用戶:Dr3939/沙盒
使用者:Dr3939/VideoWiki/痛風 草稿:VideoWiki:Gout
| A | B | C |
|---|---|---|
| 1 | 2 | 3 |
歷史
[編輯]User:Dr3939/翻譯史 [3] 摘要 - 色素沉着絨毛結節性滑膜炎 費爾蒂症候群 單株抗體治療 自體免疫
User:Dr3939/VideoWiki/痛風 User:Dr3939/沙盒/Test
- 口周皮膚炎, 顳顎關節功能障礙 - 2024/11/14, 摘要
- 梨狀肌症候群, 前十字韌帶受傷 - 2024/11/13, 摘要
- 髖關節發育不良, 醋甲唑胺, 胃輕癱, 乙型阻斷劑中毒 - 2024/11/12, 摘要
- 缺血性壞死, 硬化性苔癬, 托吡酯, 氟氫可體松, 肛門疼痛, 尿素乳膏 - 2024/11/10, 摘要
- 姿勢性心博過速症候群, 外陰搔癢 - 2024/11/09, 摘要
- 氯沙坦, 淋巴水腫, 感覺異常, 子宮破裂 - 2024/11/08, 摘要
- 巴瑞替尼, 托法替尼, 依奇珠單抗 - 2024/11/06, 摘要
- 古塞奇尤單抗, 利生奇珠單抗, 羅莫珠單抗, 阿伏利尤單抗 - 2024/11/05, 摘要
- 司庫奇尤單抗, 伊班膦酸, 來氟米特 - 2024/11/04, 摘要
- 烏帕替尼, 阿巴西普, 類A酸, 冷凍沉澱品, 舒林酸 - 2024/11/03, 摘要
- Template:腫瘤伴隨症候群 - 2024/11/02, 範本, 全
- 抗NMDA受體腦炎, 絨毛羊膜炎, 妊娠多形疹 - 2024/11/02, 摘要
- 腎上腺功能不全, 複雜性局部疼痛症候群, 暫時性滑膜炎 - 2024/11/01, 摘要
- 藥物疹合併嗜伊紅血症及全身症狀, 脂肪乳, 鷹嘴突滑囊炎, 血小板減少症, 髕骨肌腱炎, 結節性紅斑, 壞疽性膿皮症, 心包積液 - 2024/10/31, 摘要
- 髕前滑囊炎, 靜脈炎, 氫羥腎上腺皮質素, 硫酸鋅 (藥品), 那曲酮, 磺達肝癸鈉 - 2024/10/30, 摘要
- 風濕性多肌痛症 - 2024/10/29, 摘要
- 肋軟骨炎 - 2024/10/28, 摘要
- 掌腱膜攣縮症 - 2024/10/27, 摘要
- 藥物不良反應 - 2024/01/14, 全
- NUDT15 - 2023/12/24, 全
- 前景問題格式, 證據等級, Category:實證應用, Template:實證應用, Template:實證醫學應用 - 2022/4/24-5/08, WikiMed X TEBMA, 全, 編修+審查
- 二水合焦磷酸鈣晶體沉積病 - 2021/12/06-12/08, 全
- Template:骨軟骨病 - 2021/11/28, 範本, 全-更新
- 羥氯喹 - 2020/04/12, 醫週譯小時, 摘要
- 磺胺嘧啶, 結節病, Template:Sarcoidosis - 2020/04/04, 醫週譯小時, 全-摘要-範本
- 自體免疫, 軟組織疾病, 黃房子 (梵高) - 2020/01/27, 長條目, 摘要/全/擴充
- 風濕, 單株抗體治療, 單克隆抗體治療 - 2020/01/26, 長條目, 全/摘要.
- Template:兒童關節炎 - 2020/01/23, 範本, 全;鋅缺乏 - 2020/01/23, 審查
- 幼年特發性關節炎 - 2020/01/15, 長條目, 全
- Template:滑液檢查 - 2020/01/12, 範本, 全
- 敗血性關節炎 - 2020/01/08, 長條目, 全
- Template:骨軟骨病 - 2020/1/6, 範本, 全
- v 反覆性風濕症 - 2020/1/3, 小作品級, 全
- 骨贅, 骨質增生 - 2020/1/1, 小作品級, 全
- 反應性關節炎, 萊特氏症候群 - 2019/12/22, 長條目, 全
- v 成人史迪爾氏病, 成人史迪爾氏症候群 - 2019/12/21, 長條目, 全
- 關節痛 - 2019/12/19, 長條目, 全
- 骨頭侵蝕, 骨侵蝕 - 2019/12/18, 長條目, 全
- 寡關節炎 - 2019/12/10, 長條目, 全
- 愛迪生氏病, 惡性貧血 - 2019/12/04, 醫週譯小時
- 著骨點炎, 接骨點炎, 附着部炎, 著骨點病變, 接骨點病變, 附着部病變 - 2019/11/18, 長條目, 全
- 脊椎炎, 脊椎病變, 薦腸關節炎, 椎間盤炎, 脊椎椎間盤炎, 博特氏病 - 2019/11/17, 長條目, 全
- 關節僵硬, 關節積血, 絨毛結節性滑膜炎, 色素沉着絨毛結節性滑膜炎 (摘要), Category:風濕病學, v 風濕病學 - 2019/11/16, 長條目, 全
- v 希伯登氏結節, 布夏氏結節 - 2019/11/15, 小作品級, 全
- 單關節炎, 多關節炎, 費爾蒂症候群, 結晶性關節病變, 軟骨鈣質沈積病 - 2019/11/13, 小作品級, 全
- SpA, 脊椎關節炎, 血清陰性脊椎關節病變, SPA (消歧義), v 乾癬性關節炎 - 2019/11/12, 轉址 - 擴充 - 長條目, 全
- 關節病變, 脊椎關節病變 - 2019/11/11, 小作品級, 全
- Template:過敏及自體免疫疾病, Template:全身性結締組織疾病, Template:全身性血管炎, Template:過敏狀況 - 2019/11/10, 範本, 全
- Template:自體抗原 - 2019/11/09, 範本, 全
- Template:關節病變和相關疾病, Template:脊椎疾病 - 2019/11/08, 範本, 全
- v 反覆流產 - 2019/10/29-11/03 , 長條目, 全
- v 流產 - 2019/10/19-10/29, 長條目, 全, 補充 及 更新;
- 失智症 - 2019/10/13, 長條目, 部分, 醫週譯小時;
- v 類風濕因子, Template:自體抗體 - 2019/10/08-10/10 長條目/範本, 全
- v 抗甲狀腺自體抗體 - 2019/10/07-10/09, 長條目, 部分 及 整合
- 結節性甲狀腺腫 - 2019/10/08, 消歧義, 全
- Category:腳部疾病, Category:過度使用傷害, Category:軟組織疾病, Template:軟組織疾病 2019/09/27, 類別/範本, 全 及 補充
- v 足底筋膜炎 - 2019/09/10~9/20, 長條目, 部分, 醫週譯小時; 9/21-22 整合
- v 女性不孕症 - 2019/09/09~10/04, 長條目, 部分
- v 不孕 - 2019/09/04~9/07, 長條目, 部分
- 生酮飲食 Draft:生酮飲食 - 2019/08/20~9/03, 長條目, 部分
- 拉米夫定 - 2019/08/19, 醫週譯小時
- 肝豆狀核變性, 三環類抗抑鬱藥藥物過量 - 2019/08/16, 醫週譯小時
- v 實證醫學 - 2019/08/11~8/20, 長條目, 全
開始:實證醫學
- Evidence based medicine > 內容翻譯 > User:Dr3939/實證醫學 + Draft:實證醫學 > 實證醫學
- 8/11 8:00 上午動念要翻譯,在 facebook 上找人介紹熟 wikipedia/wikimed 的朋友,三個小時後就加入兩位好友,並加入維基醫學翻譯社群WikiMed
- 接着就克文件準備動手,8/11 23:00 左右開始使用 內容翻譯功能 翻譯到 User:Dr3939/實證醫學 8/20 23:00 左右,翻完初稿。
- 8/20 再加兩位好友,再將 User:Dr3939/實證醫學 與用 連結翻譯器 產生的 Draft:實證醫學 校稿合併
- 8/24 23:00 完工
- 首次建立帳號 2005年3月22日 (二) 14:01
重疊症候群 抗環瓜氨酸肽抗體 外生骨贅 en:Exostosis en:Template:Osteochondropathy 模板:骨軟骨病 著骨點增生 en:Inflammatory arthritis
全身發作型幼年特發性關節炎
[編輯]en:Systemic-onset juvenile idiopathic arthritis 全身發作型幼年特發性關節炎 兒童關節炎
自體免疫
[編輯]免疫耐受性
[編輯]- 株落忽視理論指出在胸腺中未顯現自身反應性的 T 細胞將成熟並遷移到週邊組織,在週邊組織內,T 細胞無法接近的組織,所以不會遇到合適的抗原。因此,逃脫株落刪除的自身反應性 B 細胞無法找到抗原或特定的輔助性T細胞來活化它們。[1]
- 抑制群體或調節T細胞理論:調節性 T 淋巴細胞(通常為 CD4+FoxP3+ 細胞)的工作在於預防、下調或限制免疫系統中的自身反應性被激活。
Tolerance can also be differentiated into "central" and "peripheral" tolerance, on whether or not the above-stated checking mechanisms operate in the central lymphoid organs (thymus and bone marrow) or the peripheral lymphoid organs (lymph node, spleen, etc., where self-reactive B-cells may be destroyed). It must be emphasised that these theories are not mutually exclusive, and evidence has been mounting suggesting that all of these mechanisms may actively contribute to vertebrate immunological tolerance.
關於上述檢查機制是在中央淋巴器官(胸腺和骨髓)還是在外周淋巴器官(淋巴結,脾臟等)中起作用,耐受性還可以分為「中心」耐受性和「外周」耐受性。 ,其中自反應性B細胞可能會被破壞)。 必須強調的是,這些理論並不是相互排斥的,並且越來越多的證據表明所有這些機制都可能積極地促進脊椎動物的免疫耐受。
A puzzling feature of the documented loss of tolerance seen in spontaneous human autoimmunity is that it is almost entirely restricted to the autoantibody responses produced by B lymphocytes. Loss of tolerance by T cells has been extremely hard to demonstrate, and where there is evidence for an abnormal T cell response it is usually not to the antigen recognised by autoantibodies. Thus, in rheumatoid arthritis there are autoantibodies to IgG Fc but apparently no corresponding T cell response. In systemic lupus there are autoantibodies to DNA, which cannot evoke a T cell response, and limited evidence for T cell responses implicates nucleoprotein antigens. In Celiac disease there are autoantibodies to tissue transglutaminase but the T cell response is to the foreign protein gliadin. This disparity has led to the idea that human autoimmune disease is in most cases (with probable exceptions including type I diabetes) based on a loss of B cell tolerance which makes use of normal T cell responses to foreign antigens in a variety of aberrant ways.[2]
自發性人類自體免疫中所見的耐受性喪失的一個令人困惑的特徵是,它幾乎完全限於B淋巴細胞產生的自身抗體反應。很難證明T細胞喪失了耐受性,並且在有證據表明存在異常的T細胞反應的情況下,通常不是針對自身抗體識別的抗原。因此,在類風濕關節炎中,存在針對IgG Fc的自身抗體,但顯然沒有相應的T細胞應答。在系統性狼瘡中,有針對DNA的自身抗體,無法引起T細胞反應,而有關T細胞反應的證據有限,這牽涉到核蛋白抗原。在乳糜瀉中,存在針對組織轉穀氨酰胺酶的自身抗體,但T細胞對外源蛋白麥醇溶蛋白的反應。這種差異導致了這樣一種觀念,即人類自體免疫性疾病在大多數情況下(可能包括I型糖尿病)(基於B細胞耐受力喪失)是基於B細胞耐受性喪失的原因,而B細胞以各種異常方式利用了正常T細胞對外源抗原的反應。
Immunodeficiency and autoimmunity
[編輯]There are a large number of immunodeficiency syndromes that present clinical and laboratory characteristics of autoimmunity. The decreased ability of the immune system to clear infections in these patients may be responsible for causing autoimmunity through perpetual immune system activation.[3]
One example is common variable immunodeficiency (CVID) where multiple autoimmune diseases are seen, e.g.: inflammatory bowel disease, autoimmune thrombocytopenia and autoimmune thyroid disease.
Familial hemophagocytic lymphohistiocytosis, an autosomal recessive primary immunodeficiency, is another example. Pancytopenia, rashes, swollen lymph nodes and enlargement of the liver and spleen are commonly seen in such individuals. Presence of multiple uncleared viral infections due to lack of perforin are thought to be responsible.
In addition to chronic and/or recurrent infections many autoimmune diseases including arthritis, autoimmune hemolytic anemia, scleroderma and type 1 diabetes mellitus are also seen in X-linked agammaglobulinemia (XLA). Recurrent bacterial and fungal infections and chronic inflammation of the gut and lungs are seen in chronic granulomatous disease (CGD) as well. CGD is a caused by decreased production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase by neutrophils. Hypomorphic RAG mutations are seen in patients with midline granulomatous disease; an autoimmune disorder that is commonly seen in patients with granulomatosis with polyangiitis and NK/T cell lymphomas.
Wiskott–Aldrich syndrome (WAS) patients also present with eczema, autoimmune manifestations, recurrent bacterial infections and lymphoma.
In autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) also autoimmunity and infections coexist: organ-specific autoimmune manifestations (e.g. hypoparathyroidism and adrenocortical failure) and chronic mucocutaneous candidiasis.
Finally, IgA deficiency is also sometimes associated with the development of autoimmune and atopic phenomena.
Genetic factors
[編輯]Certain individuals are genetically susceptible to developing autoimmune diseases. This susceptibility is associated with multiple genes plus other risk factors. Genetically predisposed individuals do not always develop autoimmune diseases.
Three main sets of genes are suspected in many autoimmune diseases. These genes are related to:
- Immunoglobulins
- T-cell receptors
- The major histocompatibility complexes (MHC).
The first two, which are involved in the recognition of antigens, are inherently variable and susceptible to recombination. These variations enable the immune system to respond to a very wide variety of invaders, but may also give rise to lymphocytes capable of self-reactivity.
- HLA DR2 is strongly positively correlated with systemic lupus erythematosus, narcolepsy[4] and multiple sclerosis, and negatively correlated with DM Type 1.
- HLA DR3 is correlated strongly with Sjögren syndrome, myasthenia gravis, SLE, and DM Type 1.
- HLA DR4 is correlated with the genesis of rheumatoid arthritis, Type 1 diabetes mellitus, and pemphigus vulgaris.
Fewer correlations exist with MHC class I molecules. The most notable and consistent is the association between HLA B27 and spondyloarthropathies like ankylosing spondylitis and reactive arthritis. Correlations may exist between polymorphisms within class II MHC promoters and autoimmune disease.
The contributions of genes outside the MHC complex remain the subject of research, in animal models of disease (Linda Wicker's extensive genetic studies of diabetes in the NOD mouse), and in patients (Brian Kotzin's linkage analysis of susceptibility to SLE).
Recently, PTPN22 has been associated with multiple autoimmune diseases including Type I diabetes, rheumatoid arthritis, systemic lupus erythematosus, Hashimoto’s thyroiditis, Graves』 disease, Addison’s disease, Myasthenia Gravis, vitiligo, systemic sclerosis juvenile idiopathic arthritis, and psoriatic arthritis.[5][需要解釋]
Sex
[編輯]| Ratio of female/male incidence of autoimmune diseases | |
|---|---|
| Hashimoto's thyroiditis | 10:1[6] |
| Graves' disease | 7:1[6] |
| Multiple sclerosis (MS) | 2:1[6] |
| Myasthenia gravis | 2:1[6] |
| Systemic lupus erythematosus (SLE) | 9:1[6] |
| Rheumatoid arthritis | 5:2[6] |
| Primary sclerosing cholangitis | 1:2 |
There is some evidence that a person's sex may also have some role in the development of autoimmunity; that is, most autoimmune diseases are sex-related. A few autoimmune diseases that men are just as or more likely to develop as women include: ankylosing spondylitis, type 1 diabetes mellitus, granulomatosis with polyangiitis, Crohn's disease, Primary sclerosing cholangitis and psoriasis.
The reasons for the sex role in autoimmunity vary. Women appear to generally mount larger inflammatory responses than men when their immune systems are triggered, increasing the risk of autoimmunity. Involvement of sex steroids is indicated by that many autoimmune diseases tend to fluctuate in accordance with hormonal changes, for example: during pregnancy, in the menstrual cycle, or when using oral contraception. A history of pregnancy also appears to leave a persistent increased risk for autoimmune disease. It has been suggested that the slight, direct exchange of cells between mothers and their children during pregnancy may induce autoimmunity.[7] This would tip the gender balance in the direction of the female.
Another theory suggests the female high tendency to get autoimmunity is due to an imbalanced X-chromosome inactivation.[8] The X-inactivation skew theory, proposed by Princeton University's Jeff Stewart, has recently been confirmed experimentally in scleroderma and autoimmune thyroiditis.[9] Other complex X-linked genetic susceptibility mechanisms are proposed and under investigation.
Environmental factors
[編輯]Infectious diseases and parasites
[編輯]An interesting inverse relationship exists between infectious diseases and autoimmune diseases. In areas where multiple infectious diseases are endemic, autoimmune diseases are quite rarely seen. The reverse, to some extent, seems to hold true. The hygiene hypothesis attributes these correlations to the immune-manipulating strategies of pathogens. While such an observation has been variously termed as spurious and ineffective, according to some studies, parasite infection is associated with reduced activity of autoimmune disease.[10][11][12]
The putative mechanism is that the parasite attenuates the host immune response in order to protect itself. This may provide a serendipitous benefit to a host that also suffers from autoimmune disease. The details of parasite immune modulation are not yet known, but may include secretion of anti-inflammatory agents or interference with the host immune signaling.
A paradoxical observation has been the strong association of certain microbial organisms with autoimmune diseases. For example, Klebsiella pneumoniae and coxsackievirus B have been strongly correlated with ankylosing spondylitis and diabetes mellitus type 1, respectively. This has been explained by the tendency of the infecting organism to produce super-antigens that are capable of polyclonal activation of B-lymphocytes, and production of large amounts of antibodies of varying specificities, some of which may be self-reactive (see below).
Chemical agents and drugs
[編輯]Certain chemical agents and drugs can also be associated with the genesis of autoimmune conditions, or conditions that simulate autoimmune diseases. The most striking of these is the drug-induced lupus erythematosus. Usually, withdrawal of the offending drug cures the symptoms in a patient.
Cigarette smoking is now established as a major risk factor for both incidence and severity of rheumatoid arthritis. This may relate to abnormal citrullination of proteins, since the effects of smoking correlate with the presence of antibodies to citrullinated peptides.
Pathogenesis of autoimmunity
[編輯]Several mechanisms are thought to be operative in the pathogenesis of autoimmune diseases, against a backdrop of genetic predisposition and environmental modulation. It is beyond the scope of this article to discuss each of these mechanisms exhaustively, but a summary of some of the important mechanisms have been described:
- T-cell bypass – A normal immune system requires the activation of B cells by T cells before the former can undergo differentiation into plasma B-cells and subsequently produce antibodies in large quantities. This requirement of a T cell can be bypassed in rare instances, such as infection by organisms producing super-antigens, which are capable of initiating polyclonal activation of B-cells, or even of T-cells, by directly binding to the β-subunit of T-cell receptors in a non-specific fashion.
- T-cell–B-cell discordance – A normal immune response is assumed to involve B and T cell responses to the same antigen, even if we know that B cells and T cells recognise very different things: conformations on the surface of a molecule for B cells and pre-processed peptide fragments of proteins for T cells. However, there is nothing as far as we know that requires this. All that is required is that a B cell recognising antigen X endocytoses and processes a protein Y (normally =X) and presents it to a T cell. Roosnek and Lanzavecchia showed that B cells recognising IgGFc could get help from any T cell responding to an antigen co-endocytosed with IgG by the B cell as part of an immune complex. In coeliac disease it seems likely that B cells recognising tissue transglutamine are helped by T cells recognising gliadin.
- Aberrant B cell receptor-mediated feedback – A feature of human autoimmune disease is that it is largely restricted to a small group of antigens, several of which have known signaling roles in the immune response (DNA, C1q, IgGFc, Ro, Con. A receptor, Peanut agglutinin receptor(PNAR)). This fact gave rise to the idea that spontaneous autoimmunity may result when the binding of antibody to certain antigens leads to aberrant signals being fed back to parent B cells through membrane bound ligands. These ligands include B cell receptor (for antigen), IgG Fc receptors, CD21, which binds complement C3d, Toll-like receptors 9 and 7 (which can bind DNA and nucleoproteins) and PNAR. More indirect aberrant activation of B cells can also be envisaged with autoantibodies to acetyl choline receptor (on thymic myoid cells) and hormone and hormone binding proteins. Together with the concept of T-cell–B-cell discordance this idea forms the basis of the hypothesis of self-perpetuating autoreactive B cells.[13] Autoreactive B cells in spontaneous autoimmunity are seen as surviving because of subversion both of the T cell help pathway and of the feedback signal through B cell receptor, thereby overcoming the negative signals responsible for B cell self-tolerance without necessarily requiring loss of T cell self-tolerance.
- Molecular mimicry – An exogenous antigen may share structural similarities with certain host antigens; thus, any antibody produced against this antigen (which mimics the self-antigens) can also, in theory, bind to the host antigens, and amplify the immune response. The idea of molecular mimicry arose in the context of rheumatic fever, which follows infection with Group A beta-haemolytic streptococci. Although rheumatic fever has been attributed to molecular mimicry for half a century no antigen has been formally identified (if anything too many have been proposed). Moreover, the complex tissue distribution of the disease (heart, joint, skin, basal ganglia) argues against a cardiac specific antigen. It remains entirely possible that the disease is due to e.g. an unusual interaction between immune complexes, complement components and endothelium.
- Idiotype cross-reaction – Idiotypes are antigenic epitopes found in the antigen-binding portion (Fab) of the immunoglobulin molecule. Plotz and Oldstone presented evidence that autoimmunity can arise as a result of a cross-reaction between the idiotype on an antiviral antibody and a host cell receptor for the virus in question. In this case, the host-cell receptor is envisioned as an internal image of the virus, and the anti-idiotype antibodies can react with the host cells.
- Cytokine dysregulation – Cytokines have been recently divided into two groups according to the population of cells whose functions they promote: Helper T-cells type 1 or type 2. The second category of cytokines, which include IL-4, IL-10 and TGF-β (to name a few), seem to have a role in prevention of exaggeration of pro-inflammatory immune responses.
- Dendritic cell apoptosis – immune system cells called dendritic cells present antigens to active lymphocytes. Dendritic cells that are defective in apoptosis can lead to inappropriate systemic lymphocyte activation and consequent decline in self-tolerance.[14]
- Epitope spreading or epitope drift – when the immune reaction changes from targeting the primary epitope to also targeting other epitopes.[15] In contrast to molecular mimicry, the other epitopes need not be structurally similar to the primary one.
- Epitope modification or Cryptic epitope exposure – this mechanism of autoimmune disease is unique in that it does not result from a defect in the hematopoietic system. Instead, disease results from the exposure of cryptic N-glycan (polysaccharide) linkages common to lower eukaryotes and prokaryotes on the glycoproteins of mammalian non-hematopoietic cells and organs[16] This exposure of phylogenically primitive glycans activates one or more mammalian innate immune cell receptors to induce a chronic sterile inflammatory state. In the presence of chronic and inflammatory cell damage, the adaptive immune system is recruited and self–tolerance is lost with increased autoantibody production. In this form of the disease, the absence of lymphocytes can accelerate organ damage, and intravenous IgG administration can be therapeutic. Although this route to autoimmune disease may underlie various degenerative disease states, no diagnostics for this disease mechanism exist at present, and thus its role in human autoimmunity is currently unknown.
The roles of specialized immunoregulatory cell types, such as regulatory T cells, NKT cells, γδ T-cells in the pathogenesis of autoimmune disease are under investigation.
Classification
[編輯]Autoimmune diseases can be broadly divided into systemic and organ-specific or localised autoimmune disorders, depending on the principal clinico-pathologic features of each disease.
- Systemic autoimmune diseases include coeliac disease, lupus erythematosus, Sjögren syndrome, sarcoidosis, scleroderma, rheumatoid arthritis, cryoglobulinemic vasculitis, and dermatomyositis. These conditions tend to be associated with autoantibodies to antigens which are not tissue specific. Thus although polymyositis is more or less tissue specific in presentation, it may be included in this group because the autoantigens are often ubiquitous t-RNA synthetases.
- Local syndromes which affect a specific organ or tissue:
- Endocrinologic: diabetes mellitus type 1, Hashimoto's thyroiditis, Addison's disease
- Gastrointestinal: Crohn's disease, pernicious anaemia
- Dermatologic: pemphigus vulgaris, vitiligo
- Haematologic: autoimmune haemolytic anaemia, idiopathic thrombocytopenic purpura
- Neurological: multiple sclerosis, myasthenia gravis, autoimmune encephalitis, gluten ataxia
Using the traditional 「organ specific」 and 「non-organ specific」 classification scheme, many diseases have been lumped together under the autoimmune disease umbrella. However, many chronic inflammatory human disorders lack the telltale associations of B and T cell driven immunopathology. In the last decade[需要解釋] it has been firmly established that tissue "inflammation against self" does not necessarily rely on abnormal T and B cell responses.[17]
This has led to the recent proposal that the spectrum of autoimmunity should be viewed along an 「immunological disease continuum,」 with classical autoimmune diseases at one extreme and diseases driven by the innate immune system at the other extreme. Within this scheme, the full spectrum of autoimmunity can be included. Many common human autoimmune diseases can be seen to have a substantial innate immune mediated immunopathology using this new scheme. This new classification scheme has implications[需要解釋] for understanding disease mechanisms and for therapy development.[17]
Diagnosis
[編輯]Diagnosis of autoimmune disorders largely rests on accurate history and physical examination of the patient, and high index of suspicion[需要解釋] against a backdrop of certain abnormalities in routine laboratory tests (example, elevated C-reactive protein).[來源請求]
In several systemic disorders,[需要解釋] serological assays which can detect specific autoantibodies can be employed.[來源請求] Localised disorders are best diagnosed by immunofluorescence of biopsy specimens.[來源請求]
Autoantibodies are used to diagnose many autoimmune diseases.[需要解釋] The levels of autoantibodies are measured to determine the progress of the disease.[來源請求]
Treatments
[編輯]Treatments for autoimmune disease have traditionally been immunosuppressive, anti-inflammatory, or palliative.[1] Managing inflammation is critical in autoimmune diseases.[18] Non-immunological therapies, such as hormone replacement in Hashimoto's thyroiditis or Type 1 diabetes mellitus treat outcomes of the autoaggressive response, thus these are palliative treatments. Dietary manipulation limits the severity of celiac disease. Steroidal or NSAID treatment limits inflammatory symptoms of many diseases. IVIG is used for CIDP and GBS. Specific immunomodulatory therapies, such as the TNFα antagonists (e.g. etanercept), the B cell depleting agent rituximab, the anti-IL-6 receptor tocilizumab and the costimulation blocker abatacept have been shown to be useful in treating RA. Some of these immunotherapies may be associated with increased risk of adverse effects, such as susceptibility to infection.
