T细胞:修订间差异
Antigng-bot(留言 | 贡献) 小 bot: expand DOI citations |
Dennisbronfan(留言 | 贡献) 翻译外文链接 标签:添加文件 |
||
| 第24行: | 第24行: | ||
| DorlandsID = |
| DorlandsID = |
||
}} |
}} |
||
t細胞或t淋巴球 is a type of [[淋巴细胞|lymphocyte]] (a subtype of [[白血球|white blood cell]]) that plays a central role in [[细胞介导免疫|cell-mediated immunity]]. T cells can be distinguished from other lymphocytes, such as [[B细胞|B cell]]s and [[自然杀伤细胞|natural killer cell]]s, by the presence of a [[T细胞受体|T-cell receptor]] on the [[細胞表面受體|cell surface]]. They are called ''T cells'' because they mature in the [[胸腺|thymus]] from [[胸腺細胞|thymocyte]]s<ref>Alberts B, Johnson A, Lewis J, Raff M, Roberts k, Walter P (2002) [https://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A4422 Molecular Biology of the Cell]. Garland Science: New York, NY pg 1367. "T cells and B cells derive their names from the organs in which they develop. T cells develop in the thymus, and B cells, in mammals, develop in the bone marrow in adults or the liver in fetuses."</ref> (although some also mature in the [[扁桃腺|tonsil]]s<ref>{{Cite journal|title = Evidence for a stepwise program of extrathymic T cell development within the human tonsil|journal = The Journal of Clinical Investigation|date = Apr 2012|issn = 1558-8238|pmc = 3314444|pmid = 22378041|pages = 1403–1415|volume = 122|issue = 4|doi = 10.1172/JCI46125|first = Susan|last = McClory|first2 = Tiffany|last2 = Hughes|first3 = Aharon G.|last3 = Freud|first4 = Edward L.|last4 = Briercheck|first5 = Chelsea|last5 = Martin|first6 = Anthony J.|last6 = Trimboli|first7 = Jianhua|last7 = Yu|first8 = Xiaoli|last8 = Zhang|first9 = Gustavo|last9 = Leone}}</ref>). The several subsets of T cells each have a distinct function. The majority of human T cells [[J节段|rearrange]] their {{tsl|en|alpha chain||alpha and beta chains}} on the cell receptor and are termed alpha beta T cells (αβ T cells) and are part of the [[后天免疫系统|adaptive immune system]]. Specialized {{tsl|en|gamma delta T cell||gamma delta T cell}}s, (a small minority of T cells in the [[人体|human body]], more frequent in [[反芻|ruminant]]s), have invariant T-cell receptors with limited diversity, that can effectively present [[抗原|antigens]] to other T cells<ref>{{Cite journal|title = Six-of-the-best: unique contributions of γδ T cells to immunology|journal = Nature Reviews. Immunology|date = Feb 2013|issn = 1474-1741|pmc = 3951794|pmid = 23348415|pages = 88–100|volume = 13|issue = 2|doi = 10.1038/nri3384|first = Pierre|last = Vantourout|first2 = Adrian|last2 = Hayday}}</ref> and are considered to be part of the [[先天免疫系統|innate immune system]]. |
|||
'''T细胞'''({{lang-en|T cell}}、T淋巴細胞/{{lang|en|T lymphocyte}})是[[淋巴细胞]]的一种,在[[免疫反應]]中扮演着重要的角色。T细胞在胸腺内分化成熟,成熟后移居于周围[[淋巴组织]]中。T是“[[胸腺]]”(thymus)而不是[[甲狀腺]](thyroid)的[[英文]][[缩写]]。T细胞膜表面分子与T细胞的功能相关,也是T细胞的[[表面标志]](cell-surface marker),可以用以分离、鉴定不同亚群的T细胞。 |
|||
== |
== Types == |
||
T细胞按照功能和表面标志可以分成很多种类: |
|||
=== Effector === |
|||
* [[细胞毒性T细胞]](或殺手T细胞)(cytotoxic T cell):消灭受感染或突變的细胞。这些细胞的功能就像一个“杀手”或细胞毒素那样,因为它们可以对产生特殊[[抗原]]反应的目标细胞进行杀灭。细胞毒性T细胞的主要表面标志是[[CD8受體|CD8]],也被稱為殺手T細胞。 |
|||
Effector cells are the superset of all the various T cell types that actively respond immediately to a stimulus, such as {{tsl|en|co-stimulation||co-stimulation}}. This includes {{tsl|en|||helper}}, {{tsl|en|||killer}}, {{tsl|en|||regulatory}}, and potentially other T cell types. Memory cells are their opposite counterpart that are longer lived to target future infections as necessary. |
|||
* [[辅助T细胞]](helper T cell)在免疫反应中扮演中间过程的角色:它可以增生扩散来激活其它类型的产生直接免疫反应的免疫细胞。辅助T细胞的主要表面标志是[[CD4受體|CD4]]。T细胞调控或“辅助”其它淋巴细胞发挥功能。它们是已知的[[人体免疫缺陷病毒|HIV病毒(一種反轉錄病毒)]]的目標细胞,在[[艾滋病]]发病时会急剧减少。協助活化B細胞產生抗體,也可協助殺傷性T細胞及巨噬細胞發揮免疫功能。輔助T細胞也可以分泌細胞激素(cytokines)活化B細胞導向體液型免疫反應,幫助B細胞分化成漿細胞(plasma cell),或是增強巨噬細胞(macrophage)的吞噬功能(endocytosis)。 |
|||
* [[调节/抑制T细胞]](regulatory/suppressor T cell):负责调节机体免疫反应。通常起着维持自身耐受和避免免疫反应过度损伤机体的重要作用。调节/抑制T细胞有很多种,目前研究最活跃的是CD25+[[CD4受體|CD4]]+T细胞。對各種T細胞和B細胞都有抑制作用,調節和控制免疫反應,維持免疫自穩性(即免疫耐受性)。 |
|||
=== Helper === |
|||
* [[记忆T细胞]](memory T cell):在再次[[免疫反應]]中起重要作用。记忆T细胞由于暂时没有非常特异的表面标志,目前还有很多未知之处。連同記憶性B細胞一起,是在抗原刺激後,保存特異抗原訊息的淋巴細胞,壽命可長達數十年。當它們再次接受與原來相同的抗原刺激後,就可以分增殖為對付抗原的功能性T細胞或能產生抗體的漿細胞。 |
|||
[[輔助型T細胞|T helper cell]]s (T<sub>H</sub> cells) assist other white blood cells in immunologic processes, including maturation of [[B细胞|B cell]]s into [[浆细胞|plasma cell]]s and [[记忆B细胞|memory B cell]]s, and activation of [[细胞毒性T细胞|cytotoxic T cells]] and [[巨噬细胞|macrophage]]s. These cells are also known as '''CD4<sup>+</sup> T cells''' because they express the [[CD4受体|CD4]] [[醣蛋白|glycoprotein]] on their surfaces. Helper T cells become activated when they are presented with [[肽|peptide]] [[抗原|antigen]]s by {{tsl|en|MHC class II||MHC class II}} molecules, which are expressed on the surface of [[抗原呈递细胞|antigen-presenting cell]]s (APCs). Once activated, they divide rapidly and secrete small proteins called [[细胞因子|cytokine]]s that regulate or assist in the active immune response. These cells can differentiate into one of several subtypes, including [[輔助型T細胞|T<sub>H</sub>1]], [[輔助型T細胞|T<sub>H</sub>2]], {{tsl|en|Th3||T<sub>H</sub>3}}, {{tsl|en|Th17||T<sub>H</sub>17}}, {{tsl|en|Th 9 cells||T<sub>H</sub>9}}, or {{tsl|en|ThF||T<sub>FH</sub>}}, which secrete different cytokines to facilitate different types of immune responses. Signalling from the APC directs T cells into particular subtypes.<ref name="pmid17476341">{{cite journal |vauthors=Gutcher I, Becher B | title = APC-derived cytokines and T cell polarization in autoimmune inflammation | journal = J. Clin. Invest. | volume = 117 | issue = 5 | pages = 1119–27 | year = 2007 | pmid = 17476341 | pmc = 1857272 | doi = 10.1172/JCI31720 }}</ref> |
|||
<big>'''Cytotoxic (Killer) CD8 +ve'''</big> |
|||
[[File:Killer T cells surround a cancer cell.png|thumb|Superresolution image of a group of killer T cells (green and red) surrounding a cancer cell (blue, center). When a killer T cell makes contact with a target cell, the killer cell attaches and spreads over the dangerous target. The killer cell then uses special chemicals housed in vesicles (red) to deliver the killing blow. This event has thus been nicknamed “the kiss of death”. After the target cell is killed, the killer T cells move on to find the next victim.]] |
|||
[[细胞毒性T细胞|Cytotoxic T cell]]s (T<sub>C</sub> cells, CTLs, T-killer cells, killer T cells) destroy virus-infected cells and tumor cells, and are also implicated in [[器官移植|transplant]] rejection. These cells are also known as '''CD8<sup>+</sup> T cells''' since they express the [[CD8受体|CD8]] glycoprotein at their surfaces. These cells recognize their targets by binding to antigen associated with [[MHC1類分子|MHC class I]] molecules, which are present on the surface of all nucleated cells. Through {{tsl|en|interleukin-10||IL-10}}, adenosine, and other molecules secreted by [[调节T细胞|regulatory T cell]]s, the CD8<sup>+</sup> cells can be inactivated to an anergic state, which prevents {{tsl|en|autoimmune||autoimmune}} diseases. |
|||
=== Memory === |
|||
Antigen-naïve T cells expand and differentiate into memory and effector T cells after they encounter their cognate antigen within the context of an MHC molecule on the surface of a professional antigen presenting cell (e.g. a dendritic cell). Appropriate co-stimulation must be present at the time of antigen encounter for this process to occur. Historically, memory T cells were thought to belong to either the effector or central memory subtypes, each with their own distinguishing set of cell surface markers (see below).<ref>{{cite journal |vauthors= Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A | title = Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. | journal = Nature | volume = 401 | pages = 708–712 | year = 1999 | pmid = 10537110 | doi = 10.1038/44385 | bibcode = 1999Natur.401..708S }}</ref> Subsequently, numerous new populations of memory T cells were discovered including tissue-resident memory T (Trm) cells, stem memory TSCM cells, and virtual memory T cells. The single unifying theme for all [[记忆T细胞|memory T cell]] subtypes is that they are long-lived and can quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen. By this mechanism they provide the immune system with "memory" against previously encountered pathogens. Memory T cells may be either CD4<sup>+</sup> or CD8<sup>+</sup> and usually express {{tsl|en|CD45||CD45RO}}.<ref name="pmid2965180">{{cite journal |vauthors=Akbar AN, Terry L, Timms A, Beverley PC, Janossy G | title = Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells | journal = J. Immunol. | volume = 140 | issue = 7 | pages = 2171–8 | date = April 1988 | pmid = 2965180 | doi = }}</ref> |
|||
Memory T cell subtypes: |
|||
* Central memory T cells (T<sub>CM</sub> cells) express CD45RO, {{tsl|en|C-C chemokine receptor type 7||C-C chemokine receptor type 7}} (CCR7), and {{tsl|en|L-selectin||L-selectin}} (CD62L). Central memory T cells also have intermediate to high expression of {{tsl|en|CD44||CD44}}. This memory subpopulation is commonly found in the [[淋巴結|lymph node]]s and in the peripheral circulation. (Note- CD44 expression is usually used to distinguish murine naive from memory T cells). |
|||
* Effector memory T cells (T<sub>EM</sub> cells and T<sub>EMRA</sub> cells) express CD45RO but lack expression of CCR7 and {{tsl|en|L-selectin||L-selectin}}. They also have intermediate to high expression of {{tsl|en|CD44||CD44}}. These memory T cells lack lymph node-homing receptors and are thus found in the peripheral circulation and tissues.<ref>{{cite journal |vauthors=Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF | title = Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. | journal = Journal of Immunology | volume = 175 | issue = 9 | pages = 5895–903 | year = 2005 | pmid = 16237082 | doi = 10.4049/jimmunol.175.9.5895 }}</ref> T<sub>EMRA</sub> stands for terminally differentiated effector memory cells re-expressing CD45RA, which is a marker usually found on naive T cells.<ref>{{cite journal |vauthors= Koch S, Larbi A, Derhovanessian E, Özcelik D, Naumova E, Pawelec G | title = Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. | journal = Immunity & Ageing | volume = 5 | issue = 6 | year = 2008 | pmid = 18657274 | doi = 10.1186/1742-4933-5-6 | pmc=2515281}}</ref> |
|||
* Tissue resident memory T cells (T<sub>RM</sub>) occupy tissues (skin, lung, etc..) without recirculating. One cell surface marker that has been associated with T<sub>RM</sub> is the integrin αeβ7.<ref>{{Cite journal|title = Tissue-resident memory T cells|url = http://onlinelibrary.wiley.com/doi/10.1111/imr.12087/abstract|journal = Immunological Reviews|date = 2013-09-01|issn = 1600-065X|pmc = 3748618|pmid = 23947354|pages = 165–181|volume = 255|issue = 1|doi = 10.1111/imr.12087|first = Haina|last = Shin|first2 = Akiko|last2 = Iwasaki}}</ref> |
|||
