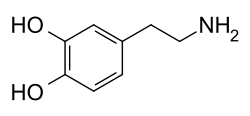
多巴胺
 多巴胺的键线式 | |
 | |
| 临床数据 | |
|---|---|
| 其他名称 |
|
| 生理学数据 | |
| 来源组织 | 黑质、腹侧被盖区等 |
| 目标组织 | 全身 |
| 受体 | D1、D2、D3、D4、D5、TAAR1[3] |
| 激动剂 | 直接:阿扑吗啡、溴隐亭 间接:可卡因、苯丙胺 |
| 拮抗剂 | 抗精神病药、甲氧氯普胺、多潘立酮 |
| 前驱物 | 苯丙氨酸、酪氨酸、L-多巴 |
| 生物合成 | 芳香族L-氨基酸脱羧酶 |
| 药物代谢 | MAO、COMT[3] |
| 识别信息 | |
| |
| CAS号 | 51-61-6 62-31-7(盐酸盐) |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.101 |
| 化学信息 | |
| 化学式 | C8H11NO2 |
| 摩尔质量 | 153.18 g·mol−1 |
| 3D模型(JSmol) | |
| |
| |
多巴胺(dopamine,DA)是一种神经调控分子和单胺类神经递质,属于儿茶酚胺和苯乙胺衍生物,占了脑中儿茶酚胺的80%。人的脑和肾脏能通过除去前体L-多巴的羧基合成多巴胺,植物和大部分动物同样能合成多巴胺。多巴胺化学名为 2-(3,4-二羟基苯基)乙胺,其英语 dopamine 是 3,4-dihydroxyphenethylamine 的缩略形式。
脑内有几条不同的多巴胺通路,其中一条在犒赏系统中发挥重要作用。大多数犒赏增加多巴胺在脑中的浓度,[4]许多成瘾药物也会增加多巴胺的分泌,或是阻止它的再摄取。[5]其它多巴胺通路则用于运动系统和控制各种激素的分泌。[5]
大众普遍认为多巴胺是产生愉悦的物质,但目前药理学研究认为多巴胺其实是记录诱因显著性的物质。[6][7][8]换句话说,多巴胺表示对某个结果的欲望或厌恶,然后推动人去使它实现,或是避免它实现。[8][9]
多巴胺在中枢神经系统以外充当局部旁分泌化学信使。在血管中,它抑制去甲肾上腺素的分泌,并使血管舒张(正常浓度下);在肾脏中,它增加钠排泄量和尿量;在胰脏中,它减少胰岛素生产;在消化系统中,它减少胃肠蠕动和保护肠胃壁;在免疫系统中,它降低淋巴细胞的活性。除了血管以外,这些多巴胺都是局部合成,局部发挥作用的。[10]
多巴胺系统的功能障碍与多种重要神经系统疾病有关,而其中一些疾病的治疗方式是改变多巴胺的作用。引起身体震颤和运动障碍的帕金森氏症是中脑黑质区中,分泌多巴胺的神经元不足所引起,而帕金森氏症最广泛使用的治疗药物L-多巴是多巴胺的代谢前体,会转化为多巴胺。有证据表明精神分裂症涉及多巴胺活性改变,因此大多数常用的抗精神病药物都是多巴胺拮抗剂,具有降低多巴胺活动的效果。[11]最有效的几种止吐剂同为多巴胺拮抗剂。不宁腿综合征与注意力不足过动症(ADHD)都与多巴胺活性降低有关。[12]高剂量多巴胺会使人上瘾,但较低剂量的多巴胺可用于治疗ADHD。多巴胺本身是静脉注射的药物,可以治疗严重的心脏衰竭或心源性休克,[13]还能治新生婴儿的低血压和败血性休克。[14]
结构
[编辑]多巴胺分子由氨基经由乙基链连接儿茶酚(有两个羟基侧基的苯环)组成。[15]因此,多巴胺是最简单的儿茶酚胺,而神经递质去甲肾上腺素和肾上腺素也同样是儿茶酚胺。[16]多巴胺中含有苯乙胺结构,因此也是苯乙胺衍生物,而许多精神药物同样是苯乙胺衍生物。[17]
多巴胺与大多数胺类似,是一种有机碱,在酸性环境中可被质子化。[18]质子化的多巴胺极易溶于水,比较稳定,但暴露于氧气或其它氧化剂下时仍会被氧化。[18]在碱性环境下,多巴胺没有被质子化,以游离碱形式存在,较难溶于水,比较活泼。[18]因为质子化的多巴胺更稳定、更易溶于水,所以用作药物的多巴胺都是它和盐酸反应产生的盐酸盐,[18]其外观为白色至黄色细粉。[19]
生物化学
[编辑]合成
[编辑]只有少部分细胞(主要是神经元和肾上腺髓质的细胞)可以合成多巴胺,[23]合成路径如下:
- 主要:L-苯丙氨酸 → L-酪氨酸 → L-多巴 → 多巴胺[20][21]
- 次要:L-苯丙氨酸 → L-酪氨酸 → 酪胺 → 多巴胺[20][21][22]
- 次要:L-苯丙氨酸 → 间酪氨酸 → 间酪胺 → 多巴胺[22][24][25]
多巴胺的直接前体L-多巴可以由必需氨基酸苯丙氨酸或是非必需氨基酸酪氨酸合成。[26]几乎所有蛋白质都含有苯丙氨酸和酪氨酸,因此很容易从食物中得到这些氨基酸。虽然食物中就有多巴胺,但因为多巴胺无法穿过血脑屏障,所以需要摄取它的前体,然后在脑中合成多巴胺。[27]
在氧气(O2)和四氢生物蝶呤作为辅因子时,L-苯丙氨酸会被苯丙氨酸羟化酶转化成L-酪氨酸;而之后四氢生物蝶呤、O2、Fe2+作为辅因子,L-酪氨酸被酪氨酸羟化酶转化成L-多巴。[26]L-多巴在芳香族L-氨基酸脱羧酶作用下,以磷酸吡哆醛为辅因子,转化为多巴胺。[26]
多巴胺是神经递质去甲肾上腺素和肾上腺素的前体。[26]多巴胺在O2和抗坏血酸作为辅因子时会被多巴胺β羟化酶转化成去甲肾上腺素,而去甲肾上腺素在S-腺苷甲硫氨酸作为辅因子时会被苯乙醇胺N-甲基转移酶转化成肾上腺素。[26]
代谢
[编辑]多巴胺会依序被单胺氧化酶(MAO)、儿茶酚-O-甲基转移酶(COMT)、醛脱氢酶(ALDH)代谢。[10]虽然多巴胺有多种代谢路径,但最终产物主要都是没有生物活性的高香草酸(HVA),会顺着血液经肾脏滤出,然后随尿液排出体外。[10]下图是多巴胺代谢成HVA的主要路径:[28]
在精神分裂症的临床研究中会测量血浆中高香草酸水平来估计脑内多巴胺水平,但这个估计方法难以分辨由去甲肾上腺素代谢产生的高香草酸。[29][30]
虽然多巴胺通常由氧化还原酶代谢,但它也可以直接和O2反应,生成醌和各种自由基。[31]反应产生的醌和自由基都会使细胞中毒,且有证据显示这就是帕金森病细胞死亡的原因。[32]
功能
[编辑]突触传导
[编辑]| 受体 | 基因 | 种类 | 机理 | |
|---|---|---|---|---|
| 类D1受体 | D1 | DRD1 | Gs偶联 | 激活腺苷酸环化酶, 增加细胞内cAMP水平 |
| D5 | DRD5 | |||
| 类D2受体 | D2 | DRD2 | Gi偶联 | 抑制腺苷酸环化酶, 降低细胞内cAMP水平 |
