-12-13 | url-status=live | access-date=2019-12-12}}</ref> |
|||
==社會與文化== |
|||
===名稱=== |
|||
阿昔洛韋最初以Zovirax品牌上市,專利於20世紀90年代到期,此後均為通用名藥物,以許多品牌在全球銷售。<ref name=Names/> |
|||
==附註== |
|||
{{Reflist|group = Note}} |
|||
== 參考文獻 == |
== 參考文獻 == |
||
{{Reflist|2}} |
{{Reflist|2}} |
||
==延伸閱讀== |
== 延伸閱讀 == |
||
{{refbegin |
{{refbegin}} |
||
* {{ |
* {{cite journal | vauthors = Hazra S, Konrad M, Lavie A | title = The sugar ring of the nucleoside is required for productive substrate positioning in the active site of human deoxycytidine kinase (dCK): implications for the development of dCK-activated acyclic guanine analogues | journal = Journal of Medicinal Chemistry | volume = 53 | issue = 15 | pages = 5792–5800 | date = August 2010 | pmid = 20684612 | pmc = 2936711 | doi = 10.1021/jm1005379 }} |
||
* {{cite book | vauthors = Harvey SC | chapter = Drug absorption, action and disposition | pages = 697–702 | veditors = Remington JP, Gennaro AR |title=Remington's Pharmaceutical Sciences |date=1990 |publisher=Mack Pub. Co |location=Easton, Pa. |isbn=978-0-912734-04-0 |edition=18th}} |
|||
* Harvey Stewart C. in Remington’s Pharmaceutical Sciences 18th edition: (ed. Gennard, Alfonso R.) Mack Publishing Company, 1990. {{ISBN|0-912734-04-3}}. |
|||
* Huovinen P |
* {{cite book | vauthors = Huovinen P, Valtonen V | title = Kliininen Farmakologia | veditors = Neuvonen PJ | location = Helsinki | language = fi | publisher = Kandidaattikustannus Oy | date = 1994 | isbn = 951-8951-09-8 }} |
||
* {{cite journal | |
* {{cite journal | vauthors = Périgaud C, Gosselin G, Imbach JL | year = 1992 | title = Nucleoside analogues as chemotherapeutic agents: a review | journal = Nucleosides and Nucleotides | volume = 11 | issue = 2–4|pages=903–945 |doi=10.1080/07328319208021748 }} |
||
* Rang |
* {{cite book | vauthors = Rang HP, Dale MM, Ritter JM | title = Pharmacology | publisher = Pearson Professional Ltd | date = 2003 | edition = 5th | isbn = 0-443-07145-4 }} |
||
{{refend}} |
{{refend}} |
||
{{模板:皮膚病用抗生素和化療藥}} |