* Virtual memory T cells differ from the other memory subsets in that they do not originate following a strong clonal expansion event. Thus, although this population as a whole is abundant within the peripheral circulation, individual virtual memory T cell clones reside at relatively low frequencies. One theory is that homeostatic proliferation gives rise to this T cell population. Although CD8 virtual memory T cells were the first to be described,<ref>{{cite journal |vauthors=Lee YJ, Jameson SC, Hogquist KA | title = Alternative memory in the CD8 T cell lineage. | journal = Trends in Immunology | volume = 32 | issue = 2 | pages = 50–56 | year = 2011 | pmid = 21288770 | doi = 10.1016/j.it.2010.12.004 | pmc=3039080}}</ref> it is now known that CD4 virtual memory cells also exist.<ref>{{cite journal |vauthors= Marusina AI, Ono Y, Merleev AA, Shimoda M, Ogawa H, Wang EA, Kondo K, Olney L, Luxardi G, Miyamura Y, Yilma TD, Villalobos IB, Bergstrom JW, Kronenberg DG, Soulika AM, Adamopoulos IE, Maverakis E | title = CD4+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity. | journal = Journal of Autoimmunity | volume = 77 | pages = 76–88 | year = 2017 | pmid = 27894837 | doi = 10.1016/j.jaut.2016.11.001 | url = http://ac.els-cdn.com/S0896841116302414/1-s2.0-S0896841116302414-main.pdf?_tid=eab200fc-1724-11e7-a9a0-00000aab0f01&acdnat=1491083478_8386d88ba0455ffaf2ebe97521819205}}</ref> |
|||
===Regulatory (suppressor)=== |
|||
[[调节T细胞|Regulatory T cell]]s (suppressor T cells) are crucial for the maintenance of [[免疫耐受|immunological tolerance]]. Their major role is to shut down T cell-mediated immunity toward the end of an immune reaction and to suppress autoreactive T cells that escaped the process of negative selection in the thymus. |
|||
Suppressor T cells along with Helper T cells can collectively be called Regulatory T cells due to their regulatory functions.<ref> |
|||
Textbook of Medical Physiology by Guyton and Hall, edition 6, pg. 448, Suppressor T cells paragraph.</ref> |
|||
Two major classes of CD4<sup>+</sup> T<sub>reg</sub> cells have been described — FOXP3<sup>+</sup> T<sub>reg</sub> cells and FOXP3<sup>−</sup> T<sub>reg</sub> cells. |
|||
Regulatory T cells can develop either during normal development in the thymus, and are then known as thymic Treg cells, or can be induced peripherally and are called peripherally derived Treg cells. These two subsets were previously called "naturally occurring", and "adaptive" or "induced", respectively.<ref name="pmid23507634">{{cite journal |vauthors=Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF | title = Regulatory T cells: recommendations to simplify the nomenclature | journal = Nat. Immunol. | volume = 14 | issue = 4 | pages = 307–8 | year = 2013 | pmid = 23507634 | doi = 10.1038/ni.2554 | url = }}</ref> Both subsets require the expression of the [[转录因子|transcription factor]] {{tsl|en|FOXP3||FOXP3}} which can be used to identify the cells. Mutations of the ''FOXP3'' gene can prevent regulatory T cell development, causing the fatal [[自體免疫性疾病|autoimmune disease]] [[IPEX症候群|IPEX]]. |
|||
Several other types of T cell have suppressive activity, but do not express FOXP3. These include Tr1 cells and Th3 cells, which are thought to originate during an immune response and act by producing suppressive molecules. {{tsl|en|Type 1 regulatory T cell||Tr1 cells}} are associated with IL-10, and Th3 cells are associated with [[转化生长因子-β|TGF-beta]]. Recently, {{tsl|en|Treg17 cells||Treg17 cells}} have been added to this list.<ref name="pmid24434314">{{cite journal |vauthors=Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E | title = Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells | journal = Indian J. Med. Res. | volume = 138 | issue = 5 | pages = 591–4 | year = 2013 | pmid = 24434314 | pmc = 3928692 | doi = }}</ref> |
|||
=== Natural killer T cell === |
|||
[[NKT細胞|Natural killer T cell]]s (NKT cells – not to be confused with [[自然杀伤细胞|natural killer cell]]s of the innate immune system) bridge the [[后天免疫系统|adaptive immune system]] with the [[先天免疫系統|innate immune system]]. Unlike conventional T cells that recognize peptide antigens presented by [[主要组织相容性复合体|major histocompatibility complex]] (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called {{tsl|en|CD1d||CD1d}}. Once activated, these cells can perform functions ascribed to both T<sub>h</sub> and T<sub>c</sub> cells (i.e., cytokine production and release of cytolytic/cell killing molecules). They are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses.<ref>{{Cite journal|last=Mallevaey|first=Thierry|last2=Fontaine|first2=Josette|last3=Breuilh|first3=Laetitia|last4=Paget|first4=Christophe|last5=Castro-Keller|first5=Alexandre|last6=Vendeville|first6=Catherine|last7=Capron|first7=Monique|last8=Leite-de-Moraes|first8=Maria|last9=Trottein|first9=François|date=2007-05-01|title=Invariant and Noninvariant Natural Killer T Cells Exert Opposite Regulatory Functions on the Immune Response during Murine Schistosomiasis|url=http://iai.asm.org/content/75/5/2171|journal=Infection and Immunity|language=en|volume=75|issue=5|pages=2171–2180|doi=10.1128/IAI.01178-06|issn=0019-9567|pmid=17353286|pmc=1865739}}</ref> |
|||
=== Mucosal associated invariant === |
|||
{{main|Mucosal associated invariant T cell}} |
|||
MAIT cells display [[先天免疫系統|innate]], effector-like qualities.<ref name=":02">{{Cite journal|last=Napier|first=Ruth J.|last2=Adams|first2=Erin J.|last3=Gold|first3=Marielle C.|last4=Lewinsohn|first4=David M.|date=2015-07-06|year=|title=The Role of Mucosal Associated Invariant T Cells in Antimicrobial Immunity|journal=Frontiers in Immunology|volume=6|pages=|doi=10.3389/fimmu.2015.00344|issn=1664-3224|pmc=4492155|via=|pmid=26217338}}</ref><ref>{{Cite journal|last=Gold|first=Marielle C.|last2=Lewinsohn|first2=David M.|date=2017-02-12|year=|title=Mucosal associated invariant T cells and the immune response to infection|journal=Microbes and infection / Institut Pasteur|volume=13|issue=8–9|pages=742–748|doi=10.1016/j.micinf.2011.03.007|issn=1286-4579|pmc=3130845|via=|pmid=21458588}}</ref> In humans, MAIT cells are found in the blood, liver, lungs, and [[黏膜|mucosa]], defending against microbial activity and infection.<ref name=":02" /> The [[MHC1類分子|MHC class I]]-like protein, {{tsl|en|MR1 (gene)||MR1}}, is responsible for presenting bacterially-produced [[维生素B|vitamin B]] metabolites to MAIT cells.<ref name=":4">{{Cite journal|last=Eckle|first=Sidonia B. G.|last2=Corbett|first2=Alexandra J.|last3=Keller|first3=Andrew N.|last4=Chen|first4=Zhenjun|last5=Godfrey|first5=Dale I.|last6=Liu|first6=Ligong|last7=Mak|first7=Jeffrey Y. W.|last8=Fairlie|first8=David P.|last9=Rossjohn|first9=Jamie|date=2015-12-18|year=|title=Recognition of Vitamin B Precursors and Byproducts by Mucosal Associated Invariant T Cells|journal=The Journal of Biological Chemistry|volume=290|issue=51|pages=30204–30211|doi=10.1074/jbc.R115.685990|issn=0021-9258|pmc=4683245|via=|pmid=26468291}}</ref><ref name=":6">{{Cite journal|last=Ussher|first=James E.|last2=Klenerman|first2=Paul|last3=Willberg|first3=Chris B.|date=2014-10-08|year=|title=Mucosal-Associated Invariant T-Cells: New Players in Anti-Bacterial Immunity|journal=Frontiers in Immunology|volume=5|pages=|doi=10.3389/fimmu.2014.00450|issn=1664-3224|pmc=4189401|via=|pmid=25339949}}</ref><ref name=":12">{{Cite journal|last=Howson|first=Lauren J.|last2=Salio|first2=Mariolina|last3=Cerundolo|first3=Vincenzo|date=2015-06-16|year=|title=MR1-Restricted Mucosal-Associated Invariant T Cells and Their Activation during Infectious Diseases|journal=Frontiers in Immunology|volume=6|pages=|doi=10.3389/fimmu.2015.00303|issn=1664-3224|pmc=4468870|via=|pmid=26136743}}</ref> After the presentation of foreign antigen by MR1, MAIT cells secretes pro-inflammatory [[细胞因子|cytokine]]s and are capable of {{tsl|en|Lysis||lysing}} bacterially-infected cells.<ref name=":02" /><ref name=":12" /> MAIT cells can also be activated through MR1-independent signaling.<ref name=":12" /> In addition to possessing innate-like functions, this T cell subset supports the [[后天免疫系统|adaptive]] immune response and has a memory-like phenotype.<ref name=":02" /> Furthermore, MAIT cells are thought to play a role in [[自體免疫性疾病|autoimmune disease]]s, such as [[多发性硬化症|multiple sclerosis]], arthritis and [[炎症性肠病|inflammatory bowel disease]],<ref name=":5">{{Cite journal|last=Hinks|first=Timothy S. C.|date=|year=2016|title=Mucosal‐associated invariant T cells in autoimmunity, immune‐mediated diseases and airways, disease|journal=Immunology|volume=148|issue=1|pages=1–12|doi=10.1111/imm.12582|issn=0019-2805|pmc=4819138|via=|pmid=26778581}}</ref><ref name=":9">{{Cite journal|last=Bianchini|first=Elena|last2=De Biasi|first2=Sara|last3=Simone|first3=Anna Maria|last4=Ferraro|first4=Diana|last5=Sola|first5=Patrizia|last6=Cossarizza|first6=Andrea|last7=Pinti|first7=Marcello|date=2017-03-01|title=Invariant natural killer T cells and mucosal-associated invariant T cells in multiple sclerosis|url=http://www.sciencedirect.com/science/article/pii/S0165247816303443|journal=Immunology Letters|volume=183|pages=1–7|doi=10.1016/j.imlet.2017.01.009}}</ref> although definitive evidence is yet to be published.<ref>{{cite journal |pmid=24450998 | doi=10.1111/cei.12277 | volume=176 | title=Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases | pmc=3992039 | year=2014 | journal=Clin. Exp. Immunol. | pages=266–74 |vauthors=Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E }}</ref><ref>{{Cite journal|last=Huang|first=Shouxiong|last2=Martin|first2=Emmanuel|last3=Kim|first3=Sojung|last4=Yu|first4=Lawrence|last5=Soudais|first5=Claire|last6=Fremont|first6=Daved H.|last7=Lantz|first7=Olivier|last8=Hansen|first8=Ted H.|date=2009-05-19|title=MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution|journal=Proceedings of the National Academy of Sciences of the United States of America|volume=106|issue=20|pages=8290–8295|doi=10.1073/pnas.0903196106|issn=1091-6490|pmc=2688861|pmid=19416870|via=|bibcode=2009PNAS..106.8290H}}</ref><ref>{{Cite journal|last=Chua|first=Wei-Jen|last2=Hansen|first2=Ted H.|date=November 2010|title=Bacteria, mucosal-associated invariant T cells and MR1|journal=Immunology and Cell Biology|volume=88|issue=8|pages=767–769|doi=10.1038/icb.2010.104|issn=1440-1711|pmid=20733595}}</ref><ref>{{cite journal |pmid=23051753 | doi=10.1038/nature11605 | volume=491 | title=MR1 presents microbial vitamin B metabolites to MAIT cells | year=2012 | journal=Nature | pages=717–23 |vauthors=Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O'Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J | bibcode=2012Natur.491..717K }}</ref> |
|||
=== Gamma delta T cells === |
|||
{{tsl|en|Gamma delta T cell||Gamma delta T cell}}s (γδ T cells) represent a small subset of T cells that possess a distinct [[T细胞受体|T cell receptor]] (TCR) on their surfaces. A majority of T cells have a [[T细胞受体|TCR]] composed of two [[醣蛋白|glycoprotein]] chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common in humans and mice (about 2% of total T cells); and are found mostly in the gut [[黏膜|mucosa]], within a population of lymphocytes known as {{tsl|en|intraepithelial lymphocyte||intraepithelial lymphocyte}}s. In rabbits, sheep, and chickens, the number of γδ T cells can be as high as 60% of total T cells. The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC-restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on [[抗原呈递细胞|APCs]]. Some {{tsl|en|murinae||murine}} γδ T cells recognize MHC class IB molecules, though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of nonpeptidic phosphorylated [[类萜|isoprenoid]] precursors, collectively named {{tsl|en|phosphoantigen||phosphoantigen}}s, which are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are {{tsl|en|isopentenyl pyrophosphate||isopentenyl pyrophosphate}} (IPP) and its isomer {{tsl|en|dimethylallyl pyrophosphate||dimethylallyl pyrophosphate}} (DMPP). Many microbes produce the highly active compound hydroxy-DMAPP ({{tsl|en|HMB-PP||HMB-PP}}) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and {{tsl|en|bisphosphonates||aminobisphosphonates}}, which upregulate endogenous IPP/DMAPP. |