| D3 | DRD3 | |||
| D4 | DRD4 | |||
| TAAR | TAAR1 | TAAR1 | Gs偶联 Gq偶联 |
增加细胞内cAMP和钙水平 |
多巴胺通过结合并激活细胞表面受体来发挥其作用。[23]多巴胺在人体内会和各种多巴胺受体以及痕量胺相关受体1(hTAAR1)结合。[3][33]哺乳动物有D1至D5这五种多巴胺受体,[23]全是代谢型的G蛋白偶联受体,通过复杂的第二信使系统发挥作用。[34]这五种多巴胺受体可分为类D1受体和类D2受体。[23]激活类D1受体(D1、D5)会激活或抑制受体所在的神经元,而激活类D2受体(D2、D3、D4)则会抑制受体所在的神经元。[34]在人的神经系统中,多巴胺受体D1最多,多巴胺受体D2次之,剩下的多巴胺受体都很少。[34]
储存、释放、再摄取
[编辑]多巴胺在脑内充当神经递质和神经调节剂,受所有单胺类神经递质共有的机制控制。[23]多巴胺在合成之后,会被溶质载体VMAT2从胞质溶胶运输到突触囊泡。[35]多巴胺会储存在这些突触囊泡里,直到因胞吐作用或痕量胺相关受体TAAR1的活动而被释放到突触间隙。[33]
多巴胺会和突触中的多巴胺受体结合并激活它们。[23][36]多巴胺受体在被激活后,会产生动作电位,然后多巴胺就会离开多巴胺受体。这些多巴胺会通过多巴胺转运体或细胞膜单胺类转运体回到胞质溶胶,[37]之后部分多巴胺会被单胺氧化酶代谢,剩下的则会被VMAT2运输到突触囊泡,等待下一次释放。[35]
中枢神经系统
[编辑]
脑中的多巴胺在管控功能、运动控制、动机、唤醒、增强、犒赏系统、哺乳、性高潮、恶心中起到重要作用。多巴胺细胞群和多巴胺通路一同组成了神经调节的多巴胺系统。人脑中可以产生多巴胺的神经元很少,只有约400,000个,[38]而且它们的细胞体只出现在脑的少数区域中。[39]但是,它们的轴突可以一直延伸到脑的其它区域,而且可以对传导对象造成强大影响。[39]这些神经元最早在1964年由安妮卡·达尔斯特罗姆和谢尔·富克塞标绘出来,并给予这些区域A开头的名字。[40]在他们的模型中,A1-A7区包含去甲肾上腺素,A8-A14区则包含多巴胺。包含多巴胺的区域包括黑质(A8、A9)、腹侧被盖区(A10)、下丘脑后叶(A11)、弓状核(A12)、未定区(A13)、脑室旁核(A14)。[40]
黑质是中脑基底核的一部分。黑质中的多巴胺神经元主要出现在黑质致密部这部分(A8)和其周围(A9)。[39]它们会通过黑质纹状体通路延伸到纹状体。这条通路在运动控制和学习新的动作技能中非常重要。[41]若失去大部分此区域的多巴胺神经元,将导致帕金森氏症。[42]
腹侧被盖区(VTA)是中脑的另一部分。它的多巴胺神经元大多通过中脑皮层通路延伸到前额叶皮质,另外一小部分则通过中脑边缘通路延伸到伏隔核,[39][41]这两条通路主要和犒赏、动机的功能相关。[41]此外,VTA也有一些多巴胺神经元会将轴突延伸到杏仁核、扣带皮层、海马体、嗅球。[39][41]越来越多文献表明,多巴胺通过影响脑的多个区域,在厌恶学习中发挥着至关重要的作用。[43][44][45]
下丘脑后叶的多巴胺神经元会一直延伸到脊髓,但其功能尚未明确。[46]有证据显示这个部分的问题和不宁腿综合症(因为强烈想要让腿部移动而难以入睡的综合症)有关。[46]
弓形核和脑室旁核都位于下丘脑。它们的多巴胺神经元通过结节漏斗通路延伸到脑下垂体前叶,抑制催乳素的分泌。[47]产生催乳素的乳促细胞在没有多巴胺的情况下会不断产生催乳素,而多巴胺则会抑制催乳素的产生。[47]
位于底丘脑未定区多巴胺神经元延伸到下丘脑许多部分,参与促性腺激素释放激素的控制。青春期后生殖系统的发育需要促性腺激素释放激素的参与。[47]
眼睛视网膜中有一些可以产生多巴胺的神经元。[48]它们是无长突细胞,没有轴突。[48]它们只在白天活跃,会在细胞外液分泌多巴胺。[48]这些多巴胺可以抑制视杆细胞,同时增强视锥细胞的活动,使人在亮光下对颜色更敏感,但在光线昏暗时相反。[48]
基底核
[编辑]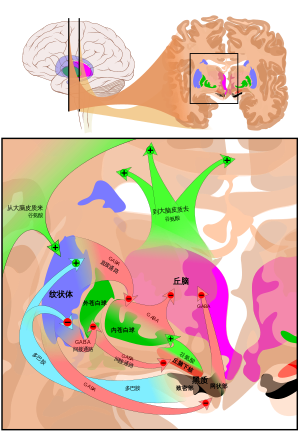
脑内最大、最重要的多巴胺来源是黑质和腹侧被盖区,它们都位于中脑,彼此密切相关且在许多方面功能相似。[39]它们都会向基底核最大的部分——纹状体[49]分泌多巴胺。[39]
目前了解基底核功能的进展缓慢,[49]最常见的假说认为基底核在行为选择扮演主要角色。[50]此假说认为当一个人或动物有多种行为可以选择执行时,基底核的活动就会决定去执行哪种行为。[51]换句话说,基底核是生物的决策系统。[51]
多巴胺会以两种方式参与行为选择的过程。首先,它决定了执行行为的阈值。[50]多巴胺活性越高,去做特定行为所需的动力就越低。[50]因此,多巴胺水平高会导致运动和冲动变多,而多巴胺水平低则会导致蛰伏及反应减慢。[50]会导致行动僵硬迟缓的帕金森病就是因黑质中多巴胺大量减少而起。[52]相反,可卡因和苯丙胺等可以增加多巴胺分泌的药物会导致多巴胺活动提高,甚至导致精神躁动和刻板症。[53]
此外,多巴胺还有“教学”的作用。[50]在选择行为后,如果多巴胺活性提升,那么基底核神经回路就会改变,使得下次出现类似情况时更容易做出相同的选择。[50]这是操作性条件反射的例子。[51]
犒赏
[编辑]
犒赏是极具诱惑的刺激,能够引导出满足欲望的行为。[54]愉悦、学习(经典条件反射和操作性条件反射)、趋向行为都是犒赏的产物。[54]愉悦是犒赏的产物,因此可通过刺激能不能带来愉悦,以确定这种刺激是不是犒赏。[54]不过,虽然所有带来愉悦的刺激都是犒赏,但有些犒赏(如钱财等外在犒赏)不会直接带来愉悦。[54][55]犒赏的动机或欲望通过它们引起的接近行为反映出来,而内在犒赏的愉悦则是得到犒赏后的结果。[54]诱因理论中区分了内在犒赏的两个成分,即反映在趋向行为的欲望,以及反映在完成行为的愉悦。[54][6][56]吸毒者的这两个成分会分离,他们对毒品的欲望越来越强,但感受到的愉悦会因为药物耐受性而越来越少。[6]
多巴胺是全脑的犒赏信号,脑对犒赏的多巴胺反应就含有显著性、价值、犒赏本身的信息。[54]此外,多巴胺还充当“犒赏预测误差”信号,即犒赏的意外程度。[54]有假说[57]认为当实际犒赏大于预测犒赏,或是得到意外的犒赏时,突触的多巴胺会短暂上升;当实际犒赏小于预测犒赏时,多巴胺的分泌水平会下降到初始值。[54]