|||
==外部連結== |
|||
{{模板:抗DNA病毒藥物}} |
|||
{{Portal|药理学}} |
|||
{{模板:葛蘭素史克}} |
|||
{{Portal|病毒}} |
|||
{{Portal bar | Medicine | Viruses}} |
|||
* [http://druginfo.nlm.nih.gov/drugportal/dpdirect.jsp?name=Acyclovir U.S. National Library of Medicine: Drug Information Portal–Aciclovir] {{Wayback|url=http://druginfo.nlm.nih.gov/drugportal/dpdirect.jsp?name=Acyclovir |date=20160409214311 }} |
|||
{{Authority control}} |
|||
{{抗DNA病毒药物}} |
|||
{{抗病毒药物}} |
|||
{{国家基本药物目录/抗微生物药}} |
|||
{{GlaxoSmithKline}} |
|||
[[分類:嘌呤]] |
|||
[[Category:抗病毒药物]] |
|||
[[ |
[[分類:世界衛生組織基本藥物]] |
||
[[分類:RTT]] |
|||
[[Category:Anti-herpes virus drugs]] |
|||
[[Category:CYP1A2 inhibitors]] |
|||
[[Category:Nephrotoxins]] |
|||
2024年3月7日 (四) 08:33的版本
 | |
 | |
| 臨床資料 | |
|---|---|
| 读音 | /eɪˈsaɪkloʊvɪər/ |
| 商品名 | Zovirax及其他[1] |
| 其他名稱 | Acycloguanosine, acyclovir (BAN UK), acyclovir (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a681045 |
| 核准狀況 | |
| 懷孕分級 |
|
| 给药途径 | 靜脈注射, 口服給藥, 外用藥物, 眼部給藥 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 15–20% (口服)[2] |
| 血漿蛋白結合率 | 9–33%[2] |
| 药物代谢 | 肝臟 |
| 生物半衰期 | 2–4小時 |
| 排泄途徑 | 腎臟 (62–90%保持原形式) |
| 识别信息 | |
| |
| CAS号 | 59277-89-3 |
| PubChem CID | |
| PubChem SID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB配體ID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.059 |
| 化学信息 | |
| 化学式 | C8H11N5O3 |
| 摩尔质量 | 225.21 g·mol−1 |
| 3D模型(JSmol) | |
| 熔点 | 256.5 °C(493.7 °F) |
| |
| |
阿昔洛韋 (英語:Aciclovir,簡稱ACV,也可拼寫為acyclovir),[3]是一種抗病毒藥物。[4]主要用於治療單純皰疹病毒(HSV)、水痘和帶狀皰疹的感染。[5]其他用途包括預防器官移植後巨細胞病毒屬感染,和預防人類疱疹病毒第四型(EBV)感染之後的嚴重併發症。[5][6]它有口服給藥、塗抹用乳霜或靜脈注射的形式。[5]
使用後常見的副作用有噁心和腹瀉。[5]可能的的嚴重副作用有腎臟問題和血小板減少。[5]肝臟或腎臟功能較差的人使用時,應更為謹慎。[5]一般認為個體在懷孕期間使用對胎兒屬於安全,尚未觀察到任何危害案例。[5][7]使用個體進行母乳哺育,對於嬰兒似乎屬於安全。[8][9]阿昔洛韋是一種模仿鳥苷的核苷類似物,[5]其作用是抑制病毒生產DNA的能力。[5]
阿昔洛韋於1974年由葛蘭素史克藥業取得美國專利,並於1981年於美國獲准用於醫療用途。[10]此藥物已列入世界衛生組織基本藥物標準清單中。[11][12]它有通用名藥物,且以許多品牌在全球銷售。[1]它是美國於2020年排名第162最常使用的處方藥,開立的處方箋數量超過300萬張。[13][14]
醫療用途

此藥物主要用於治療單純皰疹病毒、水痘和帶狀皰疹感染,包括:[2][15][16]
- 生殖器單純皰疹病毒(治療和預防)
- 新生兒單純疱疹病毒感染