|||
== Development== |
|||
{{see also|Thymocyte}} |
|||
All T cells originate from [[造血干细胞|haematopoietic stem cell]]s in the bone marrow. Haematopoietic progenitors (lymphoid progenitor cells) from [[造血干细胞|haematopoietic stem cell]]s populate the thymus and expand by cell division to generate a large population of immature [[胸腺細胞|thymocytes]].<ref name="pmid16448533">{{cite journal |vauthors=Schwarz BA, Bhandoola A | title = Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis | journal = Immunol. Rev. | volume = 209 | issue = | pages = 47–57 | date = February 2006 | pmid = 16448533 | doi = 10.1111/j.0105-2896.2006.00350.x }}</ref> The earliest thymocytes express neither CD4 nor CD8, and are therefore classed as double-negative (CD4<sup>−</sup>CD8<sup>−</sup>) cells. As they progress through their development, they become double-positive thymocytes (CD4<sup>+</sup>CD8<sup>+</sup>), and finally mature to ''single-positive'' (CD4<sup>+</sup>CD8<sup>−</sup> or CD4<sup>−</sup>CD8<sup>+</sup>) thymocytes that are then released from the thymus to peripheral tissues. There is some evidence of double-positive T-cells in the periphery, though their prevalence and function is uncertain.<ref>{{cite journal|last1=Overgaard|first1=Nana H.|last2=Jung|first2=Ji-Won|last3=Steptoe|first3=Raymond J.|last4=Wells|first4=James W.|title=CD4+/CD8+ double-positive T cells: more than just a developmental stage?|journal=Journal of Leukocyte Biology|date=1 January 2015|volume=97|issue=1|pages=31–38|doi=10.1189/jlb.1RU0814-382|issn=1938-3673|pmid=25360000}}</ref><ref>{{cite journal|last1=Cruz|first1=Jazmina L.G.|last2=Overgaard|first2=Nana H.|last3=Bridge|first3=Jenniger A.|last4=Nel|first4=Hendrik J.|last5=Frazer|first5=Ian Ian H.|last6=La Gruta|first6=Nicole|last7=Blumentral|first7= Antje|last8=Steptoe|first8=Raymond J.|last9=Wells|first9=James W.|title=CD4+CD8β+ double‐positive T cells in skin‐draining lymph nodes respond to inflammatory signals from the skin|journal=Journal of Leukocyte Biology|date=21 June 2017|volume=102|issue=3|pages=837–844|doi=10.1189/jlb.1AB0217-065R |issn=1938-3673}}</ref> |
|||
About 98% of thymocytes die during the development processes in the thymus by failing either positive selection or negative selection, whereas the other 2% survive and leave the thymus to become mature immunocompetent T cells. Increasing evidence indicates [[小分子核糖核酸|microRNAs]], which are small [[非編碼核糖核酸|noncoding regulatory RNAs]], could impact the clonal selection process during thymic development. For example, {{tsl|en|miR-181a||miR-181a}} was found to play a role in the positive selection of T lymphocytes.<ref name="pmid17382377">{{cite journal |vauthors=Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ | title = miR-181a is an intrinsic modulator of T cell sensitivity and selection | journal = Cell | volume = 129 | issue = 1 | pages = 147–61 | year = 2007 | pmid = 17382377 | doi = 10.1016/j.cell.2007.03.008 }}</ref> |
|||
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3%<ref name="pmid10837068">{{cite journal |vauthors=Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP | title = The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection | journal = Annu. Rev. Immunol. | volume = 18 | issue = | pages = 529–60 | year = 2000 | pmid = 10837068 | doi = 10.1146/annurev.immunol.18.1.529 }}</ref> a year throughout middle age, a corresponding fall in the thymic production of {{tsl|en|naïve T cell||naïve T cell}}s occurs, leaving peripheral T cell expansion to play a greater role in protecting older subjects. |
|||
=== Beta selection === |
|||
Common lymphoid precursor cells that migrate to the thymus become known as T-cell precursors (or thymocytes) and do not express a T cell receptor. Broadly speaking, the double negative (DN) stage is focused on producing a functional β-chain whereas the double positive (DP) stage is focused on producing a functional α-chain, ultimately producing a functional αβ T cell receptor. As the developing thymocyte progresses through the four DN stages (DN1, DN2, DN3, and DN4), the T cell expresses an invariant α-chain but rearranges the β-chain locus. If the rearranged β-chain successfully pairs with the invariant α-chain, signals are produced which cease rearrangement of the β-chain (and silence the alternate allele) and result in proliferation of the cell.<ref>{{Cite book|title = Immunobiology|last = Janeway|first = Charles|publisher = Garland Science|year = 2012|isbn = 9780815342434|location = |pages = 301–305}}</ref> Although these signals require this pre-TCR at the cell surface, they are independent of ligand binding to the pre-TCR. These thymocytes will then express both CD4 and CD8 and progresses to the double positive (DP) stage where selection of the α-chain takes place. If a rearranged β-chain does not lead to any signalling (e.g. as a result of an inability to pair with the invariant α-chain), the cell may die by neglect (lack of signalling). |
|||
=== Positive selection === |
|||
Positive selection "selects for" T cells capable of interacting with MHC. Positive selection involves the production of a signal by double-positive precursors that express either MHC Class I or II restricted receptors. The signal produced by these thymocytes result in RAG gene repression, long-term survival and migration into the medulla, as well as differentiation into mature T cells. The process of positive selection takes a number of days.<ref>{{Cite journal|last=Timothy K. Starr|last2=Stephen C. Jameson|last3=Hogquist|first3=and Kristin A.|date=2003-01-01|title=Positive and Negative Selection of T Cells|url=https://dx.doi.org/10.1146/annurev.immunol.21.120601.141107|journal=Annual Review of Immunology|volume=21|issue=1|pages=139–176|doi=10.1146/annurev.immunol.21.120601.141107|pmid=12414722}}</ref> |
|||
Double-positive [[胸腺細胞|thymocyte]]s (CD4<sup>+</sup>/CD8<sup>+</sup>) move deep into the thymic {{tsl|en|cortex (anatomy)||cortex}}, where they are presented with self-[[抗原|antigen]]s. These self-antigens are expressed by thymic cortical epithelial cells on [[主要组织相容性复合体|MHC]] molecules on the surface of cortical epithelial cells. Only those thymocytes that interact with MHC-I or MHC-II appropriately (i.e., not too strongly or too weakly) will receive a vital "survival signal". All that cannot (i.e., if they do not interact strongly enough, or if they bind too strongly) will die by "death by neglect" (no survival signal). This process ensures that the selected T-cells will have an MHC affinity that can serve useful functions in the body (i.e., the cells must be able to interact with MHC and peptide complexes to effect immune responses). The vast majority of all thymocytes will die during this process. |
|||
A thymocyte's fate is determined during positive selection. Double-positive cells (CD4<sup>+</sup>/CD8<sup>+</sup>) that interact well with MHC class II molecules will eventually become CD4<sup>+</sup> cells, whereas thymocytes that interact well with MHC class I molecules mature into CD8<sup>+</sup> cells. A T cell becomes a CD4<sup>+</sup> cell by down-regulating expression of its CD8 cell surface receptors. If the cell does not lose its signal, it will continue downregulating CD8 and become a CD4<sup>+</sup>, single positive cell.<ref>{{Cite journal|last=Zerrahn|first=Jens|last2=Held|first2=Werner|last3=Raulet|first3=David H.|date=1997-03-07|title=The MHC Reactivity of the T Cell Repertoire Prior to Positive and Negative Selection|url=http://www.cell.com/article/S0092867400819054/abstract|journal=Cell|language=English|volume=88|issue=5|pages=627–636|doi=10.1016/S0092-8674(00)81905-4|issn=0092-8674|pmid=9054502 }}</ref> But, if there is a signal interruption, the cell stops downregulating CD8 and switches over to downregulating CD4 molecules, instead, eventually becoming a CD8<sup>+</sup>, single positive cell. |
|||
This process does not remove thymocytes that may cause {{tsl|en|autoimmunity||autoimmunity}}. The potentially autoimmune cells are removed by the process of negative selection, which occurs in the thymic medulla (discussed below). |
|||
=== Negative selection === |
|||
Negative selection removes thymocytes that are capable of strongly binding with "self" MHC peptides. Thymocytes that survive positive selection migrate towards the boundary of the cortex and medulla in the thymus. While in the medulla, they are again presented with a self-antigen presented on the MHC complex of medullary thymic epithelial cells (mTECs).<ref name="pmid20431619">{{cite journal |vauthors=Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L | title = Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance | journal = Nat. Immunol. | volume = 11 | issue = 6 | pages = 512–9 | date = June 2010 | pmid = 20431619 | doi = 10.1038/ni.1874 | url = http://mediatum.ub.tum.de/doc/1073501/document.pdf }}</ref> mTECs must be [[自體免疫調節因子|AIRE]]<sup>+</sup> to properly express self-antigens from all tissues of the body on their MHC class I peptides. Some mTECs are phagocytosed by thymic dendritic cells; this allows for presentation of self-antigens on MHC class II molecules (positively selected CD4<sup>+</sup> cells must interact with MHC class II molecules, thus APCs, which possess MHC class II, must be present for CD4<sup>+</sup> T-cell negative selection). Thymocytes that interact too strongly with the self-antigen receive an [[细胞凋亡|apoptotic]] signal that leads to cell death. However, some of these cells are selected to become [[调节T细胞|Treg]] cells. The remaining cells exit the thymus as immature {{tsl|en|naïve T cell||naïve T cell}}s (also known as recent thymic emigrants <ref>{{Cite journal|last=Pekalski|first=Marcin L.|last2=Rubio García|first2=Arcadio|last3=Ferreira|first3=Ricardo C.|last4=Rainbow|first4=Daniel B.|last5=Smyth|first5=Deborah J.|last6=Mashar|first6=Meghavi|last7=Brady|first7=Jane|last8=Savinykh|first8=Natalia|last9=Dopico|first9=Xaquin Castro|date=2017-08-17|title=Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2|url=https://doi.org/10.1172/jci.insight.93739|journal=JCI Insight|language=en|volume=2|issue=16|pages=|doi=10.1172/jci.insight.93739|issn=0021-9738|via=}}</ref>). This process is an important component of {{tsl|en|central tolerance||central tolerance}} and serves to prevent the formation of self-reactive T cells that are capable of inducing autoimmune diseases in the host. |
|||