动物大脑的微电极记录显示在有犒赏时,VTA和黑质的多巴胺神经元会变得很活跃。[54]它们与犒赏相关的认知至关重要,是犒赏系统的核心结构。[6][58][59]多巴胺在这里的作用与轴突的延伸方向有关。[6]从VTA延伸到伏隔核的壳的轴突会为各种犒赏设置其诱因显着性;从VTA延伸到前额叶皮质的轴突根据各种犒赏的诱因显着性,不断更新不同目标的价值;从VTA延伸到杏仁核或海马体的轴突巩固与犒赏相关的记忆的巩固;从VTA延伸到伏隔核的核以及从黑质延伸到纹状体的轴突则用于学习有助于获得犒赏的运动。[6][60]
愉悦
[编辑]虽然多巴胺在引起反映在趋向行为的欲望的方面很重要,但是更深入的研究发现多巴胺不能简单与反映在完成行为的愉悦画上等号,[55]因为介导愉悦的快感中心不仅存在于多巴胺系统中(如伏隔核的壳),也存在于多巴胺系统外(如腹侧苍白球和臂旁核)。[55][56][61]通过直接电击脑的多巴胺通路,许多动物都会感受到愉悦,且愿意为了得到愉悦感做事。[62]降低多巴胺水平的抗精神病药物会造成失乐,即对原本能够带来愉悦的活动失去兴趣的现象。[63]诸如性交、饮食、玩电子游戏等带来愉悦的活动都能增加多巴胺的分泌。[64]所有会上瘾的药物都会直接或间接影响伏隔核的多巴胺神经传递,[6][62]导致对这些药物的欲望增加。[56]冰毒和可卡因等兴奋剂会增加突触间隙的多巴胺水平,导致对它们的欲望增加,但感受到的愉悦并没有显著改变。[56][62]海洛因和吗啡等类阿片则不同,除了增加欲望,也会增加感受到的愉悦。[56]
2019年1月的临床研究评估了多巴胺前体L-多巴,多巴胺拮抗剂维思通,以及安慰剂对音乐上的抖颤诱发的愉悦程度的影响,发现操纵多巴胺的神经传递可以调节愉悦的认知。[65][66]该研究证明多巴胺神经传递的增加是音乐造成愉悦的必要条件。[65][66]1998年的另一研究则发现玩电子游戏时,纹状体会分泌多巴胺,而这些多巴胺与学习、行为增强、整合感觉-动作关系有关。[67]据该研究,玩电子游戏的潜在问题与人格特征有关,如低自尊、低自我效能、焦虑、攻击性,以及有抑郁症和焦虑症的临床症状。[68]
中枢神经系统以外
[编辑]因为多巴胺无法通过血脑屏障,所以脑外多巴胺的合成和功能基本独立于脑内多巴胺的合成和功能。[27]血液中含有相当量的多巴胺,但其作用尚未完全清楚。[10]人的血浆中的多巴胺水平与肾上腺素水平相近,但其中超过95%都以硫酸多巴胺的形式存在,是肠系膜的SULT1A3酶作用于多巴胺产生的。[10]血浆中的多巴胺水平在饭后可达饭前的五十倍以上,因此人体为了消除这些过量的多巴胺,就会把游离的多巴胺转化成硫酸多巴胺。[10]硫酸多巴胺没有生物作用,会随尿液排出体外。[10]
血液中剩下一小部分的游离多巴胺可能是交感神经、消化系统或其它器官合成的。[10]它们可能会和周围组织的多巴胺受体结合、被代谢掉,或是被多巴胺β羟化酶转化成去甲肾上腺素,然后通过肾上腺髓质分泌到循环系统。[10]多巴胺会和位于动脉壁的多巴胺受体结合,充当血管舒张剂。[69]颈动脉体会在低氧条件下分泌多巴胺来激活这些受体,但目前不知道这些多巴胺受体有没有其它功能。[69]
此外,多巴胺还能通过外分泌或旁分泌影响免疫系统、肾脏、胰脏。[10]
免疫系统
[编辑]免疫细胞可以制造和分泌多巴胺。[70]多巴胺可以影响脾脏、骨髓、循环系统的免疫细胞,[71]也可以和淋巴细胞上的受体结合,[70]抑制淋巴细胞的活性。这个功能的用途不明,可能是神经系统和免疫系统之间相互作用的途径,也可能与某些自身免疫性疾病相关。[71]
肾脏
[编辑]肾脏的多巴胺系统位于肾单位的细胞,其中含有所有种类的多巴胺受体。[72]肾小管的细胞可以合成多巴胺,之后分泌到肾小管液。多巴胺在此能增加肾的血液供应、提高肾功能,并增加钠离子的排泄。当肾脏的多巴胺功能缺失时,会导致钠离子的排泄减少,造成高血压。有证据表明肾脏多巴胺系统出问题会导致氧化应激、水肿、高血压等疾病。[73]基因问题或高血压有可能使肾脏多巴胺系统产生缺陷。[74]
胰脏
[编辑]多巴胺在胰脏的功能比较复杂。胰脏可分为两部分,即外分泌腺部分和内分泌腺部分。外分泌腺会合成消化酶和包括多巴胺在内的其它物质,然后分泌到小肠。[75]这些被分泌到小肠的多巴胺功能不是很明确,可能包括保护肠胃壁以及减少胃肠蠕动。[75]
胰脏的内分泌腺部分就是胰岛。它会合成胰岛素,然后分泌到循环系统。[75]有证据显示制造胰岛素的胰岛β细胞有多巴胺受体,它们受到多巴胺作用时降低胰岛素的释放。[75]这些和胰岛β细胞受体结合的多巴胺的来源还没有厘清的很清楚,可能源自交感神经系统,然后顺着血流来到胰岛,也有可能是其它胰脏细胞合成的。[75]
医疗用途
[编辑]
多巴胺是列于世界卫生组织基本药物标准清单的药物[76]。它通过静脉注射给药,最常用于治疗严重低血压、心跳过缓、心搏停止,如心肌梗死、心力衰竭所引起的心源性休克,可增加心排血量、提高心率、增强心肌收缩力,对新生儿的治疗更为重要[77][14]。由于多巴胺在血浆中的生物半衰期很短(成年人一分钟、新生婴儿两分钟、早产儿五分钟),所以注射多巴胺需要滴注[78]。
多巴胺对心血管的影响源自它对α1、β1、β2肾上腺素受体的作用[79][80],可以增加钠排泄量和尿量[78]。此外,多巴胺效应依剂量而定,低剂量会提高每搏输出量和心率,进而提高心输出量和血压[81]。更高的剂量还能造成血管收缩,进一步提高血压[81][82]。较旧的文献称极低剂量的多巴胺可在没有副作用的情况下增强肾功能,但最近的研究得出的结论认为这种剂量无效,甚至可能有害[83]。
多巴胺的副作用包括影响肾功能和心律失常[81]。多巴胺的半数致死量为59mg/kg(小鼠,静脉注射)、95mg/kg(小鼠,腹腔注射)、163mg/kg(大鼠,腹腔注射)、79mg/kg(狗,静脉注射)[84]。
疾病与药理学
[编辑]多巴胺系统和许多疾病有关,包括帕金森病、注意力不足多动症、妥瑞症、精神分裂症、双相情感障碍、成瘾。除了多巴胺以外,很多药物也可以和人体各处的多巴胺系统产生作用,其中一些被用作药品或毒品。神经化学家已开发了许多试验药物,其中一些和多巴胺受体的亲和力高,是它们的激动剂或拮抗剂。多巴胺转运体抑制剂、VMAT抑制剂、酶抑制剂等药物也都可以影响多巴胺系统。[85]
大脑老化