- 唇瘡
- 帶狀泡疹
- 免疫缺陷者的急性水痘感染
- 單純皰疹病毒腦炎
- 免疫缺陷者的急性皮膚黏膜交界處單純皰疹病毒
- 單純疱疹性角膜炎和單純皰疹病毒瞼緣炎
- 為免疫能力低下族群(如接受癌症化學治療者)提供皰疹病毒預防[17]
其治療EBV病毒感染的效力尚不清楚。[5]尚未發現它對治療EBV引起的傳染性單核細胞增多症有效。[18]伐昔洛韋和阿昔洛韋透過抑制病毒的DNA複製而發揮作用,但截至2016年,幾乎沒有證據顯示其對治療EBV病毒有效。此藥物價格昂貴,有引起抗病毒藥物抗藥性的風險,並且(在1%至10%的病例中)可能會導致患者發生不舒服的副作用。[19]
口服形式的阿昔洛韋似乎不會將罹患帶狀皰疹個體的疼痛風險降低。[20]使用阿昔洛韋治療眼部皰疹患者,可能比使用碘苷更為有效及安全。[21]目前尚不清楚含阿昔洛韋眼藥水是否比含溴夫定眼藥水更為有效。[21]
靜脈注射形式的阿昔洛韋可有效治療不同種類皰疹病毒引起的嚴重狀況,包括嚴重局部感染、嚴重生殖器感染、水痘和皰疹病毒腦炎。它對全身性或創傷性皰疹感染、皰疹性濕疹和單純皰疹病毒腦炎的治療也有效。對20世紀80年代發表的研究報告審查,顯示如果於疫情爆發初期使用阿昔洛韋,可在減少發病數量和持續時間方面發揮一定作用。[22]研究顯示外用阿昔洛韋在疫情爆發初期和晚期使用均有效,這項審查採用更嚴格的方法和更大的樣本量,因此具有更高的可信度。[23]在試驗中顯示藥物對預防HIV傳播並無作用,但可幫助未接受抗反轉錄病毒藥物(ART)治療的人群減緩HIV疾病的進展。這項發現強調對HIV感染者中測試簡單、廉價的非ART處理策略(例如使用阿昔洛韋和複方新諾明)的重要性。[24]
懷孕
阿昔洛韋被美國食品藥物管理局(FDA)歸類為B類藥物(藥物在動物研究中並未顯示出對胎兒的風險,但尚無足够的懷孕婦女研究来確定其安全性),[25]美國疾病管制與預防中心(CDC)和其他機構已於2005年宣布在生殖器皰疹嚴重復發或首次發作期間,可使用阿昔洛韋作治療。[26]對於嚴重的HSV感染(尤其是傳播性HSV),也可使用靜脈注射形式進行治療。[27]
對小鼠、兔子和大鼠(使用超過相當於人類使用劑量的10倍以上)在器官形成過程中所做的研究未證明會導致出生缺陷。[28]在對大鼠進行的研究中,於懷孕第10天給予相當於人類標準穩態濃度63倍的藥物,[Note 1]會出現頭部和尾部發生異常的結果。[28]
CDC於2006年推薦使用阿昔洛韋於治療個體於懷孕期間的水痘感染,特別是在中期和晚期。[29]
阿昔洛韋會在乳汁中出現,因此建議進行母乳哺育的婦女應予謹慎。有限的試驗研究顯示母親口服阿昔洛韋後,接受哺乳的嬰兒每天會接觸到大約0.3毫克/公斤的劑量。若哺乳期母親乳房附近或乳房有皰疹病症,應避免親自哺乳。[25][30]
不良影響
全身治療
使用阿昔洛韋作全身性治療(口服或靜脈注射)常見的藥物不良反應(≥1%的患者)包括噁心、嘔吐、腹瀉、腦病變(僅發生於靜脈注射形式)、注射部位反應和頭痛。有報導在高劑量使用時會出現幻覺。不常見的不良反應(0.1-1%的患者)有煩躁、眩暈、困惑、頭暈、水腫、關節痛、喉嚨痛、便秘、腹痛、脫髮、皮疹和虛弱。罕見的不良反應(<0.1%的患者)有昏迷、癲癇發作、嗜中性白血球低下、白血球減少症、尿結晶、厭食、疲倦、肝炎、史蒂芬斯-強森症候群、中毒性表皮壞死鬆解症、血栓性血小板減少性紫癜、過敏性休克[15]和科塔爾症候群。
由於靜脈注射阿昔洛韋會造成腎臟中尿結晶,會對5%至10%的使用者造成可逆性腎毒性。對於脫水和已存在腎功能不全的患者進行快速輸注阿昔洛韋有較高尿結晶腎病變風險。充足攝取水分、減緩輸注速度以及根據患者腎功能而調整劑量將可將這類風險降低。[31][32][33]
阿昔洛韋代謝產物9-羧基甲氧基甲基鳥嘌呤(9-CMMG) 已被證明會產生神經系統不良事件,特別是對老年人和腎功能不全的人群。[34][35][36]
外用療法
含阿昔洛韋的外用乳霜通常會導致(≥1%) 的個體皮膚乾燥、剝落或短暫刺痛/灼熱感。罕見的不良反應有紅斑或發癢。[15]當施用於眼睛時,通常會導致(≥1%)的個體發生短暫的輕微刺痛。在眼部使用阿昔洛韋,有少數(0.1-1%的患者)會發生角膜點狀上皮擦傷或是過敏反應。[15]
藥物交互作用
在體外進行複製研究發現酮康唑與阿昔洛韋併用時,對HSV-1和HSV-2的感染具有協同、劑量相關性抗病毒活性。然而這種效應尚未在臨床試驗上得到證實,因此需進行更多研究來評估協同作用的實際潛力。[37]
針對丙磺舒與阿昔洛韋同時給藥的研究顯示阿昔洛韋的生物半衰期可延長,受到尿液和腎清除的速率被降低。[25]
干擾素與阿昔洛韋合併使用時會產生協同作用,對接受靜脈注射干擾素的患者於施用阿昔洛韋時應謹慎。[38]