Helminthic therapy is an experimental approach that involves inoculation of the patient with specific parasitic intestinal nematodes (helminths). There are currently two closely related treatments available, inoculation with either Necator americanus, commonly known as hookworms, or Trichuris Suis Ova, commonly known as Pig Whipworm Eggs.[19][19][20][21][22][23]
T-cell vaccination is also being explored as a possible future therapy for autoimmune disorders.[來源請求]
Nutrition and autoimmunity
[編輯]Vitamin D/Sunlight
- Because most human cells and tissues have receptors for vitamin D, including T and B cells, adequate levels of vitamin D can aid in the regulation of the immune system.[24] Vitamin D plays a role in immune function by acting on T cells and natural killer cells.[25] Research has demonstrated an association between low serum vitamin D and autoimmune diseases, including multiple sclerosis, type 1 diabetes, and Systemic Lupus Erythematosus (commonly referred to simply as lupus).[25][26][27] However, since photosensitivity occurs in lupus, patients are advised to avoid sunlight which may be responsible for vitamin D deficiency seen in this disease.[25][26][27] Polymorphisms in the vitamin D receptor gene are commonly found in people with autoimmune diseases, giving one potential mechanism for vitamin D's role in autoimmunity.[25][26] There is mixed evidence on the effect of vitamin D supplementation in type 1 diabetes, lupus, and multiple sclerosis.[25][26][27]
Omega-3 Fatty Acids
- Studies have shown that adequate consumption of omega-3 fatty acids counteracts the effects of arachidonic acids, which contribute to symptoms of autoimmune diseases. Human and animal trials suggest that omega-3 is an effective treatment modality for many cases of Rheumatoid Arthritis, Inflammatory Bowel Disease, Asthma, and Psoriasis.[28]
- While major depression is not necessarily an autoimmune disease, some of its physiological symptoms are inflammatory and autoimmune in nature. Omega-3 may inhibit production of interferon gamma and other cytokines which cause the physiological symptoms of depression. This may be due to the fact that an imbalance in omega-3 and omega-6 fatty acids, which have opposing effects, is instrumental in the etiology of major depression.[28]
Probiotics/Microflora
- Various types of bacteria and microflora present in fermented dairy products, especially Lactobacillus casei, have been shown to both stimulate immune response to tumors in mice and to regulate immune function, delaying or preventing the onset of nonobese diabetes. This is particularly true of the Shirota strain of L. casei (LcS). The LcS strain is mainly found in yogurt and similar products in Europe and Japan, and rarely elsewhere.[29]
- It has been theorized that free radicals contribute to the onset of type-1 diabetes in infants and young children, and therefore that the risk could be reduced by high intake of antioxidant substances during pregnancy. However, a study conducted in a hospital in Finland from 1997-2002 concluded that there was no statistically significant correlation between antioxidant intake and diabetes risk.[30] This study involved monitoring of food intake through questionnaires, and estimated antioxidant intake on this basis, rather than by exact measurements or use of supplements.
自體免疫性疾病
[編輯]| Autoimmune diseases | |
|---|---|
 | |
| Young woman with the typical "butterfly rash" found in systemic lupus erythematosus | |
| 症狀 | Depends on the condition. Commonly low grade fever, feeling tired[31] |
| 起病年齡 | Adulthood[31] |
| 類型 | List of autoimmune diseases (alopecia areata, celiac disease, diabetes mellitus type 1, Graves' disease, inflammatory bowel disease, multiple sclerosis, psoriasis, rheumatoid arthritis, systemic lupus erythematosus, others)[31] |
| 藥物 | Nonsteroidal anti-inflammatory drugs, immunosuppressants, intravenous immunoglobulin[31][32] |
| 盛行率 | 24 million / 7% (USA)[31][33] |
| 分類和外部資源 | |
| 醫學專科 | Rheumatology, immunology, gastroenterology, neurology, dermatology |
An autoimmune disease is a condition arising from an abnormal immune response to a normal body part.[31] There are at least 80 types of autoimmune diseases.[31] Nearly any body part can be involved.[33] Common symptoms include low grade fever and feeling tired.[31] Often symptoms come and go.[31]
The cause is generally unknown.[33] Some autoimmune diseases such as lupus run in families, and certain cases may be triggered by infections or other environmental factors.[31] Some common diseases that are generally considered autoimmune include celiac disease, diabetes mellitus type 1, Graves' disease, inflammatory bowel disease, multiple sclerosis, psoriasis, rheumatoid arthritis, and systemic lupus erythematosus.[31][34] The diagnosis can be difficult to determine.[31]
Treatment depends on the type and severity of the condition.[31] Nonsteroidal anti-inflammatory drugs (NSAIDs) and immunosuppressants are often used.[31] Intravenous immunoglobulin may also occasionally be used.[32] While treatment usually improves symptoms, they do not typically cure the disease.[31]
About 24 million (7%) people in the United States are affected by an autoimmune disease.[31][33] Women are more commonly affected than men.[31] Often they start during adulthood.[31] The first autoimmune diseases were described in the early 1900s.[35]
Signs and symptoms
[編輯]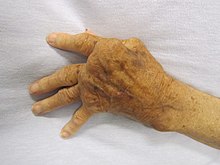
Autoimmune diseases have a wide variety of different effects. They do tend to have one of three characteristic pathological effects which characterize them as autoimmune diseases:[36]
- Damage to or destruction of tissues
- Altered organ growth
- Altered organ function
It has been estimated that autoimmune diseases are among the leading causes of death among women in the United States in all age groups up to 65 years.[37]
A substantial minority of the population suffers from these diseases, which are often chronic, debilitating, and life-threatening.[來源請求]
There are more than 80 illnesses caused by autoimmunity.[38]
Causes
[編輯]The cause is generally unknown.[33] Some autoimmune diseases such as lupus run in families, and certain cases may be triggered by infections or other environmental factors.[31] There are more than 100 autoimmune diseases.[39] Some common diseases that are generally considered autoimmune include celiac disease, diabetes mellitus type 1, Graves' disease, inflammatory bowel disease, multiple sclerosis, psoriasis, rheumatoid arthritis, and systemic lupus erythematosus.[31][34]
Pathophysiology
[編輯]The human immune system typically produces both T cells and B cells that are capable of being reactive with self-antigens, but these self-reactive cells are usually either killed prior to becoming active within the immune system, placed into a state of anergy (silently removed from their role within the immune system due to over-activation), or removed from their role within the immune system by regulatory cells. When any one of these mechanisms fail, it is possible to have a reservoir of self-reactive cells that become functional within the immune system. The mechanisms of preventing self-reactive T cells from being created takes place through negative selection process within the thymus as the T cell is developing into a mature immune cell.
Some infections, such as Campylobacter jejuni, have antigens that are similar (but not identical) to our own self-molecules. In this case, a normal immune response to C. jejuni can result in the production of antibodies that also react to a lesser degree with gangliosides of myelin sheath surrounding peripheral nerves' axons (i.e., Guillain–Barré). A major understanding of the underlying pathophysiology of autoimmune diseases has been the application of genome wide association scans that have identified a degree of genetic sharing among the autoimmune diseases.[40]
Autoimmunity, on the other hand, is the presence of self-reactive immune response (e.g., auto-antibodies, self-reactive T cells), with or without damage or pathology resulting from it.[41] This may be restricted to certain organs (e.g. in autoimmune thyroiditis) or involve a particular tissue in different places (e.g. Goodpasture's disease which may affect the basement membrane in both the lung and the kidney).
There are many theories as to how an autoimmune disease state arises. Some common ones are listed below.
Cryptic determinants/molecular sequestration
[編輯]Although it is possible for a potential autoantigen to be spatially sequestered in an immune privileged site within the body (e.g. the eye), mechanisms exist to express even these antigens in a tolerogenic fashion to the immune system. However, it is impossible to induce tolerance (immune unresponsiveness) to all aspects of an autoantigen. This is because under normal physiologic conditions some regions of a self-antigen are not expressed at a sufficient level to induce tolerance. These poorly displayed areas of an antigen are called "cryptic determinants." The immune system maintains a high-affinity repertoire to the cryptic self because the presentation of these determinants was insufficient to induce strong tolerance.[42]
Molecular mimicry
[編輯]The concept of molecular mimicry describes a situation in which a foreign antigen can initiate an immune response in which a T or B cell component cross-recognizes self. The cross reactive immune response is responsible for the autoimmune disease state.[43] Cross-reactive immune responses to self were first described for antibodies.
Altered glycan theory
[編輯]According to this theory the effector function of the immune response is mediated by the glycans (polysaccharides) displayed by the cells and humoral components of the immune system. Individuals with autoimmunity have alterations in their glycosylation profile such that a proinflammatory immune response is favored. It is further hypothesized that individual autoimmune diseases will have unique glycan signatures.[44]
Hygiene hypothesis
[編輯]According to the hygiene hypothesis, high levels of cleanliness expose children to fewer antigens than in the past, causing their immune systems to become overactive and more likely to misidentify own tissues as foreign, resulting in autoimmune or allergic conditions such as asthma.[45]
Diagnosis
[編輯]For a disease to be regarded as an autoimmune disease it needs to answer to Witebsky's postulates (first formulated by Ernest Witebsky and colleagues in 1957 and modified in 1994):[46][47]
- Direct evidence from transfer of disease-causing antibody or disease-causing T lymphocyte white blood cells
- Indirect evidence based on reproduction of the autoimmune disease in experimental animals
- Circumstantial evidence from clinical clues
Epidemiology
[編輯]The first estimate of US prevalence for autoimmune diseases as a group was published in 1997 by Jacobson, et al. They reported US prevalence to be around 9 million, applying prevalence estimates for 24 diseases to a US population of 279 million.[48] Jacobson's work was updated by Hayter & Cook in 2012.[49] This study used Witebsky's postulates, as revised by Rose & Bona,[50] to extend the list to 81 diseases and estimated overall cumulative US prevalence for the 81 autoimmune diseases at 5.0%, with 3.0% for males and 7.1% for females. The estimated community prevalence, which takes into account the observation that many people have more than one autoimmune disease, was 4.5% overall, with 2.7% for males and 6.4% for females.[49]
Research
[編輯]In both autoimmune and inflammatory diseases, the condition arises through aberrant reactions of the human adaptive or innate immune systems. In autoimmunity, the patient's immune system is activated against the body's own proteins. In chronic inflammatory diseases, neutrophils and other leukocytes are constitutively recruited by cytokines and chemokines, leading to tissue damage.
Mitigation of inflammation by activation of anti-inflammatory genes and the suppression of inflammatory genes in immune cells is a promising therapeutic approach.[51][52][53] There is a body of evidence that once the production of autoantibodies has been initialized, autoantibodies have the capacity to maintain their own production.[54]
Stem cell transplantation is being studied and has shown promising results in certain cases.[55]
History
[編輯]Traditionally it was believed that the immune system was unable to react against the body's own tissues, a concept described by the German immunologist Paul Ehrlich as "horror autotoxicus". In 1904 this theory was challenged by the discovery of a substance in the serum of patients with paroxysmal cold hemoglobinuria that reacted with red blood cells.[56]
單株抗體治療
[編輯]單株抗體治療 單克隆抗體治療 Template:細胞外化療藥物 en:Biologics for immunosuppression
Antibody structure and function
[編輯]Immunoglobulin G (IgG) antibodies are large heterodimeric molecules, approximately 150 kDa and are composed of two kinds of polypeptide chain, called the heavy (~50kDa) and the light chain (~25kDa). The two types of light chains are kappa (κ) and lambda (λ). By cleavage with enzyme papain, the Fab (fragment-antigen binding) part can be separated from the Fc (fragment constant) part of the molecule. The Fab fragments contain the variable domains, which consist of three antibody hypervariable amino acid domains responsible for the antibody specificity embedded into constant regions. The four known IgG subclasses are involved in antibody-dependent cellular cytotoxicity.[57] Antibodies are a key component of the adaptive immune response, playing a central role in both in the recognition of foreign antigens and the stimulation of an immune response to them. The advent of monoclonal antibody technology has made it possible to raise antibodies against specific antigens presented on the surfaces of tumors.[58] Monoclonal antibodies can be acquired in the immune system via passive immunity or active immunity.The advantage of active monoclonal antibody therapy is the fact that the immune system will produce antibodies long-term, with only a short-term drug administration to induce this response. However, the immune response to certain antigens may be inadequate, especially in the elderly. Additionally, adverse reactions from these antibodies may occur because of long-lasting response to antigens.[59] Passive monoclonal antibody therapy can ensure consistent antibody concentration, and can control for adverse reactions by stopping administration. However, the repeated administration and consequent higher cost for this therapy are major disadvantages.[59]
Monoclonal antibody therapy may prove to be beneficial for cancer, autoimmune diseases, and neurological disorders that result in the degeneration of body cells, such as Alzheimer's disease. Monoclonal antibody therapy can aid the immune system because the innate immune system responds to the environmental factors it encounters by discriminating against foreign cells from cells of the body. Therefore, tumor cells that are proliferating at high rates, or body cells that are dying which subsequently cause physiological problems are generally not specifically targeted by the immune system, since tumor cells are the patient's own cells. Tumor cells, however are highly abnormal, and many display unusual antigens. Some such tumor antigens are inappropriate for the cell type or its environment. Monoclonal antibodies can target tumor cells or abnormal cells in the body that are recognized as body cells, but are debilitating to one's health.