In summary, β-selection is the first checkpoint, where the T cells that are able to form a functional pre-TCR with an invariant alpha chain and a functional beta chain are allowed to continue development in the thymus. Next, positive selection checks that T cells have successfully rearranged their TCRα locus and are capable of recognizing peptide-MHC complexes with appropriate affinity. Negative selection in the medulla then obliterates T cells that bind too strongly to self-antigens expressed on MHC molecules. These selection processes allow for tolerance of self by the immune system. Typical T cells that leave the thymus (via the corticomedullarly junction) are self-restricted, self-tolerant, and singly positive. |
|||
== Activation == |
|||
[[Image:T cell activation.svg|thumb|300px|right|The T lymphocyte activation pathway: T cells contribute to immune defenses in two major ways; some direct and regulate immune responses; others directly attack infected or cancerous cells.<ref name=NIAID>The {{tsl|en|NIAID||NIAID}} resource booklet [https://www.niaid.nih.gov/publications/immune/the_immune_system.pdf "Understanding the Immune System (pdf)"].</ref>]] |
|||
Activation of CD4<sup>+</sup> T cells occurs through the simultaneous engagement of the [[T细胞受体|T-cell receptor]] and a co-stimulatory molecule (like {{tsl|en|CD28||CD28}}, or {{tsl|en|CD278||ICOS}}) on the T cell by the major histocompatibility complex (MHCII) [[肽|peptide]] and co-stimulatory molecules on the APC. Both are required for production of an effective immune response; in the absence of {{tsl|en|co-stimulation||co-stimulation}}, T cell receptor signalling alone results in [[株落失能|anergy]]. The signalling pathways downstream from co-stimulatory molecules usually engages the {{tsl|en|phosphoinositide 3-kinase||PI3K}} pathway generating {{tsl|en|PIP3||PIP3}} at the plasma membrane and recruiting {{tsl|en|pleckstrin homology domain||PH domain}} containing signaling molecules like {{tsl|en|Phosphoinositide-dependent kinase-1||PDK1}} that are essential for the activation of {{tsl|en|PRKCQ||PKCθ}}, and eventual [[白细胞介素-2|IL-2]] production. Optimal CD8<sup>+</sup> T cell response relies on CD4<sup>+</sup> signalling.<ref>{{Cite journal|last=Williams|first=Matthew A.|last2=Bevan|first2=Michael J.|date=2007-01-01|title=Effector and Memory CTL Differentiation|url=https://dx.doi.org/10.1146/annurev.immunol.25.022106.141548|journal=Annual Review of Immunology|volume=25|issue=1|pages=171–192|doi=10.1146/annurev.immunol.25.022106.141548|pmid=17129182}}</ref> CD4<sup>+</sup> cells are useful in the initial antigenic activation of naïve CD8 T cells, and sustaining memory CD8<sup>+</sup> T cells in the aftermath of an acute infection. Therefore, activation of CD4<sup>+</sup> T cells can be beneficial to the action of CD8<sup>+</sup> T cells.<ref>{{Cite journal|last=Janssen|first=Edith M.|last2=Lemmens|first2=Edward E.|last3=Wolfe|first3=Tom|last4=Christen|first4=Urs|last5=von Herrath|first5=Matthias G.|last6=Schoenberger|first6=Stephen P.|date=2003-02-20|title=CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes|url=http://www.nature.com/nature/journal/v421/n6925/abs/nature01441.html|journal=Nature|language=en|volume=421|issue=6925|pages=852–856|doi=10.1038/nature01441|issn=0028-0836|bibcode=2003Natur.421..852J}}</ref><ref>{{Cite journal|last=Shedlock|first=Devon J.|last2=Shen|first2=Hao|date=2003-04-11|title=Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory|url=http://science.sciencemag.org/content/300/5617/337|journal=Science|language=en|volume=300|issue=5617|pages=337–339|doi=10.1126/science.1082305|issn=0036-8075|pmid=12690201|bibcode=2003Sci...300..337S}}</ref><ref>{{Cite journal|last=Sun|first=Joseph C.|last2=Williams|first2=Matthew A.|last3=Bevan|first3=Michael J.|date=2004-09-01|title=CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection|url=http://www.nature.com/ni/journal/v5/n9/abs/ni1105.html|journal=Nature Immunology|language=en|volume=5|issue=9|pages=927–933|doi=10.1038/ni1105|issn=1529-2908|pmc=2776074|pmid=15300249}}</ref> |
|||
The first signal is provided by binding of the T cell receptor to its cognate peptide presented on MHCII on an APC. MHCII is restricted to so-called professional [[抗原呈递细胞|antigen-presenting cell]]s, like dendritic cells, B cells, and macrophages, to name a few. The peptides presented to CD8<sup>+</sup> T cells by MHC class I molecules are 8–13 amino acids in length; the peptides presented to CD4<sup>+</sup> cells by MHC class II molecules are longer, usually 12–25 amino acids in length,<ref>Jennifer Rolland and Robyn O'Hehir, "Turning off the T cells: Peptides for treatment of allergic Diseases," Today's life science publishing, 1999, Page 32</ref> as the ends of the binding cleft of the MHC class II molecule are open. |
|||
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as [[壞死|necrotic]]-bodies or [[热休克蛋白|heat shock proteins]]. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the {{tsl|en|CD80||CD80}} and {{tsl|en|CD86||CD86}} proteins, which together constitute the {{tsl|en|B7 (protein)||B7}} protein, (B7.1 and B7.2, respectively) on the APC. Other receptors are expressed upon activation of the T cell, such as {{tsl|en|OX40||OX40}} and ICOS, but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes [[株落失能|anergic]], and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation. Once a T cell has been appropriately activated (i.e. has received signal one and signal two) it alters its cell surface expression of a variety of proteins. Markers of T cell activation include CD69, CD71 and CD25 (also a marker for Treg cells), and HLA-DR (a marker of human T cell activation). CTLA-4 expression is also up-regulated on activated T cells, which in turn outcompetes CD28 for binding to the B7 proteins. This is a checkpoint mechanism to prevent over activation of the T cell. Activated T cells also change their cell surface glycosylation profile.<ref>{{cite journal |vauthors=Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB | title = Glycans in the immune system and The Altered Glycan Theory of Autoimmunity | journal = J Autoimmun | volume = 57 | issue = 6 | pages = 1–13 | year = 2015 | pmid = 25578468 | doi = 10.1016/j.jaut.2014.12.002 | pmc=4340844}}</ref> |
|||
The [[T细胞受体|T cell receptor]] exists as a complex of several proteins. The actual T cell receptor is composed of two separate peptide chains, which are produced from the independent T cell receptor alpha and beta (''TCRα'' and ''TCRβ'') genes. The other proteins in the complex are the {{tsl|en|CD3 (immunology)||CD3}} proteins: CD3εγ and CD3εδ heterodimers and, most important, a CD3ζ homodimer, which has a total of six {{tsl|en|immunoreceptor tyrosine-based activation motif||ITAM}} motifs. The ITAM motifs on the CD3ζ can be phosphorylated by {{tsl|en|Lck||Lck}} and in turn recruit {{tsl|en|ZAP70||ZAP-70}}. Lck and/or ZAP-70 can also phosphorylate the [[酪氨酸|tyrosines]] on many other molecules, not least CD28, {{tsl|en|Linker of activated T cells||LAT}} and {{tsl|en|Lymphocyte cytosolic protein 2||SLP-76}}, which allows the aggregation of signalling complexes around these proteins. |
|||
Phosphorylated {{tsl|en|linker of activated T cells||LAT}} recruits SLP-76 to the membrane, where it can then bring in {{tsl|en|Phosphoinositide phospholipase C||PLC-γ}}, {{tsl|en|VAV1||VAV1}}, {{tsl|en|ITK (gene)||Itk}} and potentially {{tsl|en|phosphoinositide 3-kinase||PI3K}}. PLC-γ cleaves PI(4,5)P2 on the inner leaflet of the membrane to create the active intermediaries diacylglycerol ({{tsl|en|Diglyceride||DAG}}), inositol-1,4,5-trisphosphate ([[肌醇三磷酸|IP3]]); PI3K also acts on PIP2, phosphorylating it to produce phosphatidlyinositol-3,4,5-trisphosphate (PIP3). DAG binds and activates some PKCs. Most important in T cells is PKCθ, critical for activating the transcription factors [[NF-κB]] and AP-1. [[肌醇三磷酸|IP3]] is released from the membrane by PLC-γ and diffuses rapidly to activate calcium channel receptors on the [[内质网|ER]], which induces the release of [[鈣質|calcium]] into the cytosol. Low calcium in the endoplasmic reticulum causes STIM1 clustering on the ER membrane and leads to activation of cell membrane CRAC channels that allows additional calcium to flow into the cytosol from the extracellular space. This aggregated cytosolic calcium binds calmodulin, which can then activate [[钙调磷酸酶|calcineurin]]. Calcineurin, in turn, activates {{tsl|en|NFAT||NFAT}}, which then translocates to the nucleus. NFAT is a [[转录因子|transcription factor]] that activates the transcription of a pleiotropic set of genes, most notable, IL-2, a cytokine that promotes long-term proliferation of activated T cells. |
|||
PLCγ can also initiate the [[NF-κB|NF-κB pathway]]. DAG activates PKCθ, which then phosphorylates CARMA1, causing it to unfold and function as a scaffold. The cytosolic domains bind an adapter {{tsl|en|BCL10||BCL10}} via {{tsl|en|CARD domain||CARD}} (Caspase activation and recruitment domains) domains; that then binds TRAF6, which is ubiquitinated at K63.{{rp|513–523}}<ref name="isbn0-12-289632-7"/> This form of ubiquitination does not lead to degradation of target proteins. Rather, it serves to recruit NEMO, IKKα and -β, and TAB1-2/ TAK1.<ref name="pmid14579250">{{cite journal |vauthors=Wu H, Arron JR | title = TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology | journal = BioEssays | volume = 25 | issue = 11 | pages = 1096–105 | date = November 2003 | pmid = 14579250 | doi = 10.1002/bies.10352 }}</ref> TAK 1 phosphorylates IKK-β, which then phosphorylates IκB allowing for K48 ubiquitination: leads to proteasomal degradation. Rel A and p50 can then enter the nucleus and bind the NF-κB response element. This coupled with NFAT signaling allows for complete activation of the IL-2 gene.<ref name="isbn0-12-289632-7">{{cite book |vauthors=Tatham P, Gomperts BD, Kramer IM | title = Signal transduction | publisher = Elsevier Academic Press | location = Amsterdam | year = 2003 | pages = | isbn = 0-12-289632-7 }}</ref> |
|||
While in most cases activation is dependent on TCR recognition of antigen, alternative pathways for activation have been described. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter.<ref name="pmid21045195">{{cite journal |vauthors=Milstein O, Hagin D, Lask A, Reich-Zeliger S, Shezen E, Ophir E, Eidelstein Y, Afik R, Antebi YE, Dustin ML, Reisner Y | title = CTLs respond with activation and granule secretion when serving as targets for T cell recognition | journal = Blood | volume = 117 | issue = 3 | pages = 1042–52 | date = January 2011 | pmid = 21045195 | pmc = 3035066 | doi = 10.1182/blood-2010-05-283770 }}</ref> |
|||
In spring 2014, the [[T细胞|T-Cell Activation in Space]] (TCAS) experiment was launched to the [[国际空间站|International Space Station]] on the {{tsl|en|SpaceX CRS-3||SpaceX CRS-3}} mission to study how "deficiencies in the human immune system are affected by a microgravity environment".<ref name=nsf20140414prelaunchArticle>{{cite news |last=Graham|first=William |title=SpaceX ready for CRS-3 Dragon launch and new milestones |url=http://www.nasaspaceflight.com/2014/04/spacex-crs-3-dragon-new-milestones/ |accessdate=2014-04-14 |newspaper=NASAspaceflight.com |date=2014-04-14 }}</ref> |
|||
T cell activation is modulated by [[活性氧类|reactive oxygen species]].<ref>{{cite journal|last1=Belikov|first1=Aleksey V.|last2=Schraven|first2=Burkhart|last3=Simeoni|first3=Luca|title=T cells and reactive oxygen species|journal=Journal of Biomedical Science|date=1 January 2015|volume=22|pages=85|doi=10.1186/s12929-015-0194-3|pmid=26471060|url=https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-015-0194-3|accessdate=29 April 2017|issn=1423-0127|pmc=4608155}}</ref> |