雖然HIV感染者經常會將齊多夫定與阿昔洛韋一起使用,但至少一名患者在接受靜脈注射阿昔洛韋30-60天後出現極度嗜睡和昏睡的神經毒性,停用阿昔洛韋後症狀得到舒緩。[39]
體液中檢測
可對血漿或血清中的阿昔洛韋進行數量檢測,以確認腎功能不全患者的藥物蓄積,或是診斷急性用藥過量中毒狀況。[40]
作用機制


阿昔洛韋由病毒的胸苷激酶轉化為阿昔洛韋單磷酸,然後由宿主細胞激酶轉化為阿昔洛韋三磷酸(ACV-TP,也稱為阿昔洛-GTP)。[28]ACV-TP是種非常有效的病毒DNA複製抑制劑。 ACV-TP會競爭性抑制並將病毒DNA聚合酶滅活。[41]其單磷酸鹽也會摻入病毒DNA之中,導致蛋白質生物合成終止。[28][42][43]
抗藥性
免疫系統健康的人發生阿昔洛韋抗藥性的案例很少見,但在長期接受抗病毒預防的免疫缺陷人群(接受器官移植者、因HIV感染而患有後天免疫缺陷症候群者)中較為常見(高達10%)。 對HSV的抗藥性機制有病毒胸苷激酶缺陷,以及病毒胸苷激酶或DNA聚合酶的突變,而將基質敏感性改變。[44][45]
微生物學
阿昔洛韋對皰疹病毒科中的大多數都有活性,如下(依活性降冪方式排列):[46][47]
- 單純皰疹病毒 I 型 (HSV-1)
- 單純皰疹病毒 II 型 (HSV-2)
- 水痘帶狀皰疹病毒(VZV)
- EBV病毒
- 巨細胞病毒屬(HCMV) – 活性最低
藥物動力學
阿昔洛韋的水溶性不佳,口服形式的生物利用度不高(15-30%),因此如果需要高濃度的效果,則需透過靜脈注射的形式。口服給藥後1-2小時後出現最大血藥濃度。根據美國生物製藥分類系統(BCS),阿昔洛韋屬於BCS III 類藥物,即具可溶性但腸道滲透性低。[48]阿昔洛韋分佈率高,報導的蛋白質結合率為9%至33%。[49]阿昔洛韋的生物半衰期(t½)取決於年齡:新生兒為4小時、1-12歲兒童為2-3小時,而成人為3小時。[2]
歷史
阿昔洛韋被視為是種啟動抗病毒藥物新時代的先發者,它具有極高的選擇性和低細胞毒性。[4]它在1970年代中期被發現後,即開始用作有效治療皰疹病毒家族中大多數(包括單純皰疹病毒和水痘帶狀皰疹病毒)引起感染的有效藥物。合成阿昔洛韋的基礎是一種由加勒比海綿Cryptotethya crypta分離出的核苷。[50][51][52]它是由Howard Schaeffer與羅伯·文思、S. Bittner和S. Gurwara共同發現的腺苷類似物 - 無環腺苷,具有不錯的抗病毒活性。[53]後來Schaeffer加入葛蘭素史克,與藥理學家 格特魯德·B·埃利恩繼續開發此藥物。[54]一項於1979年發佈關於阿昔洛韋的美國專利中將Schaeffer列為發明人。[55]羅伯·文思後來發明阿巴卡韋- 一種用於HIV患者的[逆轉錄酶抑制劑]]。[56]埃利恩則因使用開創性藥物設計於新藥物開發而榮獲1988年諾貝爾生理學或醫學獎。
伐昔洛韋是阿昔洛韋的一種前藥,於1995年核准用於醫療用途,經攝取後在體內轉化為阿昔洛韋。[57]
阿昔洛韋加上氫羥腎上腺皮質素的乳霜複方藥於2009年在美國獲得核准上市(商品名為Xerese),用於治療早期復發性唇瘡,以減少成人和兒童(6歲以上)發展成瘍性唇瘡的可能性,並將癒合時間縮短。[58][59]
社會與文化
名稱
阿昔洛韋最初以Zovirax品牌上市,專利於20世紀90年代到期,此後均為通用名藥物,以許多品牌在全球銷售。[1]
附註
- ^ Subject to the same conditions as before
參考文獻
- ^ 1.0 1.1 1.2 Aciclovir. Drugs.com. [2015-09-06]. (原始内容存档于2015-09-23).
- ^ 2.0 2.1 2.2 2.3 Zovirax (acyclovir) dosing, indications, interactions, adverse effects, and more. Medscape Reference. WebMD. [2014-02-05]. (原始内容存档于2014-02-19).
- ^ Kevin ES. The Aciclovir. Kevin. [2017-05-25] (德语).
- ^ 4.0 4.1 de Clercq E, Field HJ. Antiviral prodrugs – the development of successful prodrug strategies for antiviral chemotherapy. British Journal of Pharmacology 147 (1) (Wiley-Blackwell). 2005-10-05: 1–11 (January 2006). PMC 1615839