[编辑]许多研究发现年龄与大脑纹状体和纹外皮层[86]多巴胺合成量、多巴胺受体数量的减少有关。[87]多巴胺受体D1、D2、D3的减少已有充分记录。[88][89][90]多巴胺随年龄的减少可能与许多和年龄正相关的神经系统疾病有关,如肢体僵硬。[91]
多发性硬化症
[编辑]有研究报告称多巴胺失衡导致了多发性硬化症的疲劳症状。[92]多发性硬化症病人体内的多巴胺抑制了IL-17和IFN-γ的合成。[93]
帕金森病
[编辑]帕金森病是一种与年龄相关的疾病,其症状是身体僵硬、行动迟缓、四肢颤抖,[52]到了晚期还会发展出痴呆症,最终死亡。[52]这些症状导因于黑质里分泌多巴胺的细胞死亡。[94]这些细胞很脆弱,脑炎、多次脑震荡、MPTP中毒都可以使它们大量死亡,导致症状与帕金森病相似的帕金森综合症。[95]不过,大部分帕金森病案例都是病因不明症,无法得知细胞死亡的原因。[95]
因为L-多巴在人体内会被转化成多巴胺,[26]所以帕金森综合症最常用L-多巴治疗。[27]不直接使用多巴胺是因为它不能通过血脑屏障,但L-多巴可以。[27]它通常会和卡比多巴或苄丝肼等脱羧酶抑制剂合并使用,减少在脑外就被转化成多巴胺的量,增加进入脑内的L-多巴含量。[27]虽然长期使用L-多巴会导致异动症等副作用,但它仍是长期治疗大多数帕金森病病例的最佳选择。[27]
L-多巴无法补回已经死去的多巴胺细胞,但它可以使其它多巴胺细胞分泌更多多巴胺来弥补。[27]不过到了晚期,已经死亡的多巴胺细胞已经多到其它多巴胺细胞都无法弥补足够的多巴胺。[27]
用于治疗帕金森病的此方法有时与多巴胺失调综合症的发展有关。[96][97]
药物成瘾
[编辑]
可卡因、苯丙胺衍生物(如甲基苯丙胺)、阿得拉尔、哌甲酯以及各种兴奋剂都会通过多种机制来增加脑中的多巴胺水平,发挥作用。[98]可卡因和哌甲酯都是多巴胺再摄取抑制剂,[99]会非竞争性抑制多巴胺的再摄取,造成突触间隙的多巴胺水平增加。[100][101]:54–58苯丙胺衍生物同样可以增加突触间隙的多巴胺水平,但机理不同。[102][101]:147–150
兴奋剂会使人心率、体温、出汗增加,警觉性、注意力、耐力提高,且犒赏带来的快乐增加,但大剂量的兴奋剂会导致烦躁、焦虑,甚至与现实失去联系。[98]兴奋剂因会直接激活脑内的犒赏系统而极易成瘾,[98]但小剂量兴奋剂可以治疗注意力不足多动症(ADHD)和发作性嗜睡病。[103][104]

许多成瘾药物会增加与犒赏系统相关的多巴胺活性。[98]尼古丁、可卡因、冰毒等兴奋剂都会提升多巴胺的水平,而这似乎是导致这些药物成瘾的主要因素。不过对类阿片海洛因来说,犒赏系统中多巴胺水平的提升并非成瘾的主要因素。[105]当已经对兴奋剂上瘾的人试图戒掉它们时,他们并不会有像戒酒或戒类阿片的身体上的痛苦,而是会强烈渴望它们,产生烦躁、不安及其它由精神依赖引起的症状。[106]
多巴胺系统在成瘾机制中发挥着至关重要的作用。首先,脑内多巴胺受体的基因差别就足以预测一个人以后认为兴奋剂有吸引力,还是令人厌恶。[107]此外,在使用兴奋剂后,脑内多巴胺水平就会在接下来几分钟到几小时提高。[98]最后,长期大剂量兴奋剂会导致多巴胺慢性升高,引发脑一系列严重的结构变化,导致成瘾。[108]治疗这种药物成瘾非常困难,因为就算停止使用它们,精神依赖带来的渴望也不会停止;就算渴望看上去停止了,在面对与药物相关的刺激(如朋友、地点、情况)时仍可能重新出现。[106]
思觉失调和抗精神病药物
[编辑]1950年代初,精神科医生发现了一系列被称作典型抗精神病药物(又称主要镇静剂)的药物可以有效减轻精神分裂症患者的思觉失调症状,第一个被广泛使用的抗精神病药物氯丙嗪就让许多精神分裂症患者出院。[109]1970年代,研究者了解到这些典型抗精神病药物都是D2受体的拮抗剂。[109][110]此发现导致精神分裂症的多巴胺假说的出现,它认为精神分裂症因多巴胺功能亢进造成。[111]由于冰毒等可以增强多巴胺功能的兴奋剂会加剧思觉失调,而且正常人大量使用这些兴奋剂也会产生类似的症状,这个假说得到了更多支持。[111]
然而,之后的研究对经典的多巴胺假说提出了质疑,因为精神分裂症患者脑内的多巴胺活性通常不会有明显增加。[111]虽然如此,但是许多精神科医生和神经科学家仍然认为精神分裂症涉及到多巴胺系统的某种异常。[109]随着时间的推移,多巴胺假说演变,它所假设的各种功能障碍也往往变得越来越微妙、复杂。[109]
精神药理学家斯蒂芬·史达在2018年的一篇综述中指出在许多精神分裂症病例中,基于多巴胺、血清素、谷氨酸的三个相互关联的网络的问题导致了纹状体中的多巴胺受体D2过度兴奋。[112]
注意力不足多动症
[编辑]多巴胺神经传递的改变与注意力不足多动症(ADHD)有关。[113]多巴胺和ADHD的关系涉及到治疗ADHD用的药,因为最有效的ADHD治疗药物哌甲酯和苯丙胺都可以提升脑中多巴胺和去甲肾上腺素的水平。[114]这些药物治疗ADHD的机理是间接激动前额叶皮质的多巴胺受体和去甲肾上腺素受体,具体来说分别是多巴胺受体D1和肾上腺素受体α2。[113][115][116]
疼痛
[编辑]多巴胺会在中枢神经系统处理疼痛时发挥作用。[117]帕金森病经常出现的疼痛症状和多巴胺水平降低有关,而灼口综合症、纤维肌痛、不宁腿综合症等痛苦的疾病也都与多巴胺系统异常有关。[117]
恶心
[编辑]恶心号呕吐主要取决于脑干延髓脑极后区的活动,而这个区域也含有大量多巴胺受体D2。[118]因此,激活多巴胺受体D2的药物(如帕金森病药物及阿扑吗啡等多巴胺受体激动剂[119])很可能会导致呕吐。[118]反过来说,甲氧氯普胺等多巴胺受体D2拮抗剂则可用作止吐剂。[118]
其它生物中的多巴胺
[编辑]微生物
[编辑]至今仍没有古细菌中有多巴胺的报告,不过在某些细菌以及一种叫四膜虫的原生动物中已检测到多巴胺存在。[120]细菌和动物合成多巴胺所用的酶同源,因此有说法称动物的多巴胺合成路径来自细菌的基因水平转移,而这有可能是细菌和真核生物共生,产生线粒体的结果。[121]
动物
[编辑]大部分多细胞生物都把多巴胺当作神经递质。[122]目前只有一份关于海绵中多巴胺的报告,而且没有说明其功能。[123]不过,其它径向对称物种(如水母、水螅、珊瑚等刺胞动物)中的神经系统中都有多巴胺的存在。[124]多巴胺作为神经递质的功能可追溯到5亿年前的寒武纪。多巴胺在脊椎动物、棘皮动物、节肢动物、软体动物、某些蠕虫里都是神经递质。[125][126]