|||
=== Antigen discrimination === |
|||
A unique feature of T cells is their ability to discriminate between healthy and abnormal (e.g. infected or cancerous) cells in the body.<ref name="Feinerman_2008">{{cite journal |vauthors=Feinerman O, Germain RN, Altan-Bonnet G | title = Quantitative challenges in understanding ligand discrimination by alphabeta T cells | journal = Mol. Immunol. | volume = 45 | issue = 3 | pages = 619–31 | year = 2008 | pmid = 17825415 | pmc = 2131735 | doi = 10.1016/j.molimm.2007.03.028 }}</ref> Healthy cells typically express a large number of self derived pMHC on their cell surface and although the T cell antigen receptor can interact with at least a subset of these self pMHC, the T cell generally ignores these healthy cells. However, when these very same cells contain even minute quantities of pathogen derived pMHC, T cells are able to become activated and initiate immune responses. The ability of T cells to ignore healthy cells but respond when these same cells contain pathogen (or cancer) derived pMHC is known as antigen discrimination. The molecular mechanisms that underlie this process are controversial.<ref name="Feinerman_2008"/><ref name="pmid24636916">{{cite journal |vauthors=Dushek O, van der Merwe PA | title = An induced rebinding model of antigen discrimination | journal = Trends Immunol. | volume = 35 | issue = 4 | pages = 153–8 | year = 2014 | pmid = 24636916 | pmc = 3989030 | doi = 10.1016/j.it.2014.02.002 }}</ref> |
|||
==Clinical significance== |
|||
=== Deficiency === |
|||
{{Main|T cell deficiency}} |
|||
Causes of {{tsl|en|T cell deficiency||T cell deficiency}} include {{tsl|en|lymphocytopenia||lymphocytopenia}} of T cells and/or defects on function of individual T cells. Complete insufficiency of T cell function can result from {{tsl|en|hereditary condition||hereditary condition}}s such as [[嚴重複合型免疫缺乏症|severe combined immunodeficiency]] (SCID), {{tsl|en|Omenn syndrome||Omenn syndrome}}, and {{tsl|en|cartilage–hair hypoplasia||cartilage–hair hypoplasia}}.<ref name=Schwartz2011>[http://emedicine.medscape.com/article/888372-overview Medscape > T-cell Disorders]. Author: Robert A Schwartz, MD, MPH; Chief Editor: Harumi Jyonouchi, MD. Updated: May 16, 2011</ref> Causes of partial insufficiencies of T cell function include [[艾滋病|acquired immune deficiency syndrome]] (AIDS), and hereditary conditions such as [[迪喬治症候群|DiGeorge syndrome]] (DGS), {{tsl|en|chromosomal breakage syndrome||chromosomal breakage syndrome}}s (CBSs), and B-cell and T-cell combined disorders such as [[共濟失調微血管擴張症候群|ataxia-telangiectasia]] (AT) and {{tsl|en|Wiskott–Aldrich syndrome||Wiskott–Aldrich syndrome}} (WAS).<ref name=Schwartz2011/> |
|||
The main pathogens of concern in T cell deficiencies are [[細胞內寄生物|intracellular pathogen]]s, including ''[[单纯疱疹病毒|Herpes simplex virus]]'', ''[[分枝杆菌属|Mycobacterium]]'' and ''{{tsl|en|Listeria||Listeria}}''.<ref name="isbn1-4051-2665-5">{{cite book |veditors=Jones J, Bannister BA, Gillespie SH | title = Infection: Microbiology and Management | publisher = Wiley-Blackwell | location = | year = 2006 | page = 435 | isbn = 1-4051-2665-5 | url = https://books.google.com/books?id=iPuvQDcqW88C&pg=PA435 }}</ref> Also, [[真菌病|fungal infections]] are also more common and severe in T cell deficiencies.<ref name = "isbn1-4051-2665-5"/> |
|||
===Cancer=== |
|||
{{Further|T-cell lymphoma}} |
|||
[[癌症|Cancer]] of T cells is termed [[T细胞淋巴瘤|T-cell lymphoma]], and accounts for perhaps one in ten cases of [[非霍奇金氏淋巴瘤|non-Hodgkin lymphoma]].<ref name="The Lymphomas">{{cite web |url=http://www.leukemia-lymphoma.org/attachments/National/br_1161891669.pdf |title=The Lymphomas |accessdate=2008-04-07 |date=May 2006 |format=PDF |publisher=The Leukemia & Lymphoma Society |page=2}}</ref> The main forms of T cell lymphoma are: |
|||
* {{tsl|en|Extranodal T cell lymphoma||Extranodal T cell lymphoma}} |
|||
* {{tsl|en|Cutaneous T cell lymphoma||Cutaneous T cell lymphoma}}s: {{tsl|en|Sézary syndrome||Sézary syndrome}} and {{tsl|en|Mycosis fungoides||Mycosis fungoides}} |
|||
* {{tsl|en|Anaplastic large cell lymphoma||Anaplastic large cell lymphoma}} |
|||
* {{tsl|en|Angioimmunoblastic T cell lymphoma||Angioimmunoblastic T cell lymphoma}} |
|||
===Exhaustion=== |
|||
T cell exhaustion is the progressive loss of T cell function. It can occur during sepsis and after other acute or chronic infections.<ref name=Yi2010>{{cite journal| title=T cell exhaustion: characteristics, causes and conversion| journal=Immunology| date= Apr 2010 |volume= 129| issue=4| pages= 474–481 |doi= 10.1111/j.1365-2567.2010.03255.x| PMC= 2842494 | vauthors = Yi JS, Cox MA, Zajac AJ | pmid=20201977}}</ref><ref>{{cite journal| title=Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach| vauthors = Hotchkiss RS, Monneret G, Payen D|journal=Lancet Infect Dis| date= Mar 2013 | volume=13| issue=3| pages=260–8| doi= 10.1016/S1473-3099(13)70001-X| pmc=3798159}}</ref> |
|||
[[T cell exhaustion]] is mediated by several inhibitory receptors including {{tsl|en|Programmed cell death 1||programmed cell death protein 1}} (PD1), TIM3, and {{tsl|en|LAG3||lymphocyte activation gene 3 protein}} (LAG3).<ref name=Mahoney2015>{{cite journal |vauthors = Mahoney KM, Rennert PD, Freeman GJ |title=Combination cancer immunotherapy and new immunomodulatory targets |journal=Nat Rev Drug Discov |volume=14 |issue=8 |pages=561–84 |year=2015 |pmid=26228759 |doi=10.1038/nrd4591}}</ref> |
|||
<br>CD8+ T cell exhaustion occurs in some tumours, and can be partly reversed by blocking the inhibitory receptors (e.g. PD1).<ref name=Pauken2015>{{cite journal |vauthors = Pauken KE, Wherry EJ |title = Overcoming T cell exhaustion in infection and cancer |journal = Trends Immunol. |volume = 36 |issue = 4 |pages = 265–76 |year = 2015 |pmid = 25797516 |pmc = 4393798 |doi = 10.1016/j.it.2015.02.008}}</ref> |
|||
T cell exhaustion is associated with [[表觀遺傳學|epigenetic]] changes in the T cells.<ref>{{Cite news|url=http://www.genengnews.com/gen-news-highlights/epigenetic-reprogramming-may-boost-immunotherapy/81254578|title=Epigenetic Reprogramming May Boost Immunotherapy|last=|first=|date=2017-06-27|work=Genetic Engineering & Biotechnology News|access-date=2017-08-08|archive-url=|archive-date=|dead-url=}}</ref> |
|||
( See also {{tsl|en|Immunosenescence||Immunosenescence#T cell functional dysregulation as a biomarker for immunosenescence}} ). |
|||
==Research== |
|||
=== Genetic engineering === |
|||
In 2015, a team of researchers led by Dr. Alexander Marson<ref>{{Cite news|url=https://www.independent.co.uk/news/science/crispr-breakthrough-announced-in-technique-of-editing-dna-to-fight-off-deadly-illnesses-10420050.html|title=Breakthrough announced in 'editing' DNA to fight off deadly illness|date=2015-07-27|work=The Independent|access-date=2017-08-08|language=en-GB}}</ref> at the [[加利福尼亚大学旧金山分校|University of California, San Francisco]] successfully edited the genome of human T cells using a {{tsl|en|Cas9||Cas9}} ribonucleoprotein delivery method.<ref name=":0">{{Cite journal|title = Generation of knock-in primary human T cells using Cas9 ribonucleoproteins|journal = Proceedings of the National Academy of Sciences of the United States of America|date = 2015-08-18|issn = 1091-6490|pmc = 4547290|pmid = 26216948|pages = 10437–10442|volume = 112|issue = 33|doi = 10.1073/pnas.1512503112|first = Kathrin|last = Schumann|first2 = Steven|last2 = Lin|first3 = Eric|last3 = Boyer|first4 = Dimitre R.|last4 = Simeonov|first5 = Meena|last5 = Subramaniam|first6 = Rachel E.|last6 = Gate|first7 = Genevieve E.|last7 = Haliburton|first8 = Chun J.|last8 = Ye|first9 = Jeffrey A.|last9 = Bluestone|bibcode = 2015PNAS..11210437S}}</ref> This advancement has potential for applications in treating "{{tsl|en|Cancer immunotherapy||cancer immunotherapies}} and cell-based therapies for HIV, {{tsl|en|Primary immunodeficiency||primary immune deficiencies}}, and [[自體免疫性疾病|autoimmune disease]]s".<ref name=":0" /> |
|||
== 参见 == |
== 参见 == |
||
2018年5月25日 (五) 02:16的版本
| 此條目需要編修,以確保標點符號使用恰当。 (2018年1月) |
| T细胞 | |
|---|---|
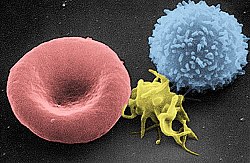
 人类T细胞的扫描电子显微镜图片 | |
 | |
| 基本信息 | |
| 系統 | 免疫系统 |
| 标识字符 | |
| 拉丁文 | lymphocytus T |
| MeSH | D013601 |
| TH | H2.00.04.1.02007 |
| FMA | FMA:62870 |
| 《解剖學術語》 [在维基数据上编辑] | |
t細胞或t淋巴球 is a type of lymphocyte (a subtype of white blood cell) that plays a central role in cell-mediated immunity. T cells can be distinguished from other lymphocytes, such as B cells and natural killer cells, by the presence of a T-cell receptor on the cell surface. They are called T cells because they mature in the thymus from thymocytes[1] (although some also mature in the tonsils[2]). The several subsets of T cells each have a distinct function. The majority of human T cells rearrange their alpha and beta chains on the cell receptor and are termed alpha beta T cells (αβ T cells) and are part of the adaptive immune system. Specialized gamma delta T cells, (a small minority of T cells in the human body, more frequent in ruminants), have invariant T-cell receptors with limited diversity, that can effectively present antigens to other T cells[3] and are considered to be part of the innate immune system.
Types
Effector
Effector cells are the superset of all the various T cell types that actively respond immediately to a stimulus, such as co-stimulation. This includes helper, killer, regulatory, and potentially other T cell types. Memory cells are their opposite counterpart that are longer lived to target future infections as necessary.
Helper
T helper cells (TH cells) assist other white blood cells in immunologic processes, including maturation of B cells into plasma cells and memory B cells, and activation of cytotoxic T cells and macrophages. These cells are also known as CD4+ T cells because they express the CD4 glycoprotein on their surfaces. Helper T cells become activated when they are presented with peptide antigens by MHC class II molecules, which are expressed on the surface of antigen-presenting cells (APCs). Once activated, they divide rapidly and secrete small proteins called cytokines that regulate or assist in the active immune response. These cells can differentiate into one of several subtypes, including TH1, TH2, TH3, TH17, TH9, or TFH, which secrete different cytokines to facilitate different types of immune responses. Signalling from the APC directs T cells into particular subtypes.[4]
Cytotoxic (Killer) CD8 +ve
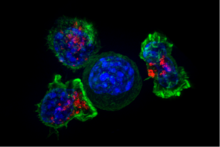
Cytotoxic T cells (TC cells, CTLs, T-killer cells, killer T cells) destroy virus-infected cells and tumor cells, and are also implicated in transplant rejection. These cells are also known as CD8+ T cells since they express the CD8 glycoprotein at their surfaces. These cells recognize their targets by binding to antigen associated with MHC class I molecules, which are present on the surface of all nucleated cells. Through IL-10, adenosine, and other molecules secreted by regulatory T cells, the CD8+ cells can be inactivated to an anergic state, which prevents autoimmune diseases.