History
[編輯]
Immunotherapy developed in the 1970s following the discovery of the structure of antibodies and the development of hybridoma technology, which provided the first reliable source of monoclonal antibodies.[61][62] These advances allowed for the specific targeting of tumors both in vitro and in vivo. Initial research on malignant neoplasms found mAb therapy of limited and generally short-lived success with blood malignancies.[63][64] Treatment also had to be tailored to each individual patient, which was impracticable in routine clinical settings.
Four major antibody types that have been developed are murine, chimeric, humanised and human. Antibodies of each type are distinguished by suffixes on their name.
Murine
[編輯]Initial therapeutic antibodies were murine analogues (suffix -omab). These antibodies have: a short half-life in vivo (due to immune complex formation), limited penetration into tumour sites and inadequately recruit host effector functions.[65] Chimeric and humanized antibodies have generally replaced them in therapeutic antibody applications.[66] Understanding of proteomics has proven essential in identifying novel tumour targets.
Initially, murine antibodies were obtained by hybridoma technology, for which Jerne, Köhler and Milstein received a Nobel prize. However the dissimilarity between murine and human immune systems led to the clinical failure of these antibodies, except in some specific circumstances. Major problems associated with murine antibodies included reduced stimulation of cytotoxicity and the formation complexes after repeated administration, which resulted in mild allergic reactions and sometimes anaphylactic shock.[65] Hybridoma technology has been replaced by recombinant DNA technology, transgenic mice and phage display.[66]
Chimeric and humanized
[編輯]To reduce murine antibody immunogenicity (attacks by the immune system against the antibody), murine molecules were engineered to remove immunogenic content and to increase immunologic efficiency.[65] This was initially achieved by the production of chimeric (suffix -ximab) and humanized antibodies (suffix -zumab). Chimeric antibodies are composed of murine variable regions fused onto human constant regions. Taking human gene sequences from the kappa light chain and the IgG1 heavy chain results in antibodies that are approximately 65% human. This reduces immunogenicity, and thus increases serum half-life.
Humanised antibodies are produced by grafting murine hypervariable regions on amino acid domains into human antibodies. This results in a molecule of approximately 95% human origin. Humanised antibodies bind antigen much more weakly than the parent murine monoclonal antibody, with reported decreases in affinity of up to several hundredfold.[67][68] Increases in antibody-antigen binding strength have been achieved by introducing mutations into the complementarity determining regions (CDR),[69] using techniques such as chain-shuffling, randomization of complementarity-determining regions and antibodies with mutations within the variable regions induced by error-prone PCR, E. coli mutator strains and site-specific mutagenesis.[70]
Human monoclonal antibodies
[編輯]Human monoclonal antibodies (suffix -umab) are produced using transgenic mice or phage display libraries by transferring human immunoglobulin genes into the murine genome and vaccinating the transgenic mouse against the desired antigen, leading to the production of appropriate monoclonal antibodies.[66] Murine antibodies in vitro are thereby transformed into fully human antibodies.[58]
The heavy and light chains of human IgG proteins are expressed in structural polymorphic (allotypic) forms. Human IgG allotype is one of the many factors that can contribute to immunogenicity.[71][72]
Targeted conditions
[編輯]Cancer
[編輯]Anti-cancer monoclonal antibodies can be targeted against malignant cells by several mechanisms. Ramucirumab is a recombinant human monoclonal antibody and is used in the treatment of advanced malignancies.[73] In childhood lymphoma, phase I and II studies have found a positive effect of using antibody therapy.[74]
Autoimmune diseases
[編輯]Monoclonal antibodies used for autoimmune diseases include infliximab and adalimumab, which are effective in rheumatoid arthritis, Crohn's disease and ulcerative colitis by their ability to bind to and inhibit TNF-α.[75] Basiliximab and daclizumab inhibit IL-2 on activated T cells and thereby help preventing acute rejection of kidney transplants.[75] Omalizumab inhibits human immunoglobulin E (IgE) and is useful in moderate-to-severe allergic asthma.
Alzheimer's disease
[編輯]Alzheimer’s disease (AD) is a multi-faceted, age-dependent, progressive neurodegenerative disorder, and is a major cause of dementia.[76] According to the Amyloid hypothesis, the accumulation of extracellular amyloid betapeptides (Aβ) into plaques via oligomerization leads to hallmark symptomatic conditions of AD through synaptic dysfunction and neurodegeneration.[77] Immunotherapy via exogenous monoclonal antibody (MAB) administration has been known to treat various central nervous disorders, such as AD, by inhibiting Aβ-oligomerization thereby preventing neurotoxicity. However, MABs are large for passive protein channels and are therefore inefficient due to the blood-brain barrier preventing MAB passage into the brain. However, the Peripheral Sink hypothesis proposes a mechanism where MABs may not need to cross the blood-brain barrier.[78] Therefore, many research studies are being conducted from failed attempts to treat AD in the past.[77]
However, anti-Aβ vaccines can promote antibody-mediated clearance of Aβ plaques in transgenic mice models with amyloid precursor proteins (APP), and can reduce cognitive impairments.[76] Vaccines can stimulate the immune system to produce its own antibodies,[79] in this case by introducing Aβ into transgenic animal models, known as active immunization. They can also introduce antibodies into animal models, known as passive immunization. In mice expressing APP, both active and passive immunization of anti-Aβ antibodies has been shown to be effective in clearing plaques, and can improve cognitive function.[77]. Therefore, several clinical trials using passive and active immunization approaches by development of certain drugs approved by the FDA are currently underway, and are expected to yield results in a couple of years.[77] The implementation of these drugs is during the onset of AD. Other research and drug development for early intervention and AD prevention is ongoing. Various drugs that are under research to treat AD include Bapineuzumab, Solanezumab, and Gautenerumab.
Bapineuzumab
[編輯]Bapineuzumab, a humanized anti-aβ MAB, is directed against the N-terminus of Aβ. Phase II clinical trials of Bapineuzumab in mild to moderate AD patients resulted in reduced Aβ concentration in the brain. However, in patients with increased apolipoprotein (APOE) e4 carriers, Bapineuzumab treatment is also accompanied by vasogenic edema,[80] a cytotoxic condition where the blood brain barrier has been disrupted thereby affecting white matter from excess accumulation of fluid from capillaries in intracellular and extracellular spaces of the brain.[81] In Phase III clinical trials, Bapineuzumab treatment is associated with reduced rate of accumulation of Aβ in the brain in APOE e4 patients, and no significant reduction of Aβ concentration in APOE e4 patients and non-APOE e4 patients. Therefore, Aβ plaque concentration was not reduced, and there is no significant clinical benefits in cognitive functioning. Bapineuzumab was discontinued after failing in Phase III clinical trial[81]
Solanezumab
[編輯]Solanezumab, an anti-aβ MAB, targets the N-terminus of Aβ. In Phase I and Phase II of clinical trials, Solanezumab treatment resulted in cerebrospinal fluid elevation of Aβ, thereby showing a reduced concentration of Aβ plaques. Additionally, there are no associated adverse side effects. Phase III clinical trials of Solanezumab brought about significant reduction in cognitive impairment in patients with mild AD, but not in patients with severe AD. However, Aβ concentration did not significantly change, along with other AD biomarkers, including phospho-tau expression, and hippocampal volume. Phase III clinical trials are currently ongoing.[78]
Preventive trials
[編輯]Failure of several drugs in Phase III clinical trials has led to AD prevention and early intervention for onset AD treatment endeavours. Passive anti-Aβ MAB treatment can be used for preventive attempts to modify AD progression before it causes extensive brain damage and symptoms. Trials using MAB treatment for patients positive for genetic risk factors, and elderly patients positive for indicators of AD are underway. This includes anti-AB treatment in Asymptomatic Alzheimer's Disease (A4), the Alzheimer’s Prevention Initiative (API), and DIAN-TU.[78] The A4 study on older individuals who are positive for indicators of AD but are negative for genetic risk factors will test Solanezumab in Phase III Clinical Trials, as a follow up of previous Solanezumab studies.[78] DIAN-TU, launched in December 2012, focuses on young patients positive for genetic mutations that are risks for AD. This study uses Solanezumab and Gautenerumab. Gautenerumab, the first fully human MAB that preferentially interacts with oligomerized Aβ plaques in the brain, caused significant reduction in Aβ concentration in Phase I clinical trials, preventing plaque formation and concentration without altering plasma concentration of the brain. Phase II and III clinical trials are currently being conducted.[78]
Therapy types
[編輯]Radioimmunotherapy
[編輯]Radioimmunotherapy (RIT) involves the use of radioactively-conjugated murine antibodies against cellular antigens. Most research involves their application to lymphomas, as these are highly radio-sensitive malignancies. To limit radiation exposure, murine antibodies were chosen, as their high immunogenicity promotes rapid tumor clearance. Tositumomab is an example used for non-Hodgkin's lymphoma.
Antibody-directed enzyme prodrug therapy
[編輯]Antibody-directed enzyme prodrug therapy (ADEPT) involves the application of cancer-associated monoclonal antibodies that are linked to a drug-activating enzyme. Systemic administration of a non-toxic agent results in the antibody's conversion to a toxic drug, resulting in a cytotoxic effect that can be targeted at malignant cells. The clinical success of ADEPT treatments is limited.[82]
Antibody-drug conjugates
[編輯]Antibody-drug conjugates (ADCs) are antibodies linked to one or more drug molecules. Typically when the ADC meets the target cell (e.g. a cancerous cell) the drug is released to kill it. Many ADCs are in clinical development. 截至2016年[update] a few have been approved.
Immunoliposome therapy
[編輯]Immunoliposomes are antibody-conjugated liposomes. Liposomes can carry drugs or therapeutic nucleotides and when conjugated with monoclonal antibodies, may be directed against malignant cells. Immunoliposomes have been successfully used in vivo to convey tumour-suppressing genes into tumours, using an antibody fragment against the human transferrin receptor. Tissue-specific gene delivery using immunoliposomes has been achieved in brain and breast cancer tissue.[83]
Checkpoint therapy
[編輯]Checkpoint therapy uses antibodies and other techniques to circumvent the defenses that tumors use to suppress the immune system. Each defense is known as a checkpoint. Compound therapies combine antibodies to suppress multiple defensive layers. Known checkpoints include CTLA-4 targeted by ipilimumab, PD-1 targeted by nivolumab and pembrolizumab and the tumor microenvironment.[84]
The tumor microenvironment (TME) features prevents the recruitment of T cells to the tumor. Ways include chemokine CCL2 nitration, which traps T cells in the stroma. Tumor vasculature helps tumors preferentially recruit other immune cells over T cells, in part through endothelial cell (EC)–specific expression of FasL, ETBR, and B7H3. Myelomonocytic and tumor cells can up-regulate expression of PD-L1, partly driven by hypoxic conditions and cytokine production, such as IFNβ. Aberrant metabolite production in the TME, such as the pathway regulation by IDO, can affect T cell functions directly and indirectly via cells such as Treg cells. CD8 cells can be suppressed by B cells regulation of TAM phenotypes. Cancer-associated fibroblasts (CAFs) have multiple TME functions, in part through extracellular matrix (ECM)–mediated T cell trapping and CXCL12-regulated T cell exclusion.[85]
FDA-approved therapeutic antibodies
[編輯]The first FDA-approved therapeutic monoclonal antibody was a murine IgG2a CD3 specific transplant rejection drug, OKT3 (also called muromonab), in 1986. This drug found use in solid organ transplant recipients who became steroid resistant.[86] Hundreds of therapies are undergoing clinical trials. Most are concerned with immunological and oncological targets.