 . PMID 16284630. doi:10.1038/sj.bjp.0706446.
. PMID 16284630. doi:10.1038/sj.bjp.0706446.
- ^ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 Acyclovir. The American Society of Health-System Pharmacists. [2015-01-01]. (原始内容存档于2015-01-05).
- ^ Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. Journal of Clinical Virology. November 2010, 49 (3): 151–157. PMID 20739216. doi:10.1016/j.jcv.2010.07.008.
- ^ Prescribing medicines in pregnancy database. Australian Government. 2014-03-03 [2014-04-22]. (原始内容存档于2014-04-08).
- ^ Hamilton R. Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015: 59. ISBN 9781284057560.
- ^ Acyclovir use while Breastfeeding. 2015-03-10 [2016-03-08]. (原始内容存档于2016-03-05).
Even with the highest maternal dosages, the dosage of acyclovir in milk is only about 1% of a typical infant dosage and would not be expected to cause any adverse effects in breastfed infants
- ^ Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. 2006: 504. ISBN 9783527607495.
- ^ World Health Organization. World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. hdl:10665/325771
 . WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization. World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. hdl:10665/345533
 . WHO/MHP/HPS/EML/2021.02.
. WHO/MHP/HPS/EML/2021.02.
- ^ The Top 300 of 2020. ClinCalc. [2022-10-07].
- ^ Acyclovir - Drug Usage Statistics. ClinCalc. [2022-10-07].
- ^ 15.0 15.1 15.2 15.3 Rossi, S (编). Australian Medicines Handbook 2013. Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ^ Joint Formulary Committee. British National Formulary (BNF)
 65. London: Pharmaceutical Press. 2013. ISBN 978-0-85711-084-8.
65. London: Pharmaceutical Press. 2013. ISBN 978-0-85711-084-8.
- ^ Elad S, Zadik Y, Hewson I, Hovan A, Correa ME, Logan R, Elting LS, Spijkervet FK, Brennan MT. A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea. Supportive Care in Cancer. August 2010, 18 (8): 993–1006. PMID 20544224. S2CID 2969472. doi:10.1007/s00520-010-0900-3.