Memory
Antigen-naïve T cells expand and differentiate into memory and effector T cells after they encounter their cognate antigen within the context of an MHC molecule on the surface of a professional antigen presenting cell (e.g. a dendritic cell). Appropriate co-stimulation must be present at the time of antigen encounter for this process to occur. Historically, memory T cells were thought to belong to either the effector or central memory subtypes, each with their own distinguishing set of cell surface markers (see below).[5] Subsequently, numerous new populations of memory T cells were discovered including tissue-resident memory T (Trm) cells, stem memory TSCM cells, and virtual memory T cells. The single unifying theme for all memory T cell subtypes is that they are long-lived and can quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen. By this mechanism they provide the immune system with "memory" against previously encountered pathogens. Memory T cells may be either CD4+ or CD8+ and usually express CD45RO.[6]
Memory T cell subtypes:
- Central memory T cells (TCM cells) express CD45RO, C-C chemokine receptor type 7 (CCR7), and L-selectin (CD62L). Central memory T cells also have intermediate to high expression of CD44. This memory subpopulation is commonly found in the lymph nodes and in the peripheral circulation. (Note- CD44 expression is usually used to distinguish murine naive from memory T cells).
- Effector memory T cells (TEM cells and TEMRA cells) express CD45RO but lack expression of CCR7 and L-selectin. They also have intermediate to high expression of CD44. These memory T cells lack lymph node-homing receptors and are thus found in the peripheral circulation and tissues.[7] TEMRA stands for terminally differentiated effector memory cells re-expressing CD45RA, which is a marker usually found on naive T cells.[8]
- Tissue resident memory T cells (TRM) occupy tissues (skin, lung, etc..) without recirculating. One cell surface marker that has been associated with TRM is the integrin αeβ7.[9]
- Virtual memory T cells differ from the other memory subsets in that they do not originate following a strong clonal expansion event. Thus, although this population as a whole is abundant within the peripheral circulation, individual virtual memory T cell clones reside at relatively low frequencies. One theory is that homeostatic proliferation gives rise to this T cell population. Although CD8 virtual memory T cells were the first to be described,[10] it is now known that CD4 virtual memory cells also exist.[11]
Regulatory (suppressor)
Regulatory T cells (suppressor T cells) are crucial for the maintenance of immunological tolerance. Their major role is to shut down T cell-mediated immunity toward the end of an immune reaction and to suppress autoreactive T cells that escaped the process of negative selection in the thymus. Suppressor T cells along with Helper T cells can collectively be called Regulatory T cells due to their regulatory functions.[12]
Two major classes of CD4+ Treg cells have been described — FOXP3+ Treg cells and FOXP3− Treg cells.
Regulatory T cells can develop either during normal development in the thymus, and are then known as thymic Treg cells, or can be induced peripherally and are called peripherally derived Treg cells. These two subsets were previously called "naturally occurring", and "adaptive" or "induced", respectively.[13] Both subsets require the expression of the transcription factor FOXP3 which can be used to identify the cells. Mutations of the FOXP3 gene can prevent regulatory T cell development, causing the fatal autoimmune disease IPEX.
Several other types of T cell have suppressive activity, but do not express FOXP3. These include Tr1 cells and Th3 cells, which are thought to originate during an immune response and act by producing suppressive molecules. Tr1 cells are associated with IL-10, and Th3 cells are associated with TGF-beta. Recently, Treg17 cells have been added to this list.[14]
Natural killer T cell
Natural killer T cells (NKT cells – not to be confused with natural killer cells of the innate immune system) bridge the adaptive immune system with the innate immune system. Unlike conventional T cells that recognize peptide antigens presented by major histocompatibility complex (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called CD1d. Once activated, these cells can perform functions ascribed to both Th and Tc cells (i.e., cytokine production and release of cytolytic/cell killing molecules). They are also able to recognize and eliminate some tumor cells and cells infected with herpes viruses.[15]
Mucosal associated invariant
MAIT cells display innate, effector-like qualities.[16][17] In humans, MAIT cells are found in the blood, liver, lungs, and mucosa, defending against microbial activity and infection.[16] The MHC class I-like protein, MR1, is responsible for presenting bacterially-produced vitamin B metabolites to MAIT cells.[18][19][20] After the presentation of foreign antigen by MR1, MAIT cells secretes pro-inflammatory cytokines and are capable of lysing bacterially-infected cells.[16][20] MAIT cells can also be activated through MR1-independent signaling.[20] In addition to possessing innate-like functions, this T cell subset supports the adaptive immune response and has a memory-like phenotype.[16] Furthermore, MAIT cells are thought to play a role in autoimmune diseases, such as multiple sclerosis, arthritis and inflammatory bowel disease,[21][22] although definitive evidence is yet to be published.[23][24][25][26]
Gamma delta T cells
Gamma delta T cells (γδ T cells) represent a small subset of T cells that possess a distinct T cell receptor (TCR) on their surfaces. A majority of T cells have a TCR composed of two glycoprotein chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common in humans and mice (about 2% of total T cells); and are found mostly in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes. In rabbits, sheep, and chickens, the number of γδ T cells can be as high as 60% of total T cells. The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC-restricted and seem to be able to recognize whole proteins rather than requiring peptides to be presented by MHC molecules on APCs. Some murine γδ T cells recognize MHC class IB molecules, though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a set of nonpeptidic phosphorylated isoprenoid precursors, collectively named phosphoantigens, which are produced by virtually all living cells. The most common phosphoantigens from animal and human cells (including cancer cells) are isopentenyl pyrophosphate (IPP) and its isomer dimethylallyl pyrophosphate (DMPP). Many microbes produce the highly active compound hydroxy-DMAPP (HMB-PP) and corresponding mononucleotide conjugates, in addition to IPP and DMAPP. Plant cells produce both types of phosphoantigens. Drugs activating human Vγ9/Vδ2 T cells comprise synthetic phosphoantigens and aminobisphosphonates, which upregulate endogenous IPP/DMAPP.
Development
All T cells originate from haematopoietic stem cells in the bone marrow. Haematopoietic progenitors (lymphoid progenitor cells) from haematopoietic stem cells populate the thymus and expand by cell division to generate a large population of immature thymocytes.[27] The earliest thymocytes express neither CD4 nor CD8, and are therefore classed as double-negative (CD4−CD8−) cells. As they progress through their development, they become double-positive thymocytes (CD4+CD8+), and finally mature to single-positive (CD4+CD8− or CD4−CD8+) thymocytes that are then released from the thymus to peripheral tissues. There is some evidence of double-positive T-cells in the periphery, though their prevalence and function is uncertain.[28][29]
About 98% of thymocytes die during the development processes in the thymus by failing either positive selection or negative selection, whereas the other 2% survive and leave the thymus to become mature immunocompetent T cells. Increasing evidence indicates microRNAs, which are small noncoding regulatory RNAs, could impact the clonal selection process during thymic development. For example, miR-181a was found to play a role in the positive selection of T lymphocytes.[30]
The thymus contributes fewer cells as a person ages. As the thymus shrinks by about 3%[31] a year throughout middle age, a corresponding fall in the thymic production of naïve T cells occurs, leaving peripheral T cell expansion to play a greater role in protecting older subjects.
Beta selection
Common lymphoid precursor cells that migrate to the thymus become known as T-cell precursors (or thymocytes) and do not express a T cell receptor. Broadly speaking, the double negative (DN) stage is focused on producing a functional β-chain whereas the double positive (DP) stage is focused on producing a functional α-chain, ultimately producing a functional αβ T cell receptor. As the developing thymocyte progresses through the four DN stages (DN1, DN2, DN3, and DN4), the T cell expresses an invariant α-chain but rearranges the β-chain locus. If the rearranged β-chain successfully pairs with the invariant α-chain, signals are produced which cease rearrangement of the β-chain (and silence the alternate allele) and result in proliferation of the cell.[32] Although these signals require this pre-TCR at the cell surface, they are independent of ligand binding to the pre-TCR. These thymocytes will then express both CD4 and CD8 and progresses to the double positive (DP) stage where selection of the α-chain takes place. If a rearranged β-chain does not lead to any signalling (e.g. as a result of an inability to pair with the invariant α-chain), the cell may die by neglect (lack of signalling).
Positive selection
Positive selection "selects for" T cells capable of interacting with MHC. Positive selection involves the production of a signal by double-positive precursors that express either MHC Class I or II restricted receptors. The signal produced by these thymocytes result in RAG gene repression, long-term survival and migration into the medulla, as well as differentiation into mature T cells. The process of positive selection takes a number of days.[33]
Double-positive thymocytes (CD4+/CD8+) move deep into the thymic cortex, where they are presented with self-antigens. These self-antigens are expressed by thymic cortical epithelial cells on MHC molecules on the surface of cortical epithelial cells. Only those thymocytes that interact with MHC-I or MHC-II appropriately (i.e., not too strongly or too weakly) will receive a vital "survival signal". All that cannot (i.e., if they do not interact strongly enough, or if they bind too strongly) will die by "death by neglect" (no survival signal). This process ensures that the selected T-cells will have an MHC affinity that can serve useful functions in the body (i.e., the cells must be able to interact with MHC and peptide complexes to effect immune responses). The vast majority of all thymocytes will die during this process.
A thymocyte's fate is determined during positive selection. Double-positive cells (CD4+/CD8+) that interact well with MHC class II molecules will eventually become CD4+ cells, whereas thymocytes that interact well with MHC class I molecules mature into CD8+ cells. A T cell becomes a CD4+ cell by down-regulating expression of its CD8 cell surface receptors. If the cell does not lose its signal, it will continue downregulating CD8 and become a CD4+, single positive cell.[34] But, if there is a signal interruption, the cell stops downregulating CD8 and switches over to downregulating CD4 molecules, instead, eventually becoming a CD8+, single positive cell.
This process does not remove thymocytes that may cause autoimmunity. The potentially autoimmune cells are removed by the process of negative selection, which occurs in the thymic medulla (discussed below).
Negative selection
Negative selection removes thymocytes that are capable of strongly binding with "self" MHC peptides. Thymocytes that survive positive selection migrate towards the boundary of the cortex and medulla in the thymus. While in the medulla, they are again presented with a self-antigen presented on the MHC complex of medullary thymic epithelial cells (mTECs).[35] mTECs must be AIRE+ to properly express self-antigens from all tissues of the body on their MHC class I peptides. Some mTECs are phagocytosed by thymic dendritic cells; this allows for presentation of self-antigens on MHC class II molecules (positively selected CD4+ cells must interact with MHC class II molecules, thus APCs, which possess MHC class II, must be present for CD4+ T-cell negative selection). Thymocytes that interact too strongly with the self-antigen receive an apoptotic signal that leads to cell death. However, some of these cells are selected to become Treg cells. The remaining cells exit the thymus as immature naïve T cells (also known as recent thymic emigrants [36]). This process is an important component of central tolerance and serves to prevent the formation of self-reactive T cells that are capable of inducing autoimmune diseases in the host.
In summary, β-selection is the first checkpoint, where the T cells that are able to form a functional pre-TCR with an invariant alpha chain and a functional beta chain are allowed to continue development in the thymus. Next, positive selection checks that T cells have successfully rearranged their TCRα locus and are capable of recognizing peptide-MHC complexes with appropriate affinity. Negative selection in the medulla then obliterates T cells that bind too strongly to self-antigens expressed on MHC molecules. These selection processes allow for tolerance of self by the immune system. Typical T cells that leave the thymus (via the corticomedullarly junction) are self-restricted, self-tolerant, and singly positive.
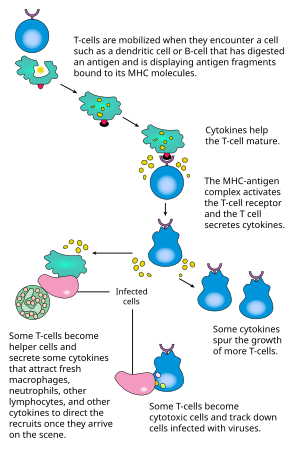
Activation
Activation of CD4+ T cells occurs through the simultaneous engagement of the T-cell receptor and a co-stimulatory molecule (like CD28, or ICOS) on the T cell by the major histocompatibility complex (MHCII) peptide and co-stimulatory molecules on the APC. Both are required for production of an effective immune response; in the absence of co-stimulation, T cell receptor signalling alone results in anergy. The signalling pathways downstream from co-stimulatory molecules usually engages the PI3K pathway generating PIP3 at the plasma membrane and recruiting PH domain containing signaling molecules like PDK1 that are essential for the activation of PKCθ, and eventual IL-2 production. Optimal CD8+ T cell response relies on CD4+ signalling.[38] CD4+ cells are useful in the initial antigenic activation of naïve CD8 T cells, and sustaining memory CD8+ T cells in the aftermath of an acute infection. Therefore, activation of CD4+ T cells can be beneficial to the action of CD8+ T cells.[39][40][41]
The first signal is provided by binding of the T cell receptor to its cognate peptide presented on MHCII on an APC. MHCII is restricted to so-called professional antigen-presenting cells, like dendritic cells, B cells, and macrophages, to name a few. The peptides presented to CD8+ T cells by MHC class I molecules are 8–13 amino acids in length; the peptides presented to CD4+ cells by MHC class II molecules are longer, usually 12–25 amino acids in length,[42] as the ends of the binding cleft of the MHC class II molecule are open.