多巴胺会影响所有动物的运动。[122]它会使扁形动物螺旋运动,还能使水蛭用爬行代替游泳。多巴胺可以激活多种脊椎动物行为的转换和选择。[122][127]此外,多巴胺也会影响所有动物的犒赏系统。[122]所有脊椎动物以及线虫动物、扁形动物、软体动物、黑腹果蝇等无脊椎动物如果在做一个动作后多巴胺水平持续增加,那么都可以重复那个动作。[122]多巴胺还能调节猴子[128]和黑腹果蝇[129]的短期和长期记忆。
节肢动物中的多巴胺长期以来都被认为是例外,因为它负责的是厌恶而不是犒赏,而犒赏则是章胺调节的。[130]不过,最近的研究发现多巴胺确实在黑腹果蝇的犒赏学习中发挥作用,而章胺的功能也是因为激活了当时未发现的多巴胺神经元。[130]
植物
[编辑]
许多植物都能合成多巴胺。[131]香蕉的多巴胺含量最高,1公斤小果野蕉果肉里含有42毫克的多巴胺,而1公斤红皮蕉果肉则含有55毫克的多巴胺。鳄梨、马铃薯、西兰花、可可豆、甘蓝的多巴胺含量则较少,1公斤含有约1至7毫克的多巴胺。橙、番茄、茄子、菠菜、菜豆、豌豆的多巴胺含量更低,1公斤含有的多巴胺不到1毫克。[131]这些多巴胺都是从酪氨酸开始合成的,合成路径和动物一样。[131]它们可被代谢成黑色素和各种生物碱。[131]植物中多巴胺的功能尚不明确,但有证据表明它们会对细菌感染等应激源做出反应,有时充当生长因子,以及改变糖的代谢路径。介导这些作用的受体,以及这些受体的激活机制都尚未确定。[131]
不过,因为多巴胺无法穿过血脑屏障,所以从这些植物中摄取的多巴胺都无法被大脑所用。[27]但是,许多植物也含有多巴胺的代谢前体L-多巴。[132]黎豆属植物的L-多巴含量最高,其中的一个种——刺毛黧豆的L-多巴含量更高。[133]蚕豆的L-多巴含量也很高,但豆里的L-多巴含量要比植物的其它部分少。[134]腊肠树属和羊蹄甲属的种子也含有相当量的L-多巴。[132]
一种叫Ulvaria obscura的绿藻的多巴胺含量极高,预估占了的净重的4.4%。有证据表明这些多巴胺是它们抵御被蜗牛和等足目吃的手段。[135]
黑色素的前体
[编辑]黑色素是存在于许多生物中的一系列深色物质。[136]它们的化学性质与多巴胺相似,且多巴胺经酪氨酸酶氧化后就会产生多巴胺黑色素。[136]多巴胺黑色素不导致皮肤变黑,[136]但有证据表明黑质的黑色源自多巴胺黑色素。[137]除了人以外,其它生物中也含有多巴胺黑色素。植物中的多巴胺很可能是多巴胺黑色素的前体。[138]蝴蝶翅膀上复杂的图案,以及幼虫身上的黑白条纹也都因多巴胺黑色素所致。[139]
历史与发展
[编辑]多巴胺最早于1910年由乔治·巴格和詹姆斯·尤恩在英国伦敦惠康实验室合成,[140]之后于1957年由凯瑟琳·蒙塔古首次在人脑中鉴定出来。因为它是L-多巴合成出来的单胺,所以被命名为多巴胺。1958年,阿尔维德·卡尔森和尼尔斯-奥克·希拉普在瑞典国家心脏研究所化学药理学实验室中最先发现多巴胺作为神经递质的功能。[141]卡尔森发现多巴胺不仅是去甲肾上腺素和肾上腺素的前体,自身也是一种神经递质,因此被授予2000年诺贝尔生理学或医学奖。[142]
聚多巴胺
[编辑]于2007年对无孔贻贝生物粘合剂的研究促使了聚多巴胺的发现。大多数材料如果放入弱碱性多巴胺溶液里,就会被一层多巴胺聚合而成的聚多巴胺覆盖。[143][144]聚多巴胺因多巴胺的氧化产生,[145]通常由多巴胺盐酸盐在作为碱的三羟甲基氨基甲烷水溶液里聚合而成,结构不明。[144]聚多巴胺的性质使它有多种潜在应用,例如防止遇光损坏、输送药物的胶囊材料,甚至可作为生物传感器的基质。[145]
参考文献
[编辑]- ^ Cruickshank L, Kennedy AR, Shankland N. CSD Entry TIRZAX: 5-(2-Ammonioethyl)-2-hydroxyphenolate, Dopamine. Cambridge Structural Database: Access Structures (Cambridge Crystallographic Data Centre). 2013. doi:10.5517/cc10m9nl
 .
.
- ^ Cruickshank L, Kennedy AR, Shankland N. Tautomeric and ionisation forms of dopamine and tyramine in the solid state. J. Mol. Struct. 2013, 1051: 132–36. Bibcode:2013JMoSt1051..132C. doi:10.1016/j.molstruc.2013.08.002.
- ^ 3.0 3.1 3.2 3.3 Dopamine: Biological activity. IUPHAR/BPS guide to pharmacology. International Union of Basic and Clinical Pharmacology. [2016-01-29]. (原始内容存档于2016-02-05).
- ^ Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007-04, 191 (3): 391–431. PMID 17072591. S2CID 468204. doi:10.1007/s00213-006-0578-x.
- ^ 5.0 5.1 Wise RA, Robble MA. Dopamine and Addiction. Annual Review of Psychology. 2020-01-04, 71 (1): 79–106. PMID 31905114. S2CID 210043316. doi:10.1146/annurev-psych-010418-103337
 .
.
- ^ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Malenka RC, Nestler EJ, Hyman SE. Sydor A, Brown RY , 编. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 147–48, 366–67, 375–76. ISBN 978-0-07-148127-4.