- ^ Gershburg E, Pagano JS. Epstein-Barr virus infections: prospects for treatment. The Journal of Antimicrobial Chemotherapy. August 2005, 56 (2): 277–281. CiteSeerX 10.1.1.320.6721
 . PMID 16006448. doi:10.1093/jac/dki240.
. PMID 16006448. doi:10.1093/jac/dki240.
- ^ De Paor M, O'Brien K, Fahey T, Smith SM. Antiviral agents for infectious mononucleosis (glandular fever). The Cochrane Database of Systematic Reviews. December 2016, 2016 (12): CD011487. PMC 6463965
 . PMID 27933614. doi:10.1002/14651858.CD011487.pub2.
. PMID 27933614. doi:10.1002/14651858.CD011487.pub2.
- ^ Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. The Cochrane Database of Systematic Reviews. February 2014, 2014 (2): CD006866. PMC 10583132
 . PMID 24500927. doi:10.1002/14651858.CD006866.pub3.
. PMID 24500927. doi:10.1002/14651858.CD006866.pub3.
- ^ 21.0 21.1 Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. The Cochrane Database of Systematic Reviews. January 2015, 1 (1): CD002898. PMC 4443501
 . PMID 25879115. doi:10.1002/14651858.CD002898.pub5.
. PMID 25879115. doi:10.1002/14651858.CD002898.pub5.
- ^ Worrall G. Acyclovir in recurrent herpes labialis. BMJ. January 1996, 312 (7022): 6. PMC 2349724
 . PMID 8555890. doi:10.1136/bmj.312.7022.6. (原始内容存档于2007-05-15). – Editorial
. PMID 8555890. doi:10.1136/bmj.312.7022.6. (原始内容存档于2007-05-15). – Editorial
- ^ Spruance SL, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrobial Agents and Chemotherapy. July 2002, 46 (7): 2238–2243. PMC 127288
 . PMID 12069980. doi:10.1128/aac.46.7.2238-2243.2002.
. PMID 12069980. doi:10.1128/aac.46.7.2238-2243.2002.
- ^ Mascolinli M, Kort R. 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention: summary of key research and implications for policy and practice - biomedical prevention. Journal of the International AIDS Society. June 2010, 13 (Suppl 1): S4. PMC 2880255
 . PMID 20519025. doi:10.1186/1758-2652-13-S1-S4
. PMID 20519025. doi:10.1186/1758-2652-13-S1-S4  .
.
- ^ 25.0 25.1 25.2 Acyclovir label (PDF). GSK. FDA. 2005. (原始内容 (PDF)存档于2017-09-08).
- ^ Drugs for non-HIV viral infections. Treatment Guidelines from the Medical Letter. April 2005, 3 (32): 23–32. PMID 15767977.
- ^ Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR. Recommendations and Reports. April 2009, 58 (RR-4): 1–207; quiz CE1–4. PMID 19357635.
- ^ 28.0 28.1 28.2 28.3 PRODUCT INFORMATION NAME OF THE DRUG OZVIR TABLETS (PDF). TGA eBusiness Services. Ranbaxy Australia Pty Ltd. 2011-08-26 [2014-02-06]. (原始内容存档于2016-08-20).
- ^ Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 9th. Public Health Foundation. January 2006: 171–192.
- ^ Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, Eidelman AI. Breastfeeding and the use of human milk. Pediatrics. February 2005, 115 (2): 496–506. PMID 15687461. S2CID 5791615. doi:10.1542/peds.2004-2491
 .
.
- ^ Razonable RR. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clinic Proceedings. October 2011, 86 (10): 1009–1026. PMC 3184032
 . PMID 21964179. doi:10.4065/mcp.2011.0309.