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as necrotic-bodies or heat shock proteins. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the CD80 and CD86 proteins, which together constitute the B7 protein, (B7.1 and B7.2, respectively) on the APC. Other receptors are expressed upon activation of the T cell, such as OX40 and ICOS, but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes anergic, and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation. Once a T cell has been appropriately activated (i.e. has received signal one and signal two) it alters its cell surface expression of a variety of proteins. Markers of T cell activation include CD69, CD71 and CD25 (also a marker for Treg cells), and HLA-DR (a marker of human T cell activation). CTLA-4 expression is also up-regulated on activated T cells, which in turn outcompetes CD28 for binding to the B7 proteins. This is a checkpoint mechanism to prevent over activation of the T cell. Activated T cells also change their cell surface glycosylation profile.[43]
The T cell receptor exists as a complex of several proteins. The actual T cell receptor is composed of two separate peptide chains, which are produced from the independent T cell receptor alpha and beta (TCRα and TCRβ) genes. The other proteins in the complex are the CD3 proteins: CD3εγ and CD3εδ heterodimers and, most important, a CD3ζ homodimer, which has a total of six ITAM motifs. The ITAM motifs on the CD3ζ can be phosphorylated by Lck and in turn recruit ZAP-70. Lck and/or ZAP-70 can also phosphorylate the tyrosines on many other molecules, not least CD28, LAT and SLP-76, which allows the aggregation of signalling complexes around these proteins.
Phosphorylated LAT recruits SLP-76 to the membrane, where it can then bring in PLC-γ, VAV1, Itk and potentially PI3K. PLC-γ cleaves PI(4,5)P2 on the inner leaflet of the membrane to create the active intermediaries diacylglycerol (DAG), inositol-1,4,5-trisphosphate (IP3); PI3K also acts on PIP2, phosphorylating it to produce phosphatidlyinositol-3,4,5-trisphosphate (PIP3). DAG binds and activates some PKCs. Most important in T cells is PKCθ, critical for activating the transcription factors NF-κB and AP-1. IP3 is released from the membrane by PLC-γ and diffuses rapidly to activate calcium channel receptors on the ER, which induces the release of calcium into the cytosol. Low calcium in the endoplasmic reticulum causes STIM1 clustering on the ER membrane and leads to activation of cell membrane CRAC channels that allows additional calcium to flow into the cytosol from the extracellular space. This aggregated cytosolic calcium binds calmodulin, which can then activate calcineurin. Calcineurin, in turn, activates NFAT, which then translocates to the nucleus. NFAT is a transcription factor that activates the transcription of a pleiotropic set of genes, most notable, IL-2, a cytokine that promotes long-term proliferation of activated T cells.
PLCγ can also initiate the NF-κB pathway. DAG activates PKCθ, which then phosphorylates CARMA1, causing it to unfold and function as a scaffold. The cytosolic domains bind an adapter BCL10 via CARD (Caspase activation and recruitment domains) domains; that then binds TRAF6, which is ubiquitinated at K63.:513–523[44] This form of ubiquitination does not lead to degradation of target proteins. Rather, it serves to recruit NEMO, IKKα and -β, and TAB1-2/ TAK1.[45] TAK 1 phosphorylates IKK-β, which then phosphorylates IκB allowing for K48 ubiquitination: leads to proteasomal degradation. Rel A and p50 can then enter the nucleus and bind the NF-κB response element. This coupled with NFAT signaling allows for complete activation of the IL-2 gene.[44]
While in most cases activation is dependent on TCR recognition of antigen, alternative pathways for activation have been described. For example, cytotoxic T cells have been shown to become activated when targeted by other CD8 T cells leading to tolerization of the latter.[46]
In spring 2014, the T-Cell Activation in Space (TCAS) experiment was launched to the International Space Station on the SpaceX CRS-3 mission to study how "deficiencies in the human immune system are affected by a microgravity environment".[47]
T cell activation is modulated by reactive oxygen species.[48]
Antigen discrimination
A unique feature of T cells is their ability to discriminate between healthy and abnormal (e.g. infected or cancerous) cells in the body.[49] Healthy cells typically express a large number of self derived pMHC on their cell surface and although the T cell antigen receptor can interact with at least a subset of these self pMHC, the T cell generally ignores these healthy cells. However, when these very same cells contain even minute quantities of pathogen derived pMHC, T cells are able to become activated and initiate immune responses. The ability of T cells to ignore healthy cells but respond when these same cells contain pathogen (or cancer) derived pMHC is known as antigen discrimination. The molecular mechanisms that underlie this process are controversial.[49][50]
Clinical significance
Deficiency
Causes of T cell deficiency include lymphocytopenia of T cells and/or defects on function of individual T cells. Complete insufficiency of T cell function can result from hereditary conditions such as severe combined immunodeficiency (SCID), Omenn syndrome, and cartilage–hair hypoplasia.[51] Causes of partial insufficiencies of T cell function include acquired immune deficiency syndrome (AIDS), and hereditary conditions such as DiGeorge syndrome (DGS), chromosomal breakage syndromes (CBSs), and B-cell and T-cell combined disorders such as ataxia-telangiectasia (AT) and Wiskott–Aldrich syndrome (WAS).[51]
The main pathogens of concern in T cell deficiencies are intracellular pathogens, including Herpes simplex virus, Mycobacterium and Listeria.[52] Also, fungal infections are also more common and severe in T cell deficiencies.[52]
Cancer
Cancer of T cells is termed T-cell lymphoma, and accounts for perhaps one in ten cases of non-Hodgkin lymphoma.[53] The main forms of T cell lymphoma are:
- Extranodal T cell lymphoma
- Cutaneous T cell lymphomas: Sézary syndrome and Mycosis fungoides
- Anaplastic large cell lymphoma
- Angioimmunoblastic T cell lymphoma
Exhaustion
T cell exhaustion is the progressive loss of T cell function. It can occur during sepsis and after other acute or chronic infections.[54][55]
T cell exhaustion is mediated by several inhibitory receptors including programmed cell death protein 1 (PD1), TIM3, and lymphocyte activation gene 3 protein (LAG3).[56]
CD8+ T cell exhaustion occurs in some tumours, and can be partly reversed by blocking the inhibitory receptors (e.g. PD1).[57]
T cell exhaustion is associated with epigenetic changes in the T cells.[58]
( See also Immunosenescence#T cell functional dysregulation as a biomarker for immunosenescence ).
Research
Genetic engineering
In 2015, a team of researchers led by Dr. Alexander Marson[59] at the University of California, San Francisco successfully edited the genome of human T cells using a Cas9 ribonucleoprotein delivery method.[60] This advancement has potential for applications in treating "cancer immunotherapies and cell-based therapies for HIV, primary immune deficiencies, and autoimmune diseases".[60]
参见
参考文献
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts k, Walter P (2002) Molecular Biology of the Cell. Garland Science: New York, NY pg 1367. "T cells and B cells derive their names from the organs in which they develop. T cells develop in the thymus, and B cells, in mammals, develop in the bone marrow in adults or the liver in fetuses."
- ^ McClory, Susan; Hughes, Tiffany; Freud, Aharon G.; Briercheck, Edward L.; Martin, Chelsea; Trimboli, Anthony J.; Yu, Jianhua; Zhang, Xiaoli; Leone, Gustavo. Evidence for a stepwise program of extrathymic T cell development within the human tonsil. The Journal of Clinical Investigation. Apr 2012, 122 (4): 1403–1415. ISSN 1558-8238. PMC 3314444
 . PMID 22378041. doi:10.1172/JCI46125.
. PMID 22378041. doi:10.1172/JCI46125.
- ^ Vantourout, Pierre; Hayday, Adrian. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Reviews. Immunology. Feb 2013, 13 (2): 88–100. ISSN 1474-1741. PMC 3951794
 . PMID 23348415. doi:10.1038/nri3384.
. PMID 23348415. doi:10.1038/nri3384.
- ^ Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Invest. 2007, 117 (5): 1119–27. PMC 1857272
 . PMID 17476341. doi:10.1172/JCI31720.
. PMID 17476341. doi:10.1172/JCI31720.
- ^ Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions.. Nature. 1999, 401: 708–712. Bibcode:1999Natur.401..708S. PMID 10537110. doi:10.1038/44385.
- ^ Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. April 1988, 140 (7): 2171–8. PMID 2965180.
- ^ Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets.. Journal of Immunology. 2005, 175 (9): 5895–903. PMID 16237082. doi:10.4049/jimmunol.175.9.5895.
- ^ Koch S, Larbi A, Derhovanessian E, Özcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people.. Immunity & Ageing. 2008, 5 (6). PMC 2515281
 . PMID 18657274. doi:10.1186/1742-4933-5-6.
. PMID 18657274. doi:10.1186/1742-4933-5-6.
- ^ Shin, Haina; Iwasaki, Akiko. Tissue-resident memory T cells. Immunological Reviews. 2013-09-01, 255 (1): 165–181. ISSN 1600-065X. PMC 3748618
 . PMID 23947354. doi:10.1111/imr.12087.
. PMID 23947354. doi:10.1111/imr.12087.
- ^ Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage.. Trends in Immunology. 2011, 32 (2): 50–56. PMC 3039080
 . PMID 21288770. doi:10.1016/j.it.2010.12.004.
. PMID 21288770. doi:10.1016/j.it.2010.12.004.
- ^ Marusina AI, Ono Y, Merleev AA, Shimoda M, Ogawa H, Wang EA, Kondo K, Olney L, Luxardi G, Miyamura Y, Yilma TD, Villalobos IB, Bergstrom JW, Kronenberg DG, Soulika AM, Adamopoulos IE, Maverakis E. CD4+ virtual memory: Antigen-inexperienced T cells reside in the naïve, regulatory, and memory T cell compartments at similar frequencies, implications for autoimmunity. (PDF). Journal of Autoimmunity. 2017, 77: 76–88. PMID 27894837. doi:10.1016/j.jaut.2016.11.001.
- ^ Textbook of Medical Physiology by Guyton and Hall, edition 6, pg. 448, Suppressor T cells paragraph.
- ^ Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nat. Immunol. 2013, 14 (4): 307–8. PMID 23507634. doi:10.1038/ni.2554.
- ^ Singh B, Schwartz JA, Sandrock C, Bellemore SM, Nikoopour E. Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells. Indian J. Med. Res. 2013, 138 (5): 591–4. PMC 3928692
 . PMID 24434314.
. PMID 24434314.
- ^ Mallevaey, Thierry; Fontaine, Josette; Breuilh, Laetitia; Paget, Christophe; Castro-Keller, Alexandre; Vendeville, Catherine; Capron, Monique; Leite-de-Moraes, Maria; Trottein, François. Invariant and Noninvariant Natural Killer T Cells Exert Opposite Regulatory Functions on the Immune Response during Murine Schistosomiasis. Infection and Immunity. 2007-05-01, 75 (5): 2171–2180. ISSN 0019-9567. PMC 1865739
 . PMID 17353286. doi:10.1128/IAI.01178-06 (英语).
. PMID 17353286. doi:10.1128/IAI.01178-06 (英语).
- ^ 16.0 16.1 16.2 16.3 Napier, Ruth J.; Adams, Erin J.; Gold, Marielle C.; Lewinsohn, David M. The Role of Mucosal Associated Invariant T Cells in Antimicrobial Immunity. Frontiers in Immunology. 2015-07-06, 6. ISSN 1664-3224. PMC 4492155
 . PMID 26217338. doi:10.3389/fimmu.2015.00344.
. PMID 26217338. doi:10.3389/fimmu.2015.00344.
- ^ Gold, Marielle C.; Lewinsohn, David M. Mucosal associated invariant T cells and the immune response to infection. Microbes and infection / Institut Pasteur. 2017-02-12, 13 (8–9): 742–748. ISSN 1286-4579. PMC 3130845
 . PMID 21458588. doi:10.1016/j.micinf.2011.03.007.
. PMID 21458588. doi:10.1016/j.micinf.2011.03.007.
