依託咪酯
外觀
此條目可參照英語維基百科相應條目來擴充。 (2024年3月27日) |
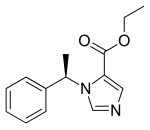 (R)-etomidate ethyl ester | |
| 臨床資料 | |
|---|---|
| 商品名 | Amidate, Hypnomidate, Tomvi |
| AHFS/Drugs.com | Monograph |
| 核准狀況 | |
| 給藥途徑 | 靜脈注射 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 血漿蛋白結合率 | 76% |
| 藥物代謝 | 在血漿和肝臟中發生酯水解 |
| 生物半衰期 | 75 分鐘 |
| 排泄途徑 | 尿液(85%),膽管 (15%) |
| 識別資訊 | |
| |
| CAS號 | 33125-97-2 |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.700 |
| 化學資訊 | |
| 化學式 | C14H16N2O2 |
| 摩爾質量 | 244.29 g·mol−1 |
| 3D模型(JSmol) | |
| 熔點 | 67 °C(153 °F) |
| 沸點 | 392 °C(738 °F) |
| |
| |
依託咪酯(英語:etomidate),別名R-(+)-1-(1-苯乙基)-1-氫-咪唑-5-甲酸乙酯、(R)-(+)-1-(1-苯乙基)-1-氫-咪唑-5-甲酸乙酯、(R)-1-(1-苯基乙基)-1H-咪唑-5-甲酸乙酯、乙苄咪唑。是一種催眠劑,用於誘導全身麻醉。
化學性質
[編輯]1-苯乙基咪唑-5-甲醇的氧化-酯化反應[2]、咪唑-5-甲酸乙酯和1-苯基乙醇的Mitsunobu偶合反應[3]及N-[1-(2-溴苯基)乙基]甘氨酸乙酯的成環-脫溴反應可用於該藥物的合成。[4]
它在氫氧化鈉溶液中水解,酸化後可以得到(R)-1-(1-苯乙基)咪唑-5-甲酸[5];它和丙醇鈉或異丙醇鈉反應,可以得到相應的丙酯或異丙酯類似物。[6]它可以和釕形成配合物。[7]
管制信息
[編輯]依託咪酯在中國是麻醉類處方藥,非法販賣涉及妨害藥品管理罪和販賣毒品罪。[8]吸食含有依託咪酯的電子煙則會被強制戒毒。[9]
參考文獻
[編輯]- ^ Summary Basis of Decision (SBD) for Tomvi. Health Canada. 23 October 2014 [29 May 2022]. (原始內容存檔於2022-05-30).
- ^ Marcus Baumann, Ian R. Baxendale. A Continuous-Flow Method for the Desulfurization of Substituted Thioimidazoles Applied to the Synthesis of Etomidate Derivatives. European Journal of Organic Chemistry, 2017. doi:10.1002/ejoc.201700833.
- ^ Joydev K. Laha and Gregory D. Cuny. Synthesis of Fused Imidazoles, Pyrroles, and Indoles with a Defined Stereocenter α to Nitrogen Utilizing Mitsunobu Alkylation Followed by Palladium-Catalyzed Cyclization. J. Org. Chem. 2011, 76, 20, 8477–8482. doi:10.1021/jo201237h.
- ^ Cor G. M. Janssen, Jos B. A. Thijssen, Willy L. M. Verluyten, Jozef J. P. Heykants. Synthesis of (R)-(+)-3H-etomidate. Journal of Labelled Compounds and Radiopharmaceuticals, 1987. 24 (8). doi:10.1002/jlcr.2580240805.
- ^ Zhao, Shizhen; et al. Design, synthesis and evaluation of 1-benzyl-1H-imidazole-5-carboxamide derivatives as potent TGR5 agonists. Bioorganic & Medicinal Chemistry. 2021. 32. 115972. doi:10.1016/j.bmc.2020.115972.
- ^ Zolle, Ilse M.; et al. New Selective Inhibitors of Steroid 11β-Hydroxylation in the Adrenal Cortex. Synthesis and Structure-Activity Relationship of Potent Etomidate Analogues. Journal of Medicinal Chemistry (2008), 51(7), 2244-2253. doi:10.1021/jm800012w
- ^ Ana Zamora; et al. Ruthenium-containing P450 inhibitors for dual enzyme inhibition and DNA damage. Dalton Trans., 2017,46, 2165-2173. doi:10.1039/C6DT04405K.
- ^ 又一种“上头电子烟”!添加了依托咪酯,危害不亚于毒品. 紹興市公安局. 2023-07-24 [2024-06-27].
- ^ 3名未成年人抽电子烟抽进戒毒所. 新浪網. 2024-06-26 [2024-06-27].
外部連結
[編輯]- Etomidate. Drug Information Portal. U.S. National Library of Medicine. [2022-06-23]. (原始內容存檔於2022-08-01).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