. PMID 21964179. doi:10.4065/mcp.2011.0309.
- ^ Brigden D, Rosling AE, Woods NC. Renal function after acyclovir intravenous injection. The American Journal of Medicine. July 1982, 73 (1A): 182–185. PMID 6285711. doi:10.1016/0002-9343(82)90087-0.
- ^ Sawyer MH, Webb DE, Balow JE, Straus SE. Acyclovir-induced renal failure. Clinical course and histology. The American Journal of Medicine. June 1988, 84 (6): 1067–1071. PMID 3376977. doi:10.1016/0002-9343(88)90313-0.
- ^ Helldén A, Odar-Cederlöf I, Diener P, Barkholt L, Medin C, Svensson JO, Säwe J, Ståhle L. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrology, Dialysis, Transplantation. June 2003, 18 (6): 1135–1141. PMID 12748346. doi:10.1093/ndt/gfg119
 .
.
- ^ Berry L, Venkatesan P. Aciclovir-induced neurotoxicity: Utility of CSF and serum CMMG levels in diagnosis. Journal of Clinical Virology. December 2014, 61 (4): 608–610. PMID 25440915. doi:10.1016/j.jcv.2014.09.001.
- ^ Chowdhury MA, Derar N, Hasan S, Hinch B, Ratnam S, Assaly R. Acyclovir-Induced Neurotoxicity: A Case Report and Review of Literature. American Journal of Therapeutics. 2016, 23 (3): e941–e943. PMID 24942005. S2CID 32983100. doi:10.1097/MJT.0000000000000093.
- ^ Pottage JC, Kessler HA, Goodrich JM, Chase R, Benson CA, Kapell K, Levin S. In vitro activity of ketoconazole against herpes simplex virus. Antimicrobial Agents and Chemotherapy. August 1986, 30 (2): 215–219. PMC 180521
 . PMID 3021048. doi:10.1128/aac.30.2.215.
. PMID 3021048. doi:10.1128/aac.30.2.215.
- ^ GlaxoSmithKline. Zovirax® (acyclovir sodium) for injection prescribing information. Research Triangle Park, NC; 2003 Nov
- ^ Bach MC. Possible drug interaction during therapy with azidothymidine and acyclovir for AIDS. The New England Journal of Medicine. February 1987, 316 (9): 547. PMID 3468354. doi:10.1056/NEJM198702263160912.
- ^ Baselt RC. Disposition of toxic drugs and chemicals in man 8th. Foster City, CA: Biomedical Publications. 2008: 29–31. ISBN 9780962652370.
- ^ VALTREX (valacyclovir hydrochloride) Caplets -GSKSource. gsksource.com. [2019-08-02].
- ^ Acyclovir (acyclovir) Capsule Acyclovir (acyclovir) Tablet [Genpharm Inc.]. DailyMed. Genpharm Inc. November 2006 [2014-02-05]. (原始内容存档于2014-02-21).
- ^ Aciclovir Tablets BP 400mg - Summary of Product Characteristics (SPC). electronic Medicines Compendium. Actavis UK Ltd. 2012-08-20 [2014-02-05]. (原始内容存档于2014-02-22).
- ^ Sweetman, S (编). Aciclovir. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 7 August 2013 [6 February 2014].
- ^ Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrobial Agents and Chemotherapy. February 2011, 55 (2): 459–472. PMC 3028810
 . PMID 21078929. doi:10.1128/AAC.00615-10.
. PMID 21078929. doi:10.1128/AAC.00615-10.
- ^ O'Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. March 1989, 37 (3): 233–309. PMID 2653790. S2CID 240858022. doi:10.2165/00003495-198937030-00002
 .
.
- ^ Wagstaff AJ, Faulds D, Goa KL. Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs. January 1994, 47 (1): 153–205. PMID 7510619. doi:10.2165/00003495-199447010-00009
 .
.