| Antibody | Brand name | Company | Approval date | Route | Type | Target | Indication (Targeted disease) |
BLA STN | Drug Label |
|---|---|---|---|---|---|---|---|---|---|
| abciximab | ReoPro | Centocor | 12/22/1994 | intravenous | chimeric Fab | GPIIb/IIIa | Percutaneous coronary intervention | 103575 | Link |
| adalimumab | Humira | Abbvie | 12/31/2002 | subcutaneous | fully human | TNF | Rheumatoid arthritis | 125057 | Link |
| adalimumab-atto | Amjevita | Amgen | 9/23/2016 | subcutaneous | fully human, biosimilar | TNF | Rheumatoid arthritis Juvenile idiopathic arthritis Psoriatic arthritis Ankylosing spondylitis Crohn's disease Ulcerative colitis Plaque psoriasis |
761024 | Link |
| ado-trastuzumab emtansine | Kadcyla | Genentech | 2/22/2013 | intravenous | humanized, antibody-drug conjugate | HER2 | Metastatic breast cancer | 125427 | Link |
| alemtuzumab | Campath, Lemtrada | Genzyme | 5/7/2001 | intravenous | humanized | CD52 | B-cell chronic lymphocytic leukemia | 103948 | Link |
| alirocumab | Praluent | Sanofi Aventis | 7/24/2015 | subcutaneous | fully human | PCSK9 | Heterozygous familial hypercholesterolemia Refractory hypercholesterolemia |
125559 | Link |
| atezolizumab | Tecentriq | Genentech | 5/18/2016 | intravenous | humanized | PD-L1 | Urothelial carcinoma | 761034 | Link |
| atezolizumab | Tecentriq | Genentech | 10/18/2016 | intravenous | humanized | PD-L1 | Urothelial carcinoma Metastatic non-small cell lung cancer |
761041 | Link |
| avelumab | Bavencio | EMD Serono | 3/23/2017 | intravenous | fully human | PD-L1 | Metastatic Merkel cell carcinoma | 761049 | Link |
| basiliximab | Simulect | Novartis | 5/12/1998 | intravenous | chimeric | IL2RA | Prophylaxis of acute organ rejection in renal transplant | 103764 | Link |
| belimumab | Benlysta | Human Genome Sciences | 3/9/2011 | intravenous | fully human | BLyS | Systemic lupus erythematosus | 125370 | Link |
| bevacizumab | Avastin | Genentech | 2/26/2004 | intravenous | humanized | VEGF | Metastatic colorectal cancer | 125085 | Link |
| bezlotoxumab | Zinplava | Merck | 10/21/2016 | intravenous | fully human | Clostridium difficile toxin B | Prevent recurrence of Clostridium difficile infection | 761046 | Link |
| blinatumomab | Blincyto | Amgen | 12/3/2014 | intravenous | mouse, bispecific | CD19 | Precursor B-cell acute lymphoblastic leukemia | 125557 | Link |
| brentuximab vedotin | Adcetris | Seattle Genetics | 9/19/2011 | intravenous | chimeric, antibody-drug conjugate | CD30 | Hodgkin lymphoma Anaplastic large-cell lymphoma |
125388 | Link |
| brodalumab | Siliq | Valeant | 2/15/2017 | subcutaneous | chimeric | IL17RA | Plaque psoriasis | 761032 | Link |
| canakinumab | Ilaris | Novartis | 6/17/2009 | subcutaneous | fully human | IL1B | Cryopyrin-associated periodic syndrome | 125319 | Link |
| capromab pendetide | ProstaScint | Cytogen | 10/28/1996 | intravenous | murine, radiolabeled | PSMA | Diagnostic imaging agent in newly diagnosed prostate cancer or post-prostatectomy | 103608 | Link |
| certolizumab pegol | Cimzia | UCB (company) | 4/22/2008 | subcutaneous | humanized | TNF | Crohn's disease | 125160 | Link |
| cetuximab | Erbitux | ImClone Systems | 2/12/2004 | intravenous | chimeric | EGFR | Metastatic colorectal carcinoma | 125084 | Link |
| daclizumab | Zenapax | Roche | 12/10/1997 | intravenous | humanized | IL2RA | Prophylaxis of acute organ rejection in renal transplant | 103749 | Link |
| daclizumab | Zinbryta | Biogen | 5/27/2016 | subcutaneous | humanized | IL2R | Multiple sclerosis | 761029 | Link |
| daratumumab | Darzalex | Janssen Biotech | 11/16/2015 | intravenous | fully human | CD38 | Multiple myeloma | 761036 | Link |
| denosumab | Prolia, Xgeva | Amgen | 6/1/2010 | subcutaneous | fully human | RANKL | Postmenopausal women with osteoporosis | 125320 | Link |
| dinutuximab | Unituxin | United Therapeutics | 3/10/2015 | intravenous | chimeric | GD2 | Pediatric high-risk neuroblastoma | 125516 | Link |
| dupilumab | Dupixent | Regeneron Pharmaceuticals | 3/28/2017 | subcutaneous | fully human | IL4RA | Atopic dermatitis, asthma | 761055 | Link |
| durvalumab | Imfinzi | AstraZeneca | 5/1/2017 | intravenous | fully human | PD-L1 | Urothelial carcinoma | 761069 | Link |
| eculizumab | Soliris | Alexion | 3/16/2007 | intravenous | humanized | Complement component 5 | Paroxysmal nocturnal hemoglobinuria | 125166 | Link |
| elotuzumab | Empliciti | Bristol-Myers Squibb | 11/30/2015 | intravenous | humanized | SLAMF7 | Multiple myeloma | 761035 | Link |
| evolocumab | Repatha | Amgen | 8/27/2015 | subcutaneous | fully human | PCSK9 | Heterozygous familial hypercholesterolemia Refractory hypercholesterolemia |
125522 | Link |
| golimumab | Simponi | Centocor | 4/24/2009 | subcutaneous | fully human | TNF | Rheumatoid arthritis Psoriatic arthritis Ankylosing spondylitis |
125289 | Link |
| golimumab | Simponi Aria | Janssen Biotech | 7/18/2013 | intravenous | fully human | TNF | Rheumatoid arthritis | 125433 | Link |
| ibritumomab tiuxetan | Zevalin | Spectrum Pharmaceuticals | 2/19/2002 | intravenous | murine, radioimmunotherapy | CD20 | Relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma | 125019 | Link |
| idarucizumab | Praxbind | Boehringer Ingelheim | 10/16/2015 | intravenous | humanized Fab | dabigatran | Emergency reversal of anticoagulant dabigatran | 761025 | Link |
| infliximab | Remicade | Centocor | 8/24/1998 | intravenous | chimeric | TNF alpha | Crohn's disease | 103772 | Link |
| infliximab-abda | Renflexis | Samsung Bioepis | 4/21/2017 | intravenous | chimeric, biosimilar | TNF | Crohn's disease Ulcerative colitis Rheumatoid arthritis Ankylosing spondylitis Psoriatic arthritis Plaque psoriasis |
761054 | Link |
| infliximab-dyyb | Inflectra | Celltrion Healthcare | 4/5/2016 | intravenous | chimeric, biosimilar | TNF | Crohn's disease Ulcerative colitis Rheumatoid arthritis Ankylosing spondylitis Psoriatic arthritis Plaque psoriasis |
125544 | Link |
| ipilimumab | Yervoy | Bristol-Myers Squibb | 3/25/2011 | intravenous | fully human | CTLA-4 | Metastatic melanoma | 125377 | Link |
| ixekizumab | Taltz | Eli Lilly | 3/22/2016 | subcutaneous | humanized | IL17A | Plaque psoriasis | 125521 | Link |
| mepolizumab | Nucala | GlaxoSmithKline | 11/4/2015 | subcutaneous | humanized | IL5 | Severe asthma | 125526 | Link |
| natalizumab | Tysabri | Biogen Idec | 11/23/2004 | intravenous | humanized | alpha-4 integrin | Multiple sclerosis | 125104 | Link |
| necitumumab | Portrazza | Eli Lilly | 11/24/2015 | intravenous | fully human | EGFR | Metastatic squamous non-small cell lung carcinoma | 125547 | Link |
| nivolumab | Opdivo | Bristol-Myers Squibb | 12/22/2014 | intravenous | fully human | PD-1 | Metastatic melanoma | 125554 | Link |
| nivolumab | Opdivo | Bristol-Myers Squibb | 3/4/2015 | intravenous | fully human | PD-1 | Metastatic squamous non-small cell lung carcinoma | 125527 | Link |
| obiltoxaximab | Anthem | Elusys Therapeutics | 3/18/2016 | intravenous | chimeric | Protective antigen of the Anthrax toxin | Inhalational anthrax | 125509 | Link |
| obinutuzumab | Gazyva | Genentech | 11/1/2013 | intravenous | humanized | CD20 | Chronic lymphocytic leukemia | 125486 | Link |
| ocrelizumab | Ocrevus | Genentech | 3/28/2017 | intravenous | humanized | CD20 | Multiple sclerosis | 761053 | Link |
| ofatumumab | Arzerra | Glaxo Grp | 10/26/2009 | intravenous | fully human | CD20 | Chronic lymphocytic leukemia | 125326 | Link |
| olaratumab | Lartruvo | Eli Lilly | 10/19/2016 | intravenous | fully human | PDGFRA | Soft tissue sarcoma | 761038 | Link |
| omalizumab | Xolair | Genentech | 6/20/2003 | subcutaneous | humanized | IgE | Moderate to severe persistent asthma | 103976 | Link |
| palivizumab | Synagis | MedImmune | 6/19/1998 | intramuscular | humanized | F protein of RSV | Respiratory syncytial virus | 103770 | Link |
| panitumumab | Vectibix | Amgen | 9/27/2006 | intravenous | fully human | EGFR | Metastatic colorectal cancer | 125147 | Link |
| pembrolizumab | Keytruda | Merck | 9/4/2014 | intravenous | humanized | PD-1 | Metastatic melanoma | 125514 | Link |
| pertuzumab | Perjeta | Genentech | 6/8/2012 | intravenous | humanized | HER2 | Metastatic breast cancer | 125409 | Link |
| ramucirumab | Cyramza | Eli Lilly | 4/21/2014 | intravenous | fully human | VEGFR2 | Gastric cancer | 125477 | Link |
| ranibizumab | Lucentis | Genentech | 6/30/2006 | intravitreal injection | humanized | VEGFR1 VEGFR2 |
Wet age-related macular degeneration | 125156 | Link |
| raxibacumab | Raxibacumab | Human Genome Sciences | 12/24/2012 | intravenous | fully human | Protective antigen of Bacillus anthracis | Inhalational anthrax | 125349 | Link |
| reslizumab | Cinqair | Teva | 3/23/2016 | intravenous | humanized | IL5 | Severe asthma | 761033 | Link |
| rituximab | Rituxan | Genentech | 11/26/1997 | intravenous | chimeric | CD20 | B-cell non-Hodgkin's lymphoma | 103705 | Link |
| secukinumab | Cosentyx | Novartis | 1/21/2015 | subcutaneous | fully human | IL17A | Plaque psoriasis | 125504 | Link |
| siltuximab | Sylvant | Janssen Biotech | 4/23/2014 | intravenous | chimeric | IL6 | Multicentric Castleman's disease | 125496 | Link |
| tocilizumab | Actemra | Genentech | 1/8/2010 | intravenous | humanized | IL6R | Rheumatoid arthritis | 125276 | Link |
| tocilizumab | Actemra | Genentech | 10/21/2013 | intravenous subcutaneous |
humanized | IL6R | Rheumatoid arthritis Polyarticular juvenile idiopathic arthritis Systemic juvenile idiopathic arthritis |
125472 | Link |
| trastuzumab | Herceptin | Genentech | 9/25/1998 | intravenous | humanized | HER2 | Metastatic breast cancer | 103792 | Link |
| ustekinumab | Stelara | Centocor | 9/25/2009 | subcutaneous | fully human | IL12 IL23 |
Plaque psoriasis | 125261 | Link |
| ustekinumab | Stelara | Janssen Biotech | 9/23/2016 | subcutaneous intravenous |
fully human | IL12 IL23 |
Plaque psoriasis Psoriatic arthritis Crohn's disease |
761044 | Link |
| vedolizumab | Entyvio | Takeda | 5/20/2014 | intravenous | humanized | integrin receptor | Ulcerative colitis Crohn's disease |
125476 | Link |
| sarilumab | Kevzara | Sanofi Aventis | 5/22/17 | subcutaneous | fully human | IL6R | Rheumatoid arthritis | 761037 | Link |
| rituximab and hyaluronidase | Rituxan Hycela | Genentech | 6/22/17 | subcutaneous | chimeric, co-formulated | CD20 | Follicular lymphoma Diffuse large B-cell lymphoma Chronic lymphocytic leukemia |
761064 | Link |
| guselkumab | Tremfya | Janssen Biotech | 7/13/17 | subcutaneous | fully human | IL23 | Plaque psoriasis | 761061 | Link |
| inotuzumab ozogamicin | Besponsa | Wyeth | 8/17/17 | intravenous | humanized, antibody-drug conjugate | CD22 | Precursor B-cell acute lymphoblastic leukemia | 761040 | Link |
| adalimumab-adbm | Cyltezo | Boehringer Ingelheim | 8/25/17 | subcutaneous | fully human, biosimilar | TNF | Rheumatoid arthritis Juvenile idiopathic arthritis Psoriatic arthritis Ankylosing spondylitis Crohn's disease Ulcerative colitis Plaque psoriasis |
761058 | Link |
| gemtuzumab ozogamicin | Mylotarg | Wyeth | 9/1/17 | intravenous | humanized, antibody-drug conjugate | CD33 | Acute myeloid leukemia | 761060 | Link |
| bevacizumab-awwb | Mvasi | Amgen | 9/14/17 | intravenous | humanized, biosimilar | VEGF | Metastatic colorectal cancer Non-squamous Non-small-cell lung carcinoma Glioblastoma Metastatic renal cell carcinoma Cervical cancer |
761028 | Link |
| benralizumab | Fasenra | Astrazeneca | 11/14/17 | subcutaneous | humanized | interleukin-5 receptor alpha subunit | Severe asthma, eosinophilic phenotype | 761070 | Link |
| emicizumab-kxwh | Hemlibra | Genentech | 11/16/17 | subcutaneous | humanized, bispecific | Factor IXa, Factor X | Hemophilia A (congenital Factor VIII deficiency) with Factor VIII inhibitors. | 761083 | Link |
| trastuzumab-dkst | Ogivri | Mylan | 12/1/17 | intravenous | humanized, biosimilar | HER2 | HER2-overexpressing breast cancer, metaststic gastric or gastroesophageal junction adenocarcinoma | 761074 | Link |
| infliximab-qbtx | Ixifi | Pfizer | 12/13/17 | intravenous | chimeric, biosimilar | TNF | Crohn's disease Ulcerative colitis Rheumatoid arthritis Ankylosing spondylitis Psoriatic arthritis Plaque psoriasis |
761072 | Link |
| ibalizumab-uiyk | Trogarzo | TaiMed Biologics | 3/6/18 | intravenous | humanized | CD4 | HIV | 761065 | Link |
| tildrakizumab-asmn | Ilumya | Merck | 3/20/18 | subcutaneous | humanized | IL23 | Plaque psoriasis | 761067 | Link |
| burosumab-twza | Crysvita | Ultragenyx | 4/17/18 | subcutaneous | fully human | FGF23 | X-linked hypophosphatemia | 761068 | Link |
| erenumab-aooe | Aimovig | Amgen | 5/17/18 | subcutaneous | fully human | CGRP receptor | Migraine headache prevention | 761077 | Link |
Tositumomab - Bexxar - 2003 - CD20
Mogamulizumab - Poteligeo - August 2018 - CCR4
Moxetumomab pasudotox - Lumoxiti - Sept 2018 - CD22
Cemiplimab - Libtayo - Sept 2018 - PD-1
Polatuzumab vedotin - Polivy - June 2019 - CD79B