- ^ Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. The Journal of Neuroscience. 2013-10-09, 33 (41): 16383–93. PMC 3792469
 . PMID 24107968. doi:10.1523/JNEUROSCI.1731-13.2013.
. PMID 24107968. doi:10.1523/JNEUROSCI.1731-13.2013.
- ^ 8.0 8.1 Wenzel JM, Rauscher NA, Cheer JF, Oleson EB. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chemical Neuroscience. 2015-01-21, 6 (1): 16–26. PMC 5820768
 . PMID 25491156. doi:10.1021/cn500255p.
. PMID 25491156. doi:10.1021/cn500255p. Thus, fear-evoking stimuli are capable of differentially altering phasic dopamine transmission across NAcc subregions. The authors propose that the observed enhancement in NAcc shell dopamine likely reflects general motivational salience, perhaps due to relief from a CS-induced fear state when the US (foot shock) is not delivered. This reasoning is supported by a report from Budygin and colleagues112 showing that, in anesthetized rats, the termination of tail pinch results in augmented dopamine release in the shell.
- ^ Puglisi-Allegra S, Ventura R. Prefrontal/accumbal catecholamine system processes high motivational salience. Front. Behav. Neurosci. 2012-06, 6: 31. PMC 3384081
 . PMID 22754514. doi:10.3389/fnbeh.2012.00031
. PMID 22754514. doi:10.3389/fnbeh.2012.00031  .
.
- ^ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacological Reviews. 2004-09, 56 (3): 331–49. PMID 15317907. S2CID 12825309. doi:10.1124/pr.56.3.1.
- ^ Moncrieff J. The myth of the chemical cure. A critique of psychiatric drug treatment. Basingstoke, UK: Palgrave MacMillan. 2008. ISBN 978-0-230-57432-8.
- ^ Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009-09, 302 (10): 1084–91. PMC 2958516
 . PMID 19738093. doi:10.1001/jama.2009.1308.
. PMID 19738093. doi:10.1001/jama.2009.1308.
- ^ Dopamine infusion (PDF). [2023-10-13]. (原始内容存档 (PDF)于2023-11-21).
- ^ 14.0 14.1 Shock and Hypotension in the Newborn Medication: Alpha/Beta Adrenergic Agonists, Vasodilators, Inotropic agents, Volume Expanders, Antibiotics, Other. emedicine.medscape.com. [2023-10-13]. (原始内容存档于2023-10-15).
- ^ Dopamine. PubChem. [2015-09-21]. (原始内容存档于2019-08-03).
- ^ Catecholamine. Britannica. [2015-09-21]. (原始内容存档于2015-07-13).
- ^ Phenylethylamine. ChemicalLand21.com. [2015-09-21]. (原始内容存档于2018-09-16).
- ^ 18.0 18.1 18.2 18.3 Carter JE, Johnson JH, Baaske DM. Dopamine Hydrochloride. Analytical Profiles of Drug Substances. 1982, 11: 257–72. ISBN 978-0122608117. doi:10.1016/S0099-5428(08)60266-X.
- ^ Specification Sheet. www.sigmaaldrich.com. [2019-09-13]. (原始内容存档于2020-07-30).
- ^ 20.0 20.1 20.2 Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010-03, 125 (3): 363–375. PMID 19948186. doi:10.1016/j.pharmthera.2009.11.005.