- ^ Karmoker JR, Hasan I, Ahmed N, Saifuddin M, Reza MS. Development and Optimization of Acyclovir Loaded Mucoadhesive Microspheres by Box -Behnken Design. Dhaka University Journal of Pharmaceutical Sciences. 2019, 18 (1): 1–12. doi:10.3329/dujps.v18i1.41421
 .
.
- ^ Aciclovir Tablets BP 400mg - Summary of Product Characteristics (SmPC) - (emc). www.medicines.org.uk.
- ^ Garrison T. Oceanography: An Invitation to Marine Science, 3rd ed.. Belmont, CA: Wadsworth Publishing Company. 1999: 471.
- ^ Sepčić K. Bioactive Alkylpyridinium Compounds from Marine Sponges. Toxin Reviews. 2000, 19 (2): 139–160. S2CID 84041855. doi:10.1081/TXR-100100318.
- ^ Laport MS, Santos OC, Muricy G. Marine sponges: potential sources of new antimicrobial drugs. Current Pharmaceutical Biotechnology. January 2009, 10 (1): 86–105. PMID 19149592. doi:10.2174/138920109787048625.
- ^ Schaeffer HJ, Gurwara S, Vince R, Bittner S. Novel substrate of adenosine deaminase. Journal of Medicinal Chemistry. April 1971, 14 (4): 367–369. PMID 5553754. doi:10.1021/jm00286a024.
- ^ Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proceedings of the National Academy of Sciences of the United States of America. December 1977, 74 (12): 5716–5720. Bibcode:1977PNAS...74.5716E. PMC 431864
 . PMID 202961. doi:10.1073/pnas.74.12.5716
. PMID 202961. doi:10.1073/pnas.74.12.5716  .
.
- ^ US 4146715,Schaeffer HJ,「2-Amido-9-(2-acyloxyethoxymethyl)hypoxanthines」,发行于1979-03-27,指定于Burrough Wellcome Co.
- ^ Vince R. A brief history of the development of Ziagen. Chemtracts. 2008, 21: 127–134.
- ^ Valacyclovir Hydrochloride Monograph for Professionals. Drugs.com. American Society of Health-System Pharmacists. [2019-03-17].
- ^ Drug Approval Package: Acyclovir and Hydrocortisone NDA #022436. U.S. Food and Drug Administration (FDA). 2019-12-13 [2019-12-13]. (原始内容存档于2019-12-13).
 本文含有此來源中屬於公有领域的内容。
本文含有此來源中屬於公有领域的内容。
- ^ Xerese- acyclovir and hydrocortisone cream. DailyMed. 2019-12-12 [2019-12-12]. (原始内容存档于2019-12-13).
延伸閱讀
- Hazra S, Konrad M, Lavie A. The sugar ring of the nucleoside is required for productive substrate positioning in the active site of human deoxycytidine kinase (dCK): implications for the development of dCK-activated acyclic guanine analogues. Journal of Medicinal Chemistry. August 2010, 53 (15): 5792–5800. PMC 2936711
 . PMID 20684612. doi:10.1021/jm1005379.
. PMID 20684612. doi:10.1021/jm1005379. - Harvey SC. Drug absorption, action and disposition. Remington JP, Gennaro AR (编). Remington's Pharmaceutical Sciences 18th. Easton, Pa.: Mack Pub. Co. 1990: 697–702. ISBN 978-0-912734-04-0.
- Huovinen P, Valtonen V. Neuvonen PJ , 编. Kliininen Farmakologia. Helsinki: Kandidaattikustannus Oy. 1994. ISBN 951-8951-09-8 (芬兰语).
- Périgaud C, Gosselin G, Imbach JL. Nucleoside analogues as chemotherapeutic agents: a review. Nucleosides and Nucleotides. 1992, 11 (2–4): 903–945. doi:10.1080/07328319208021748.
- Rang HP, Dale MM, Ritter JM. Pharmacology 5th. Pearson Professional Ltd. 2003. ISBN 0-443-07145-4.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
|