Recently, the bispecific antibodies, a novel class of therapeutic antibodies, have yielded promising results in clinical trials. In April 2009, the bispecific antibody catumaxomab was approved in the European Union.[87][88]
Economics
[編輯]Since 2000, the therapeutic market for monoclonal antibodies has grown exponentially. In 2006, the 「big 5」 therapeutic antibodies on the market were bevacizumab, trastuzumab (both oncology), adalimumab, infliximab (both autoimmune and inflammatory disorders, 『AIID』) and rituximab (oncology and AIID) accounted for 80% of revenues in 2006. In 2007, eight of the 20 best-selling biotechnology drugs in the U.S. are therapeutic monoclonal antibodies.[89] This rapid growth in demand for monoclonal antibody production has been well accommodated by the industrialization of mAb manufacturing.[90]
結核病
[編輯]色素沉着絨毛結節性滑膜炎
[編輯]Signs and symptoms
[編輯]In general, pigmented villonodular synovitis often manifests initially as sudden onset, unexplained joint swelling and pain; the joint swelling is disproportionate to the amount of pain the patient feels at first. Decreased motion and increased pain occur as the disease state progresses as well as locking of the joint. The localized form often manifests initially as a painless, slow-growing mass and progresses to the other common symptoms of PVNS. The swelling often feels warm to the touch.[91] Diffuse PVNS symptoms are often confused with those of rheumatoid arthritis.[92] While pigmented villonodular synovitis can occur in both pediatric and geriatric patients, it is more common with ages 20–50.[91]
Complications
[編輯]PVNS is locally aggressive and can spread to surrounding tissues, causing bone erosion and tissue damage. If not treated early, it can spread to areas outside the joint, extra-articular, and potentially cause permanent loss of range as well as intense pain.[93][94] The disorder also has, on average, a 45% rate of recurrence.[95]
Causes
[編輯]The exact cause is unknown. Some doctors believe it is caused by abnormal metabolism of fat. Others think it may be caused by repetitive inflammation. Some feel that blood within the joint may cause the inflammatory change.[96] Risk factors for PVNS developing are not yet understood. However, a common theme is a trauma experienced to the joint prior to the onset of symptoms.[97]
Diagnosis
[編輯]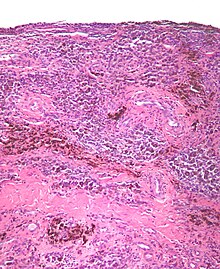
PVNS is radiologically diagnosed by magnetic resonance imaging (MRI). The disorder is difficult to identify and is often not diagnosed for four years or more after presentation due to nonspecific symptoms or a general paucity of symptoms.[95]
Classification
[編輯]Pigmented villonodular synovitis, described distinctly in 1941 by Charles J. Sutro, L. Lichtenstein, and H.L. Jafe,[95] comes in two forms: localized and diffuse. Diffuse PVNS affects the entire synovium and typically occurs in large joints such as the knee or hip. Localized, or nodular, PVNS is less common than the diffuse form and typically occurs in smaller joints such as the hands and feet. Localized PVNS often arises in the form of a large benign tumour on the tendon sheaths of the joint.[91] As the tumor grows in the joint, it damages the surrounding bone and tissues.[92] Localized PVNS is predominantly found in females and is frequently found in the fingers. Although rare, localized PVNS may develop in large joints. In either case, the knee is the most commonly affected joint (80% of cases), followed by the hip, and less commonly the ankles and shoulders.[91] PVNS is generally found more in men than women.[98] 2 cases per million population; incidence of the localized form is 9 cases per million.
Pathology
[編輯]The synovial fluid of the joint is often grossly hemorrhagic.[99]
PVNS, under the microscope, looks as the name of the condition suggests; it is composed of nodules and/or villi and has an abundant number of (pigmented) hemosiderin-laden macrophages.
Treatment
[編輯]Once PVNS is confirmed by biopsy of the synovium of an affected joint, an arthroscopic or open synovectomy of the affected area is the most common treatment. Bone lesions caused by the disorder are removed and bone grafting is performed as needed. Because diffuse PVNS has a relatively high rate of recurrence, radiation therapy or chemotherapy may be considered as a treatment option. In some cases, a total joint replacement is needed to relieve symptoms when PVNS causes significant joint destruction.[100]
費爾蒂症候群
[編輯]Signs and symptoms
[編輯]The symptoms of Felty's syndrome are similar to those of rheumatoid arthritis. Patients suffer from painful, stiff, and swollen joints, most commonly in the joints of the hands, feet, and arms. In some affected individuals, Felty's syndrome may develop during a period when the symptoms and physical findings associated with rheumatoid arthritis have subsided or are not present; in this case, Felty's syndrome may remain undiagnosed. In more rare instances, the development of Felty's syndrome may precede the development of the symptoms and physical findings associated with rheumatoid arthritis.
Felty's syndrome is also characterized by an abnormally enlarged spleen (splenomegaly) and abnormally low levels of certain white blood cells (neutropenia). As a result of neutropenia, affected individuals are increasingly susceptible to certain infections. Keratoconjunctivitis sicca may occur due to secondary Sjögren's syndrome. Individuals with Felty's syndrome may also experience fever, weight loss, and/or fatigue. In some cases, affected individuals may have discoloration (abnormal brown pigmentation) of the skin, particularly of the leg, sores (ulcers) on the lower leg, and/or an abnormally large liver (hepatomegaly). In addition, affected individuals may have abnormally low levels of circulating red blood cells (anemia), a decrease in circulating blood platelets that assist in blood clotting functions (thrombocytopenia), abnormal liver function tests and/or inflammation of the blood vessels (vasculitis).[101]
Complications
[編輯]- Recurrent infection
- Enlargement of the spleen causing too few red blood cells and platelets in the bloodstream
- Enlarged lymph nodes
- Skin hyperpigmentation
- Cutaneous ulceration
Causes
[編輯]The cause of Felty's syndrome is unknown, but it has been found to be more common in those with chronic rheumatoid arthritis. Some patients share the HLA-DR4 serotype. This syndrome is mostly present in people having extra articular manifestations of rheumatoid arthritis. People with this syndrome are at risk of infection because they have a low white blood cell count.[102]
Mechanism
[編輯]The underlying pathogenesis of Felty's syndrome is not clear.
Rheumatoid Arthritis
[編輯]
Rheumatoid arthritis is an autoimmune disease that is characterized by inflammation of the synovial joints due to attack by the body's own immune system. In this condition, the white blood cells travel through the blood stream to the synovial joints and release pro-inflammatory cytokines upon arrival. The result of this chemical release causes the synovial cells to release harmful chemicals in response as well as begin the growth of new blood vessels, forming a pannus. The pannus receives blood supply from the newly formed vessels and grows inward, invading the articular cartilage and bone within the joint. The damage to the once healthy tissue causes inflammation and ultimately fluid build-up in the joint. An accumulation of fluid results and the joints swell, slowly decreasing the space that keeps the bones from touching. If this condition is not treated, the joint space will completely narrow, causing ankylosis. At the advanced stage of ankylosis, joint mobility is completely occluded. Early presentation is commonly seen in the joints of hands and of the feet. As the disease progresses it can be seen in the knees, wrists, hips, and shoulders. This condition can affect and damage several other body systems such as the eyes, heart, lungs, and blood vessels.[103]
Rheumatoid arthritis is a condition that cannot be cured but symptoms can be treated using certain medications alone or in conjunction. Due to the increased inflammatory response of the body's immune system, this condition can cause a reduction in red and white blood cells.[104]
Neutropenia
[編輯]In Felty's syndrome, chronic activation of neutrophils progresses to neutropenia and unabated infections.[26] Neutropenia is a decreased concentration of neutrophils in the blood. Neutrophils are the most abundant cells among white blood cells and play an important role in the immune system by destroying bacteria via phagocytosis. Inflammatory chemicals draw neutrophils to the area where they congregate and fight infection. A decrease in the number of neutrophils stimulates an autoimmune response which leads to arthritis. The loss and destruction of neutrophils leading to neutropenia is therefore, inflammation-driven due to the body's need for the immune response.[26]
Splenomegaly
[編輯]Splenomegaly is a condition of the spleen causing it to be enlarged. The splenic condition involving Felty syndrome is more specifically noted as inflammatory splenomegaly. The spleen is an important lymphatic organ that is involved in filtration of the blood by discarding old and damaged red blood cells as well as maintaining platelet levels. The spleen is a lymphatic organ, which means it is largely involved in the immune system and immune responses. When the spleen becomes enlarged, it is a strong sign of infection somewhere in the body, and can be caused by inflammatory conditions such as rheumatoid arthritis. The increased need for production assistance of white blood cells to affected areas causes hyperfunction of the spleen. This increase in defense activities ultimately causes hypertrophy of the spleen, leading to splenomegaly.[105] The spleen is found in the left upper quadrant (LUQ) of the peritoneal cavity and due to its enlargement, can cause stress on neighboring organs.
Diagnosis
[編輯]This condition affects less than 1% of patients with rheumatoid arthritis.[106] The presence of three conditions: rheumatoid arthritis, an enlarged spleen (splenomegaly), and an abnormally low white blood cell count are indications that Felty's syndrome is possibly occurring. This condition as a whole is difficult to diagnose due to its complexity given a combination of disorders. It is commonly overlooked or misdiagnosed as other conditions (e.g., leukemia, systemic lupus erythrematosus)[25] because of the rarity and lack of good understanding about it. An acronym can be used to make recognizing this disease somewhat easier:
S: Splenomegaly
A: Anemia
N: Neutropenia
A: Arthritis (rheumatoid)
Conditions of the Blood
[編輯]A complete blood count (CBC) can be done to diagnose anemia (normochromic, normocytic), thrombocytopenia, and neutropenia.[107] Abnormal liver function tests are commonly used to help in diagnosis as the spleen and liver are strongly affected by one another.
Splenomegaly
[編輯]If rheumatoid arthritis is present and other symptoms occur that are not commonly found within RA itself, such as a palpable spleen, further testing should be done. A palpable spleen is not always a clinical significance, therefore CT scan, MRI, or ultrasound can be administered in order to help diagnose the condition. According to Poulin et al, dimensional guidelines for diagnosing splenomegaly are as follows:[108]
- Moderate if the largest dimension is 11–20 cm
- Severe if the largest dimension is greater than 20 cm
Rheumatoid Arthritis
[編輯]RA in patients with Felty's syndrome is chronic (after 10–15 years), and presents with increased severity along with extra articular manifestations.[26] RA can be mistaken for other conditions such as gout if not clinically diagnosed. Diagnosis can be confirmed by use of X-rays or synovial fluid analysis.[109]
Treatment
[編輯]There is no real treatment for Felty's syndrome, rather the best method in management of the disease is to control the underlying rheumatoid arthritis. Immunosuppressive therapy for RA often improves granulocytopenia and splenomegaly; this finding reflects the fact that Felty's syndrome is an immune-mediated disease. A major challenge in treating FS is recurring infection caused by neutropenia. Therefore, in order to decide upon and begin treatment, the cause and relationship of neutropenia with the overall condition must be well understood.[26] Most of the traditional medications used to treat RA have been used in the treatment of Felty's syndrome. No well-conducted, randomized, controlled trials support the use of any single agent. Most reports on treatment regimens involve small numbers of patients.[110]
Splenectomy may improve neutropenia in severe disease.
Use of rituximab[111] and leflunomide[112] have been proposed.
幼年特發性關節炎
[編輯]
參考文獻
[編輯]- ^ 1.0 1.1 Tolerance and Autoimmunity[失效連結]
- ^ Edwards JC, Cambridge G, Abrahams VM. Do self perpetuating B lymphocytes drive human autoimmune disease?. Immunology. 1999, 97 (2): 1868–1876. PMC 2326840
 . PMID 10447731. doi:10.1046/j.1365-2567.1999.00772.x.
. PMID 10447731. doi:10.1046/j.1365-2567.1999.00772.x.