- ^ 21.0 21.1 21.2 Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol. Sci. 2005-05, 26 (5): 274–281. PMID 15860375. doi:10.1016/j.tips.2005.03.007.
- ^ 22.0 22.1 22.2 22.3 Wang X, Li J, Dong G, Yue J. The endogenous substrates of brain CYP2D. Eur. J. Pharmacol. 2014-02-05, 724: 211–218. PMID 24374199. doi:10.1016/j.ejphar.2013.12.025.
The highest level of brain CYP2D activity was found in the substantia nigra ... The in vitro and in vivo studies have shown the contribution of the alternative CYP2D-mediated dopamine synthesis to the concentration of this neurotransmitter although the classic biosynthetic route to dopamine from tyrosine is active. ... Tyramine levels are especially high in the basal ganglia and limbic system, which are thought to be related to individual behavior and emotion (Yu et al., 2003c). ... Rat CYP2D isoforms (2D2/2D4/2D18) are less efficient than human CYP2D6 for the generation of dopamine from p-tyramine. The Km values of the CYP2D isoforms are as follows: CYP2D6 (87–121 μm) ≈ CYP2D2 ≈ CYP2D18 > CYP2D4 (256 μm) for m-tyramine and CYP2D4 (433 μm) > CYP2D2 ≈ CYP2D6 > CYP2D18 (688 μm) for p-tyramine
- ^ 23.0 23.1 23.2 23.3 23.4 23.5 Seeman P. Chapter 1: Historical overview: Introduction to the dopamine receptors. Neve K (编). The Dopamine Receptors. Springer. 2009: 1–22. ISBN 978-1-60327-333-6.
- ^ EC 1.14.16.2 – Tyrosine 3-monooxygenase (Homo sapiens). BRENDA. Technische Universität Braunschweig. 2016-07 [2016-10-07]. (原始内容存档于2020-08-03).
Substrate: L-phenylalanine + tetrahydrobiopterin + O2
Product: L-tyrosine + 3-hydroxyphenylalanine [(aka m-tyrosine)] + dihydropteridine + H2O
Organism: Homo sapiens
Reaction diagram (页面存档备份,存于互联网档案馆) - ^ EC 4.1.1.28 – Aromatic-L-amino-acid decarboxylase (Homo sapiens). BRENDA. Technische Universität Braunschweig. 2016-07 [2016-10-07]. (原始内容存档于2020-12-08).
Substrate: m-tyrosine
Product: m-tyramine + CO2
Organism: Homo sapiens
Reaction diagram (页面存档备份,存于互联网档案馆) - ^ 26.0 26.1 26.2 26.3 26.4 26.5 Musacchio JM. Chapter 1: Enzymes involved in the biosynthesis and degradation of catecholamines. Iverson L (编). Biochemistry of Biogenic Amines. Springer. 2013: 1–35. ISBN 978-1-4684-3171-1.
- ^ 27.0 27.1 27.2 27.3 27.4 27.5 27.6 27.7 27.8 The National Collaborating Centre for Chronic Conditions (编). Symptomatic pharmacological therapy in Parkinson's disease. Parkinson's Disease. London: Royal College of Physicians. 2006: 59–100 [2015-09-24]. ISBN 978-1-86016-283-1. (原始内容存档于2010-09-24).
- ^ Zahoor, Insha; Shafi, Amrina; Haq, Ehtishamul. Pharmacological Treatment of Parkinson's Disease. Parkinson's Disease: Pathogenesis and Clinical Aspects. Codon Publications. 2018-12-22. ISBN 978-0-9944381-6-4. doi:10.15586/codonpublications.parkinsonsdisease.2018.ch7.
- ^ Amin F, Davidson M, Davis KL. Homovanillic acid measurement in clinical research: a review of methodology. Schizophrenia Bulletin. 1992, 18 (1): 123–48. PMID 1553492. doi:10.1093/schbul/18.1.123
 .
.
- ^ Amin F, Davidson M, Kahn RS, Schmeidler J, Stern R, Knott PJ, Apter S. Assessment of the central dopaminergic index of plasma HVA in schizophrenia. Schizophrenia Bulletin. 1995, 21 (1): 53–66. PMID 7770741. doi:10.1093/schbul/21.1.53
 .
.
- ^ Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotoxicity Research. 2000-02, 1 (3): 181–95. PMID 12835101. S2CID 21892355. doi:10.1007/BF03033289.
- ^ Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself (PDF). Acta Medica Okayama. 2008-06, 62 (3): 141–50 [2023-10-14]. PMID 18596830. doi:10.18926/AMO/30942. (原始内容存档 (PDF)于2018-09-16).
- ^ 33.0 33.1 33.2 Grandy DK, Miller GM, Li JX. "TAARgeting Addiction" – The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug and Alcohol Dependence. 2016-02, 159: 9–16. PMC 4724540
 . PMID 26644139. doi:10.1016/j.drugalcdep.2015.11.014.
. PMID 26644139. doi:10.1016/j.drugalcdep.2015.11.014. TAAR1 is a high-affinity receptor for METH/AMPH and DA
- ^ 34.0 34.1 34.2 Romanelli RJ, Williams JT, Neve KA. Chapter 6: Dopamine receptor signalling: intracellular pathways to behavior. Neve KA (编). The Dopamine Receptors. Springer. 2009: 137–74. ISBN 978-1-60327-333-6.
- ^ 35.0 35.1 Eiden LE, Schäfer MK, Weihe E, Schütz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflügers Archiv. 2004-02, 447 (5): 636–40. PMID 12827358. S2CID 20764857. doi:10.1007/s00424-003-1100-5.
- ^ Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological Reviews. 2011-03, 63 (1): 182–217. PMID 21303898. S2CID 2545878. doi:10.1124/pr.110.002642.
- ^ Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nature Reviews. Neuroscience. 2003-01, 4 (1): 13–25. PMID 12511858. S2CID 21545649. doi:10.1038/nrn1008.
- ^ Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007, 30: 259–88. PMID 17600522. S2CID 13503219. doi:10.1146/annurev.neuro.28.061604.135722.
- ^ 39.0 39.1 39.2 39.3 39.4 39.5 39.6 Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends in Neurosciences. 2007-05, 30 (5): 194–202. PMID 17408759. S2CID 14239716. doi:10.1016/j.tins.2007.03.006.
- ^ 40.0 40.1 Dahlstroem A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiologica Scandinavica. Supplementum. 1964, 232 (Suppl): 1–55. PMID 14229500.
- ^ 41.0 41.1 41.2 41.3 Malenka RC, Nestler EJ, Hyman SE. Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 147–48, 154–57. ISBN 978-0-07-148127-4.