- ^ Eckle, Sidonia B. G.; Corbett, Alexandra J.; Keller, Andrew N.; Chen, Zhenjun; Godfrey, Dale I.; Liu, Ligong; Mak, Jeffrey Y. W.; Fairlie, David P.; Rossjohn, Jamie. Recognition of Vitamin B Precursors and Byproducts by Mucosal Associated Invariant T Cells. The Journal of Biological Chemistry. 2015-12-18, 290 (51): 30204–30211. ISSN 0021-9258. PMC 4683245
 . PMID 26468291. doi:10.1074/jbc.R115.685990.
. PMID 26468291. doi:10.1074/jbc.R115.685990.
- ^ Ussher, James E.; Klenerman, Paul; Willberg, Chris B. Mucosal-Associated Invariant T-Cells: New Players in Anti-Bacterial Immunity. Frontiers in Immunology. 2014-10-08, 5. ISSN 1664-3224. PMC 4189401
 . PMID 25339949. doi:10.3389/fimmu.2014.00450.
. PMID 25339949. doi:10.3389/fimmu.2014.00450.
- ^ 20.0 20.1 20.2 Howson, Lauren J.; Salio, Mariolina; Cerundolo, Vincenzo. MR1-Restricted Mucosal-Associated Invariant T Cells and Their Activation during Infectious Diseases. Frontiers in Immunology. 2015-06-16, 6. ISSN 1664-3224. PMC 4468870
 . PMID 26136743. doi:10.3389/fimmu.2015.00303.
. PMID 26136743. doi:10.3389/fimmu.2015.00303.
- ^ Hinks, Timothy S. C. Mucosal‐associated invariant T cells in autoimmunity, immune‐mediated diseases and airways, disease. Immunology. 2016, 148 (1): 1–12. ISSN 0019-2805. PMC 4819138
 . PMID 26778581. doi:10.1111/imm.12582.
. PMID 26778581. doi:10.1111/imm.12582.
- ^ Bianchini, Elena; De Biasi, Sara; Simone, Anna Maria; Ferraro, Diana; Sola, Patrizia; Cossarizza, Andrea; Pinti, Marcello. Invariant natural killer T cells and mucosal-associated invariant T cells in multiple sclerosis. Immunology Letters. 2017-03-01, 183: 1–7. doi:10.1016/j.imlet.2017.01.009.
- ^ Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, Dupas JL, Treiner E. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 2014, 176: 266–74. PMC 3992039
 . PMID 24450998. doi:10.1111/cei.12277.
. PMID 24450998. doi:10.1111/cei.12277.
- ^ Huang, Shouxiong; Martin, Emmanuel; Kim, Sojung; Yu, Lawrence; Soudais, Claire; Fremont, Daved H.; Lantz, Olivier; Hansen, Ted H. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proceedings of the National Academy of Sciences of the United States of America. 2009-05-19, 106 (20): 8290–8295. Bibcode:2009PNAS..106.8290H. ISSN 1091-6490. PMC 2688861
 . PMID 19416870. doi:10.1073/pnas.0903196106.
. PMID 19416870. doi:10.1073/pnas.0903196106.
- ^ Chua, Wei-Jen; Hansen, Ted H. Bacteria, mucosal-associated invariant T cells and MR1. Immunology and Cell Biology. November 2010, 88 (8): 767–769. ISSN 1440-1711. PMID 20733595. doi:10.1038/icb.2010.104.
- ^ Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, Purcell AW, Dudek NL, McConville MJ, O'Hair RA, Khairallah GN, Godfrey DI, Fairlie DP, Rossjohn J, McCluskey J. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012, 491: 717–23. Bibcode:2012Natur.491..717K. PMID 23051753. doi:10.1038/nature11605.
- ^ Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol. Rev. February 2006, 209: 47–57. PMID 16448533. doi:10.1111/j.0105-2896.2006.00350.x.
- ^ Overgaard, Nana H.; Jung, Ji-Won; Steptoe, Raymond J.; Wells, James W. CD4+/CD8+ double-positive T cells: more than just a developmental stage?. Journal of Leukocyte Biology. 1 January 2015, 97 (1): 31–38. ISSN 1938-3673. PMID 25360000. doi:10.1189/jlb.1RU0814-382.
- ^ Cruz, Jazmina L.G.; Overgaard, Nana H.; Bridge, Jenniger A.; Nel, Hendrik J.; Frazer, Ian Ian H.; La Gruta, Nicole; Blumentral, Antje; Steptoe, Raymond J.; Wells, James W. CD4+CD8β+ double‐positive T cells in skin‐draining lymph nodes respond to inflammatory signals from the skin. Journal of Leukocyte Biology. 21 June 2017, 102 (3): 837–844. ISSN 1938-3673. doi:10.1189/jlb.1AB0217-065R.
- ^ Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007, 129 (1): 147–61. PMID 17382377. doi:10.1016/j.cell.2007.03.008.
- ^ Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 2000, 18: 529–60. PMID 10837068. doi:10.1146/annurev.immunol.18.1.529.
- ^ Janeway, Charles. Immunobiology. Garland Science. 2012: 301–305. ISBN 9780815342434.
- ^ Timothy K. Starr; Stephen C. Jameson; Hogquist, and Kristin A. Positive and Negative Selection of T Cells. Annual Review of Immunology. 2003-01-01, 21 (1): 139–176. PMID 12414722. doi:10.1146/annurev.immunol.21.120601.141107.
- ^ Zerrahn, Jens; Held, Werner; Raulet, David H. The MHC Reactivity of the T Cell Repertoire Prior to Positive and Negative Selection. Cell. 1997-03-07, 88 (5): 627–636. ISSN 0092-8674. PMID 9054502. doi:10.1016/S0092-8674(00)81905-4 (English).
- ^ Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance (PDF). Nat. Immunol. June 2010, 11 (6): 512–9. PMID 20431619. doi:10.1038/ni.1874.
- ^ Pekalski, Marcin L.; Rubio García, Arcadio; Ferreira, Ricardo C.; Rainbow, Daniel B.; Smyth, Deborah J.; Mashar, Meghavi; Brady, Jane; Savinykh, Natalia; Dopico, Xaquin Castro. Neonatal and adult recent thymic emigrants produce IL-8 and express complement receptors CR1 and CR2. JCI Insight. 2017-08-17, 2 (16). ISSN 0021-9738. doi:10.1172/jci.insight.93739 (英语).
- ^ The NIAID resource booklet "Understanding the Immune System (pdf)".
- ^ Williams, Matthew A.; Bevan, Michael J. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007-01-01, 25 (1): 171–192. PMID 17129182. doi:10.1146/annurev.immunol.25.022106.141548.
- ^ Janssen, Edith M.; Lemmens, Edward E.; Wolfe, Tom; Christen, Urs; von Herrath, Matthias G.; Schoenberger, Stephen P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003-02-20, 421 (6925): 852–856. Bibcode:2003Natur.421..852J. ISSN 0028-0836. doi:10.1038/nature01441 (英语).
- ^ Shedlock, Devon J.; Shen, Hao. Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory. Science. 2003-04-11, 300 (5617): 337–339. Bibcode:2003Sci...300..337S. ISSN 0036-8075. PMID 12690201. doi:10.1126/science.1082305 (英语).
- ^ Sun, Joseph C.; Williams, Matthew A.; Bevan, Michael J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nature Immunology. 2004-09-01, 5 (9): 927–933. ISSN 1529-2908. PMC 2776074
 . PMID 15300249. doi:10.1038/ni1105 (英语).
. PMID 15300249. doi:10.1038/ni1105 (英语).
- ^ Jennifer Rolland and Robyn O'Hehir, "Turning off the T cells: Peptides for treatment of allergic Diseases," Today's life science publishing, 1999, Page 32
- ^ Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity. J Autoimmun. 2015, 57 (6): 1–13. PMC 4340844
 . PMID 25578468. doi:10.1016/j.jaut.2014.12.002.
. PMID 25578468. doi:10.1016/j.jaut.2014.12.002.
- ^ 44.0 44.1 Tatham P, Gomperts BD, Kramer IM. Signal transduction. Amsterdam: Elsevier Academic Press. 2003. ISBN 0-12-289632-7.
- ^ Wu H, Arron JR. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. BioEssays. November 2003, 25 (11): 1096–105. PMID 14579250. doi:10.1002/bies.10352.
- ^ Milstein O, Hagin D, Lask A, Reich-Zeliger S, Shezen E, Ophir E, Eidelstein Y, Afik R, Antebi YE, Dustin ML, Reisner Y. CTLs respond with activation and granule secretion when serving as targets for T cell recognition. Blood. January 2011, 117 (3): 1042–52. PMC 3035066
 . PMID 21045195. doi:10.1182/blood-2010-05-283770.
. PMID 21045195. doi:10.1182/blood-2010-05-283770.
- ^ Graham, William. SpaceX ready for CRS-3 Dragon launch and new milestones. NASAspaceflight.com. 2014-04-14 [2014-04-14].
- ^ Belikov, Aleksey V.; Schraven, Burkhart; Simeoni, Luca. T cells and reactive oxygen species. Journal of Biomedical Science. 1 January 2015, 22: 85 [29 April 2017]. ISSN 1423-0127. PMC 4608155
 . PMID 26471060. doi:10.1186/s12929-015-0194-3.
. PMID 26471060. doi:10.1186/s12929-015-0194-3.
- ^ 49.0 49.1 Feinerman O, Germain RN, Altan-Bonnet G. Quantitative challenges in understanding ligand discrimination by alphabeta T cells. Mol. Immunol. 2008, 45 (3): 619–31. PMC 2131735
 . PMID 17825415. doi:10.1016/j.molimm.2007.03.028.
. PMID 17825415. doi:10.1016/j.molimm.2007.03.028.
- ^ Dushek O, van der Merwe PA. An induced rebinding model of antigen discrimination. Trends Immunol. 2014, 35 (4): 153–8. PMC 3989030
 . PMID 24636916. doi:10.1016/j.it.2014.02.002.
. PMID 24636916. doi:10.1016/j.it.2014.02.002.
- ^ 51.0 51.1 Medscape > T-cell Disorders. Author: Robert A Schwartz, MD, MPH; Chief Editor: Harumi Jyonouchi, MD. Updated: May 16, 2011
- ^ 52.0 52.1 Jones J, Bannister BA, Gillespie SH (编). Infection: Microbiology and Management. Wiley-Blackwell. 2006: 435. ISBN 1-4051-2665-5.
- ^ The Lymphomas (PDF). The Leukemia & Lymphoma Society: 2. May 2006 [2008-04-07].
- ^ Yi JS, Cox MA, Zajac AJ. T cell exhaustion: characteristics, causes and conversion. Immunology. Apr 2010, 129 (4): 474–481. PMC 2842494
 . PMID 20201977. doi:10.1111/j.1365-2567.2010.03255.x.
. PMID 20201977. doi:10.1111/j.1365-2567.2010.03255.x.
- ^ Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. Mar 2013, 13 (3): 260–8. PMC 3798159
 . doi:10.1016/S1473-3099(13)70001-X.
. doi:10.1016/S1473-3099(13)70001-X.
- ^ Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015, 14 (8): 561–84. PMID 26228759. doi:10.1038/nrd4591.
- ^ Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36 (4): 265–76. PMC 4393798
 . PMID 25797516. doi:10.1016/j.it.2015.02.008.
. PMID 25797516. doi:10.1016/j.it.2015.02.008.
- ^ Epigenetic Reprogramming May Boost Immunotherapy. Genetic Engineering & Biotechnology News. 2017-06-27 [2017-08-08].
- ^ Breakthrough announced in 'editing' DNA to fight off deadly illness. The Independent. 2015-07-27 [2017-08-08] (英国英语).
- ^ 60.0 60.1 Schumann, Kathrin; Lin, Steven; Boyer, Eric; Simeonov, Dimitre R.; Subramaniam, Meena; Gate, Rachel E.; Haliburton, Genevieve E.; Ye, Chun J.; Bluestone, Jeffrey A. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proceedings of the National Academy of Sciences of the United States of America. 2015-08-18, 112 (33): 10437–10442. Bibcode:2015PNAS..11210437S. ISSN 1091-6490. PMC 4547290
 . PMID 26216948. doi:10.1073/pnas.1512503112.
. PMID 26216948. doi:10.1073/pnas.1512503112.
外部連結
- Immunobiology, 5th Edition
- niaid.nih.gov – The Immune System
- T-cell Group – Cardiff University
- (Successful!) Treatment of Metastatic Melanoma with Autologous CD4+ T Cells against NY-ESO-1.
- The Center for Modeling Immunity to Enteric Pathogens (MIEP)
- Anthony J. Davies. The tale of T cells. Immunology Today: 137–140. [2018-04-02]. doi:10.1016/0167-5699(93)90216-8.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