- ^ Grammatikos A, Tsokos G. Immunodeficiency and autoimmunity: lessons from systemic lupus erythematosus. Trends in Molecular Medicine. 2012, 18 (2): 101–108. PMC 3278563
 . PMID 22177735. doi:10.1016/j.molmed.2011.10.005.
. PMID 22177735. doi:10.1016/j.molmed.2011.10.005.
- ^ Klein J, Sato A. The HLA system. Second of two parts. New England Journal of Medicine. September 2000, 343 (11): 782–6. PMID 10984567. doi:10.1056/NEJM200009143431106.
- ^ Gregersen, Peter K.; Olsson, Lina M. Recent Advances in the Genetics of Autoimmune Disease. Annual Review of Immunology. 2009-01-01, 27: 363–391. PMC 2992886
 . PMID 19302045. doi:10.1146/annurev.immunol.021908.132653.
. PMID 19302045. doi:10.1146/annurev.immunol.021908.132653.
- ^ 6.0 6.1 6.2 6.3 6.4 6.5 Everyday Health > Women and Autoimmune Disorders By Krisha McCoy. Medically reviewed by Lindsey Marcellin, MD, MPH. Last Updated: 12/02/2009
- ^ Ainsworth, Claire (Nov. 15, 2003). The Stranger Within. New Scientist (subscription). (reprinted here [1])
- ^ Theory: High autoimmunity in females due to imbalanced X chromosome inactivation: [2]
- ^ Uz E, Loubiere LS, Gadi VK, et al. Skewed X-chromosome Inactivation in Scleroderma. Clinical Reviews in Allergy & Immunology. June 2008, 34 (3): 352–5. PMC 2716291
 . PMID 18157513. doi:10.1007/s12016-007-8044-z.
. PMID 18157513. doi:10.1007/s12016-007-8044-z.
- ^ Saunders K, Raine T, Cooke A, Lawrence C. Inhibition of Autoimmune Type 1 Diabetes by Gastrointestinal Helminth Infection. Infection and Immunity. 2007, 75 (1): 397–407. PMC 1828378
 . PMID 17043101. doi:10.1128/IAI.00664-06.
. PMID 17043101. doi:10.1128/IAI.00664-06.
- ^ Parasite Infection May Benefit Multiple Sclerosis Patients. sciencedaily.com.
- ^ Wållberg M, Harris R. Co-infection with Trypanosoma brucei brucei prevents experimental autoimmune encephalomyelitis in DBA/1 mice through induction of suppressor APCs. International Immunology. 2005, 17 (6): 721–8. PMID 15899926. doi:10.1093/intimm/dxh253.
- ^ Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nature Reviews Immunology. 2006, 6 (5): 394–403. PMID 16622478. doi:10.1038/nri1838.
- ^ Kubach J, Becker C, Schmitt E, Steinbrink K, Huter E, Tuettenberg A, Jonuleit H. Dendritic cells: sentinels of immunity and tolerance. International Journal of Hematology. 2005, 81 (3): 197–203. PMID 15814330. doi:10.1532/IJH97.04165.
- ^ Induction of autoantibodies against tyrosinase-related proteins following DNA vaccination: Unexpected reactivity to a protein paralogue 互聯網檔案館的存檔,存檔日期May 3, 2008,. Roopa Srinivasan, Alan N. Houghton, and Jedd D. Wolchok
- ^ Green R.S.; Stone E.L.; Tenno M.; Lehtonen E.; Farquhar M.G.; Marth J.D. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis (PDF). Immunity. 2007, 27 (2): 308–320. PMID 17681821. doi:10.1016/j.immuni.2007.06.008.
- ^ 17.0 17.1 McGonagle, D; McDermott, MF. A proposed classification of the immunological diseases.. PLOS Medicine. Aug 2006, 3 (8): e297. PMC 1564298
 . PMID 16942393. doi:10.1371/journal.pmed.0030297.
. PMID 16942393. doi:10.1371/journal.pmed.0030297.
- ^ Nikoopour E, Schwartz JA, Singh B. Therapeutic benefits of regulating inflammation in autoimmunity. Inflammation & Allergy Drug Targets. 2008, 7 (3): 203–210. PMID 18782028. doi:10.2174/187152808785748155.
- ^ 19.0 19.1 Zaccone P, Fehervari Z, Phillips JM, Dunne DW, Cooke A. Parasitic worms and inflammatory diseases. Parasite Immunology. 2006, 28 (10): 515–23. PMC 1618732
 . PMID 16965287. doi:10.1111/j.1365-3024.2006.00879.x.
. PMID 16965287. doi:10.1111/j.1365-3024.2006.00879.x.
- ^ Dunne DW, Cooke A. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nature Reviews Immunology. 2005, 5 (5): 420–6. PMID 15864275. doi:10.1038/nri1601.
- ^ Dittrich AM, Erbacher A, Specht S, et al. Helminth Infection with Litomosoides sigmodontis Induces Regulatory T Cells and Inhibits Allergic Sensitization, Airway Inflammation, and Hyperreactivity in a Murine Asthma Model. The Journal of Immunology. 2008, 180 (3): 1792–9. PMID 18209076. doi:10.4049/jimmunol.180.3.1792.
- ^ Wohlleben G, Trujillo C, Müller J, et al. Helminth infection modulates the development of allergen-induced airway inflammation. International Immunology. 2004, 16 (4): 585–96. PMID 15039389. doi:10.1093/intimm/dxh062.
- ^ Quinnell RJ, Bethony J, Pritchard DI. The immunoepidemiology of human hookworm infection. Parasite Immunology. 2004, 26 (11–12): 443–54. PMID 15771680. doi:10.1111/j.0141-9838.2004.00727.x.
- ^ Holick, Michael. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. The American Journal of Clinical Nutrition. December 2004, 80 (6): 1678S–1688S. PMID 15585788. doi:10.1093/ajcn/80.6.1678S.
- ^ 25.0 25.1 25.2 25.3 25.4 25.5 Yang, Chen-Yen; Leung, Patrick S. C.; Adamopoulos, Iannis E.; Gershwin, M. Eric. The Implication of Vitamin D and Autoimmunity: a Comprehensive Review. Clinical Reviews in Allergy & Immunology. 2013-10-01, 45 (2): 217–226. PMC 6047889
 . PMID 23359064. doi:10.1007/s12016-013-8361-3 (英語). 引用錯誤:帶有name屬性「:0」的
. PMID 23359064. doi:10.1007/s12016-013-8361-3 (英語). 引用錯誤:帶有name屬性「:0」的<ref>標籤用不同內容定義了多次 - ^ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 Dankers, Wendy; Colin, Edgar M.; Hamburg, Van; Piet, Jan; Lubberts, Erik. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Frontiers in Immunology. 2017, 7: 697. PMC 5247472
 . PMID 28163705. doi:10.3389/fimmu.2016.00697 (English). 引用錯誤:帶有name屬性「:1」的
. PMID 28163705. doi:10.3389/fimmu.2016.00697 (English). 引用錯誤:帶有name屬性「:1」的<ref>標籤用不同內容定義了多次 - ^ 27.0 27.1 27.2 Agmon-Levin, Nancy; Theodor, Emanuel; Segal, Ramit Maoz; Shoenfeld, Yehuda. Vitamin D in Systemic and Organ-Specific Autoimmune Diseases. Clinical Reviews in Allergy & Immunology. 2013-10-01, 45 (2): 256–266. PMID 23238772. doi:10.1007/s12016-012-8342-y (英語).
- ^ 28.0 28.1 Simopoulos, Artemis. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. Journal of the American College of Nutrition. 2002, 21 (6): 495–505. PMID 12480795. doi:10.1080/07315724.2002.10719248.
- ^ Matsuzaki, Takeshi; Akimitsu Takagi; Haruo Ikemura; Tetsuya Matsuguchi; Teruo Yokokura. Intestinal Microflora: Probiotics and Autoimmunity. The Journal of Nutrition. March 2007, 137 (3): 798S–802S. PMID 17311978. doi:10.1093/jn/137.3.798S.
- ^ Uusitalo, Liisa; Mike G Kenward; Suvi M Virtanen; Ulla Uusitalo; Jaakko Nevalainen; Sari Niinistö; Carina Kronberg-Kippilä; Marja-Leena Ovaskainen; Liisa Marjamäki; Olli Simell; Jorma Ilonen; Riitta Veijola; Mikael Knip. Intake of antioxidant vitamins and trace elements during pregnancy and risk of advanced beta cell autoimmunity in the child. The American Journal of Clinical Nutrition. August 2008, 88 (2): 458–464. PMID 18689383. doi:10.1093/ajcn/88.2.458.
- ^ 31.00 31.01 31.02 31.03 31.04 31.05 31.06 31.07 31.08 31.09 31.10 31.11 31.12 31.13 31.14 31.15 31.16 31.17 31.18 31.19 Autoimmune diseases fact sheet. OWH. 16 July 2012 [5 October 2016]. (原始內容存檔於5 October 2016).
- ^ 32.0 32.1 Katz, U; Shoenfeld, Y; Zandman-Goddard, G. Update on intravenous immunoglobulins (IVIg) mechanisms of action and off- label use in autoimmune diseases.. Current Pharmaceutical Design. 2011, 17 (29): 3166–75. PMID 21864262. doi:10.2174/138161211798157540.
- ^ 33.0 33.1 33.2 33.3 33.4 Borgelt, Laura Marie. Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. 2010: 579. ISBN 9781585281947. (原始內容存檔於2017-09-08) (英語).
- ^ 34.0 34.1 Hohlfeld, Reinhard; Dornmair, Klaus; Meinl, Edgar; Wekerle, Hartmut. The search for the target antigens of multiple sclerosis, part 1: Autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. The Lancet Neurology. 2016, 15 (2): 198–209. PMID 26724103. doi:10.1016/S1474-4422(15)00334-8.
- ^ Paniker, Ananthanarayan And. Ananthanarayan and Paniker's Textbook of Microbiology. Orient Blackswan. 2005: 169. ISBN 9788125028086. (原始內容存檔於2017-09-08) (英語).
- ^ Autoimmune disorders: MedlinePlus Medical Encyclopedia. www.nlm.nih.gov. [2016-01-21]. (原始內容存檔於2016-01-12).
- ^ Walsh, SJ; Rau, LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States.. American Journal of Public Health. September 2000, 90 (9): 1463–6. PMC 1447637
 . PMID 10983209. doi:10.2105/ajph.90.9.1463.
. PMID 10983209. doi:10.2105/ajph.90.9.1463.
- ^ MedlinePlus medical encyclopedia – autoimmune disorders. National Institutes of Health. 16 July 2014 [21 December 2014]. (原始內容存檔於5 January 2015).
- ^ Autoimmune Disease List • AARDA. AARDA. 2016-06-01 [2019-03-21] (美國英語).
- ^ Cotsapas C, Hafler DA. Immune-mediated disease genetics: the shared basis of pathogenesis. Trends in Immunology. 2013, 34 (1): 22–6. PMID 23031829. doi:10.1016/j.it.2012.09.001.
- ^ Harrison's Principles of Internal Medicine: Volumes 1 and 2, 18th Edition 18. McGraw-Hill Professional. 2011-08-11. ISBN 9780071748896 (English).
- ^ Gammon G, Sercarz E. How some T cells escape tolerance induction. Nature. 1989, 342 (6246): 183–5. Bibcode:1989Natur.342..183G. PMID 2478888. doi:10.1038/342183a0.
- ^ Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein.. Cell. 1995, 80 (5): 695–705. PMID 7534214. doi:10.1016/0092-8674(95)90348-8.
- ^ Maverakis E, Kim K, Shimoda M, Gershwin M, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity. J Autoimmun. 2015, 57 (6): 1–13. PMC 4340844
 . PMID 25578468. doi:10.1016/j.jaut.2014.12.002.
. PMID 25578468. doi:10.1016/j.jaut.2014.12.002.
- ^ Rook, Graham A. W. Hygiene Hypothesis and Autoimmune Diseases. Clinical Reviews in Allergy & Immunology. 17 November 2011, 42 (1): 5–15. PMID 22090147. doi:10.1007/s12016-011-8285-8.
- ^ Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J. Am. Med. Assoc. 1957, 164 (13): 1439–47. PMID 13448890. doi:10.1001/jama.1957.02980130015004.
- ^ Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol. Today. September 1993, 14 (9): 426–30. PMID 8216719. doi:10.1016/0167-5699(93)90244-F.
- ^ Jacobson, DL; Gange, SJ; Rose, NR; Graham, NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States.. Clinical Immunology and Immunopathology. September 1997, 84 (3): 223–43. PMID 9281381. doi:10.1006/clin.1997.4412.
- ^ 49.0 49.1 Hayter, SM; Cook, MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease.. Autoimmunity Reviews. August 2012, 11 (10): 754–65. PMID 22387972. doi:10.1016/j.autrev.2012.02.001.
- ^ Rose, NR; Bona, C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunology Today. September 1993, 14 (9): 426–30. PMID 8216719. doi:10.1016/0167-5699(93)90244-F.
- ^ Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. Nov 2009, 15 (11): 1266–72. PMC 2783696
 . PMID 19838202. doi:10.1038/nm.2048.
. PMID 19838202. doi:10.1038/nm.2048.
- ^ Roszer T, Menéndez-Gutiérrez MP, Lefterova MI, Alameda D, Núñez V, Lazar MA, Fischer T, Ricote M. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J Immunol. Jan 2011, 186 (1): 621–31. PMC 4038038
 . PMID 21135166. doi:10.4049/jimmunol.1002230.
. PMID 21135166. doi:10.4049/jimmunol.1002230.
- ^ Singh RP, Waldron RT, Hahn BH. Genes, tolerance and systemic autoimmunity. Autoimmunity Reviews. 2012, 11 (9): 664–9. PMC 3306516
 . PMID 22155015. doi:10.1016/j.autrev.2011.11.017.
. PMID 22155015. doi:10.1016/j.autrev.2011.11.017.
- ^ Böhm I. Disruption of the cytoskeleton after apoptosis induction by autoantibodies. Autoimmunity. 2003, 36 (3): 183–9. PMID 12911286. doi:10.1080/0891693031000105617.
- ^ Swart, JF; Delemarre, EM; van Wijk, F; Boelens, JJ; Kuball, J; van Laar, JM; Wulffraat, NM. Haematopoietic stem cell transplantation for autoimmune diseases.. Nature Reviews. Rheumatology. April 2017, 13 (4): 244–256. PMID 28228650. doi:10.1038/nrrheum.2017.7.
- ^ Moticka, Edward J. Historical perspective on evidence-based immunology. Elsevier Science Publishing. 2013: 300. ISBN 9780123983817.
- ^ Janeway, Charles; Paul Travers; Mark Walport; Mark Shlomchik. Immunobiology; Fifth Edition. New York and London: Garland Science. 2001. ISBN 978-0-8153-4101-7.
- ^ 58.0 58.1 Janeway CA, Jr.; et al. Immunobiology 6th. Garland Science. 2005. ISBN 978-0-443-07310-6.
- ^ 59.0 59.1 Baxter, David. Active and passive immunity, vaccine types, excipients and licensing. Occupational Medicine. December 2007, 57 (8): 552–6. PMID 18045976. doi:10.1093/occmed/kqm110.
- ^ Modified from Carter P. Improving the efficacy of antibody-based cancer therapies. Nature Reviews. Cancer. November 2001, 1 (2): 118–29. PMID 11905803. doi:10.1038/35101072.
- ^ Prof FC Breedveld. Therapeutic monoclonal antibodies. Lancet. 2000, 355 (9205): 735–740. PMID 10703815. doi:10.1016/S0140-6736(00)01034-5.
- ^ Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. August 1975, 256 (5517): 495–7. Bibcode:1975Natur.256..495K. PMID 1172191. doi:10.1038/256495a0.
- ^ Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, Antman KH, Schlossman SF. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Research. September 1980, 40 (9): 3147–54. PMID 7427932.
- ^ Ritz J, Schlossman SF. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. January 1982, 59 (1): 1–11. PMID 7032624. doi:10.1182/blood.V59.1.1.1.
- ^ 65.0 65.1 65.2 Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: present and promise. Critical Reviews in Oncology/Hematology. April 2005, 54 (1): 11–29. PMID 15780905. doi:10.1016/j.critrevonc.2004.10.011.
- ^ 66.0 66.1 66.2 Hudson PJ, Souriau C. Engineered antibodies. Nature Medicine. January 2003, 9 (1): 129–34. PMID 12514726. doi:10.1038/nm0103-129.
- ^ Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proceedings of the National Academy of Sciences of the United States of America. May 1992, 89 (10): 4285–9. Bibcode:1992PNAS...89.4285C. PMC 49066
 . PMID 1350088. doi:10.1073/pnas.89.10.4285.
. PMID 1350088. doi:10.1073/pnas.89.10.4285.
- ^ Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, Jardieu PM. Humanization of an antibody directed against IgE. Journal of Immunology. September 1993, 151 (5): 2623–32. PMID 8360482.
- ^ Chothia C, Lesk AM, Tramontano A, Levitt M, Smith-Gill SJ, Air G, Sheriff S, Padlan EA, Davies D, Tulip WR. Conformations of immunoglobulin hypervariable regions. Nature. 1989, 342 (6252): 877–83. Bibcode:1989Natur.342..877C. PMID 2687698. doi:10.1038/342877a0.
- ^ 引用錯誤:沒有為名為
Waldmann的參考文獻提供內容 - ^ Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. mAbs. July–August 2009, 1 (4): 332–8. PMC 2726606
 . PMID 20073133. doi:10.4161/mabs.1.4.9122.
. PMID 20073133. doi:10.4161/mabs.1.4.9122.
- ^ Chapman K, Pullen N, Coney L, Dempster M, Andrews L, Bajramovic J, Baldrick P, Buckley L, Jacobs A, Hale G, Green C, Ragan I, Robinson V. Preclinical development of monoclonal antibodies: considerations for the use of non-human primates. mAbs. 2009, 1 (5): 505–16. PMC 2759500
 . PMID 20065651. doi:10.4161/mabs.1.5.9676.
. PMID 20065651. doi:10.4161/mabs.1.5.9676.
- ^ Vennepureddy A, Singh P, Rastogi R, Atallah JP, Terjanian T. Evolution of ramucirumab in the treatment of cancer - A review of literature. Journal of Oncology Pharmacy Practice. June 2016, 23 (7): 525–539. PMID 27306885. doi:10.1177/1078155216655474.
- ^ de Zwart, Verena; Gouw, Samantha C; Meyer-Wentrup, Friederike AG. Antibody therapies for lymphoma in children. Cochrane Database of Systematic Reviews. 2016-01-19. ISSN 1465-1858. doi:10.1002/14651858.cd011181.pub2.
- ^ 75.0 75.1 Rang, H. P. Pharmacology. Edinburgh: Churchill Livingstone. 2003: 241. ISBN 978-0-443-07145-4.
- ^ 76.0 76.1 Pul, Refik; Dodel, Richard; Stangel, Martin. Antibody-based therapy in Alzheimer's disease. Expert Opinion on Biological Therapy. March 2011, 11 (3): 343–357. PMID 21261567. doi:10.1517/14712598.2011.552884.
- ^ 77.0 77.1 77.2 77.3 van Dyck, Christopher. Anti-Amyloid-β Monoclonal Antibodies for Alzheimer's Disease: Pitfalls and Promise. Biological Psychiatry. August 24, 2017, 83 (4): 311–319. PMC 5767539
 . PMID 28967385. doi:10.1016/j.biopsych.2017.08.010.
. PMID 28967385. doi:10.1016/j.biopsych.2017.08.010.
- ^ 78.0 78.1 78.2 78.3 78.4 Panza, F.; Imbimbo, B. P.; Logroscino, G. Amyloid-directed monoclonal antibodies for the treatment of Alzheimer's disease: The point of no return?. Expert Opinion on Biological Therapy. 2014, 14 (10): 1465–76. PMID 24981190. doi:10.1517/14712598.2014.935332.
- ^ Hanan, Eilat; Solomon, Beka. Inhibitory effect of monoclonal antibodies on Alzheimer's P-amyloid peptide aggregation. Amyloid. January 1996, 2 (3): 130–133. doi:10.3109/13506129609014365.
- ^ Goel, Ayush. Vasogenic cerebral oedema. radiopaedia.org. [2017-11-01] (英語).
- ^ 81.0 81.1 Panza, F.; Imbimbo, B.P.; D'aOnofrio, G.; Pietrarossa, G.; Seripa, Davide; Frisardi, V. Bapineuzumab: anti-β-amyloid monoclonal antibodies for the treatment of Alzheimer's disease. V. November 2010, 2 (6): 767–82. PMID 21091109. doi:10.2217/imt.10.80.
- ^ Francis RJ, Sharma SK, Springer C, Green AJ, Hope-Stone LD, Sena L, Martin J, Adamson KL, Robbins A, Gumbrell L, O'Malley D, Tsiompanou E, Shahbakhti H, Webley S, Hochhauser D, Hilson AJ, Blakey D, Begent RH. A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. British Journal of Cancer. September 2002, 87 (6): 600–7. PMC 2364249
 . PMID 12237768. doi:10.1038/sj.bjc.6600517.
. PMID 12237768. doi:10.1038/sj.bjc.6600517.
- ^ Krauss WC, Park JW, Kirpotin DB, Hong K, Benz CC. Emerging antibody-based HER2 (ErbB-2/neu) therapeutics. Breast Disease. 2000, 11: 113–24. PMID 15687597. doi:10.3233/bd-1999-11110.
- ^ 引用錯誤:沒有為名為
sa15的參考文獻提供內容 - ^ Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. April 2015, 348 (6230): 74–80. Bibcode:2015Sci...348...74J. PMID 25838376. doi:10.1126/science.aaa6204.
- ^ Hooks MA, Wade CS, Millikan WJ. Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy. 1991, 11 (1): 26–37. PMID 1902291. doi:10.1002/j.1875-9114.1991.tb03595.x (不活躍 2019-12-20).
- ^ Chames P, Baty D. Bispecific antibodies for cancer therapy: the light at the end of the tunnel?. mAbs. 2009, 1 (6): 539–47. PMC 2791310
 . PMID 20073127. doi:10.4161/mabs.1.6.10015.
. PMID 20073127. doi:10.4161/mabs.1.6.10015.
- ^ Linke, Rolf; Klein, Anke; Seimetz, Diane. Catumaxomab: Clinical development and future directions. mAbs. 2010, 2 (2): 129–136. PMC 2840231
 . PMID 20190561. doi:10.4161/mabs.2.2.11221.
. PMID 20190561. doi:10.4161/mabs.2.2.11221.
- ^ Scolnik PA. mAbs: a business perspective. mAbs. 2009, 1 (2): 179–84. PMC 2725420
 . PMID 20061824. doi:10.4161/mabs.1.2.7736.
. PMID 20061824. doi:10.4161/mabs.1.2.7736.
- ^ Kelley B. Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs. 2009, 1 (5): 443–52. PMC 2759494
 . PMID 20065641. doi:10.4161/mabs.1.5.9448.
. PMID 20065641. doi:10.4161/mabs.1.5.9448.
- ^ 91.0 91.1 91.2 91.3 Pigmented Villonodular Synovitis 於 eMedicine
- ^ 92.0 92.1 Mayclinic. Mayo Clinic. [2007-07-15]. (原始內容存檔於2007-06-10).
- ^ Clinical Study. The Stone Clinic. [2007-08-07]. (原始內容 (web journal)存檔於2007-07-01).
- ^ Jabalameli, M; Jamshidi, K; Radi, M; Hadi, H; Bagherifard, A. Surgical outcomes of 26 patients with pigmented villonodular synovitis (PVNS) of the knee at a mean follow-up of 4 years: Introducing a novel technique. Medical Journal of the Islamic Republic of Iran. 2014, 28: 123. PMC 4313448
 . PMID 25679002.
. PMID 25679002.
- ^ 95.0 95.1 95.2 Frassica FJ, Bhimani MA, McCarthy EF, Wenz J. Pigmented villonodular synovitis of the hip and knee. Am Fam Physician. October 1999, 60 (5): 1404–10; discussion 1415. PMID 10524485.
- ^ A Patient's Guide to Pigmented Villonodular Synovitis (PVNS) of the Knee (website). Medical MultiMEDIA Group. [2013-06-30].
- ^ Chang, Chong Dr. Pigmented Villo-Nodular Synovitis (PVNS) of the Knee (web blog). HC Chang Orthopaedic Surgery. [2013-06-29].
- ^ Family Doctor.org: PVNS (web journal). [2007-07-15].
- ^ Aalberg JR, Hansen P. [Pigmented villonodular synovitis]. Ugeskrift for Læger. September 1989, 151 (38): 2413–6. PMID 2800012 (Danish).
- ^ FRANK J. FRASSICA, M.D
- ^ CIGNA – Felty's Syndrome. [2008-11-07].
- ^ HowStuffWorks "Felty's Syndrome – Medical Dictionary". [2008-11-07].
- ^ Rheumatoid arthritis - Symptoms and causes - Mayo Clinic. www.mayoclinic.org. [2017-12-13] (英語).
- ^ Rheumatoid Arthritis. Nucleus Medical Media. November 4, 2017.
- ^ Radhakrishnan N, Sacher RA, Besa EC. Splenomegaly: Etiology. MedScape. Nov 12, 2017.
- ^ Felty's Syndrome Causes, Symptoms, Diagnosis and Treatment by MedicineNet.com. [2008-11-07].
- ^ Thrombocytopenia: Diagnosis. NHI; National Heart, Lung, and Blood Institute. Nov 12, 2017.
- ^ Neetu Radhakrishnan. Splenomegaly. Medscape. 2019-10-20. Updated Apr. 2012 (referring the classification system to Poulin et al.
- ^ Eggebeen AT (2007). "Gout: an update". Am Fam Physician. 76 (6): 801–8. PMID 17910294.
- ^ Keating, Richard M. eMedicine – Felty's Syndrome. [2008-11-07].
- ^ Chandra PA, Margulis Y, Schiff C. Rituximab is useful in the treatment of Felty's syndrome. Am J Ther. 2008, 15 (4): 321–2. PMID 18645332. doi:10.1097/MJT.0b013e318164bf32.
- ^ Talip F, Walker N, Khan W, Zimmermann B. Treatment of Felty's syndrome with leflunomide. J. Rheumatol. April 2001, 28 (4): 868–70. PMID 11327265.
- ^ Michael S. Clement. Blueprints Q & As for step 2. Lippincott Williams & Wilkins. 1 June 2007: 71– [14 November 2010]. ISBN 978-0-7817-7820-6.
- ^ Almoallim H, Klinkhoff A. Longterm outcome of treatment of Felty's syndrome with intramuscular gold: case reports and recommendations for management. J. Rheumatol. January 2005, 32 (1): 20–6. PMID 15630719.