- ^ Christine CW, Aminoff MJ. Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance. The American Journal of Medicine. 2004-09-15, 117 (6): 412–19. PMID 15380498. doi:10.1016/j.amjmed.2004.03.032.
- ^ Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. The Journal of Neuroscience. 2009-09, 29 (36): 11089–97. PMC 2759996
 . PMID 19741115. doi:10.1523/JNEUROSCI.1616-09.2009.
. PMID 19741115. doi:10.1523/JNEUROSCI.1616-09.2009.
- ^ Tang W, Kochubey O, Kintscher M, Schneggenburger R. A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning. The Journal of Neuroscience. 2020-04, 40 (20): JN–RM–1796–19. PMC 7219297
 . PMID 32277045. doi:10.1523/JNEUROSCI.1796-19.2020.
. PMID 32277045. doi:10.1523/JNEUROSCI.1796-19.2020.
- ^ Jo YS, Heymann G, Zweifel LS. Dopamine Neurons Reflect the Uncertainty in Fear Generalization. Neuron. 2018-11, 100 (4): 916–925.e3. PMC 6226002
 . PMID 30318411. doi:10.1016/j.neuron.2018.09.028 (英语).
. PMID 30318411. doi:10.1016/j.neuron.2018.09.028 (英语).
- ^ 46.0 46.1 Paulus W, Schomburg ED. Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis for augmentation?. Sleep Medicine Reviews. 2006-06, 10 (3): 185–96. PMID 16762808. doi:10.1016/j.smrv.2006.01.004.
- ^ 47.0 47.1 47.2 Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocrine Reviews. 2001-12, 22 (6): 724–63. PMID 11739329. doi:10.1210/er.22.6.724
 .
.
- ^ 48.0 48.1 48.2 48.3 Witkovsky P. Dopamine and retinal function. Documenta Ophthalmologica. Advances in Ophthalmology. 2004-01, 108 (1): 17–40 [2023-10-15]. PMID 15104164. S2CID 10354133. doi:10.1023/B:DOOP.0000019487.88486.0a. (原始内容存档于2020-01-09).
- ^ 49.0 49.1 Fix JD. Basal Ganglia and the Striatal Motor System. Neuroanatomy (Board Review Series) 4th. Baltimore: Wulters Kluwer & Lippincott Williams & Wilkins. 2008: 274–81. ISBN 978-0-7817-7245-7.
- ^ 50.0 50.1 50.2 50.3 50.4 50.5 Chakravarthy VS, Joseph D, Bapi RS. What do the basal ganglia do? A modeling perspective. Biological Cybernetics. 2010-09, 103 (3): 237–53 [2023-11-01]. PMID 20644953. S2CID 853119. doi:10.1007/s00422-010-0401-y. (原始内容存档于2018-09-19).
- ^ 51.0 51.1 51.2 Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annual Review of Psychology. 2015-01-03, 66: 25–52 [2023-11-01]. PMID 25251489. S2CID 28268183. doi:10.1146/annurev-psych-010213-115159. (原始内容存档于2020-04-24).
- ^ 52.0 52.1 52.2 Jankovic J. Parkinson's disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2008-04, 79 (4): 368–76 [2023-10-18]. PMID 18344392. doi:10.1136/jnnp.2007.131045
 . (原始内容存档于2015-08-19).
. (原始内容存档于2015-08-19).
- ^ Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends in Pharmacological Sciences. 2008-04, 29 (4): 192–99 [2023-11-01]. PMID 18304658. doi:10.1016/j.tips.2008.01.002. (原始内容存档于2018-09-19).
- ^ 54.00 54.01 54.02 54.03 54.04 54.05 54.06 54.07 54.08 54.09 Schultz W. Neuronal Reward and Decision Signals: From Theories to Data. Physiological Reviews. 2015-07, 95 (3): 853–951. PMC 4491543
 . PMID 26109341. doi:10.1152/physrev.00023.2014.
. PMID 26109341. doi:10.1152/physrev.00023.2014.
- ^ 55.0 55.1 55.2 Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews. 1993, 18 (3): 247–91. PMID 8401595. S2CID 13471436. doi:10.1016/0165-0173(93)90013-p. hdl:2027.42/30601
 .
.
- ^ 56.0 56.1 56.2 56.3 56.4 Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: 'liking', 'wanting', and learning. Current Opinion in Pharmacology. 2009-02, 9 (1): 65–73. PMC 2756052
 . PMID 19162544. doi:10.1016/j.coph.2008.12.014.
. PMID 19162544. doi:10.1016/j.coph.2008.12.014.
- ^ Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. The Journal of Neuroscience. 1996-03-01, 16 (5): 1936–47. PMC 6578666
 . PMID 8774460. doi:10.1523/JNEUROSCI.16-05-01936.1996
. PMID 8774460. doi:10.1523/JNEUROSCI.16-05-01936.1996  .
.
- ^ Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010-12-09, 68 (5): 815–34. PMC 3032992
 . PMID 21144997. doi:10.1016/j.neuron.2010.11.022.
. PMID 21144997. doi:10.1016/j.neuron.2010.11.022.
- ^ Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: Role in drug addiction. Neuroscience. 2015-08-20, 301: 529–41. PMC 4523218
 . PMID 26116518. doi:10.1016/j.neuroscience.2015.06.033.
. PMID 26116518. doi:10.1016/j.neuroscience.2015.06.033.
- ^ Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM. Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation. The Journal of Neuroscience. 2015-08, 35 (33): 11572–82. PMC 4540796
 . PMID 26290234. doi:10.1523/JNEUROSCI.2344-15.2015.
. PMID 26290234. doi:10.1523/JNEUROSCI.2344-15.2015.
- ^ Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015-05, 86 (3): 646–64. PMC 4425246
 . PMID 25950633. doi:10.1016/j.neuron.2015.02.018.
. PMID 25950633. doi:10.1016/j.neuron.2015.02.018.
- ^ 62.0 62.1 62.2 Wise RA. Addictive drugs and brain stimulation reward. Annual Review of Neuroscience. 1996, 19: 319–40. PMID 8833446. doi:10.1146/annurev.ne.19.030196.001535.
- ^ Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008-10, 14 (2–3): 169–83. PMC 3155128
 . PMID 19073424. doi:10.1007/BF03033808.
. PMID 19073424. doi:10.1007/BF03033808.
- ^ Arias-Carrión O, Pöppel E. Dopamine, learning and reward-seeking behavior. Acta Neurobiol Exp. 2007, 67 (4): 481–88. PMID 18320725.
- ^ 65.0 65.1 Ferreri L, Mas-Herrero E, Zatorre RJ, Ripollés P, Gomez-Andres A, Alicart H, Olivé G, Marco-Pallarés J, Antonijoan RM, Valle M, Riba J, Rodriguez-Fornells A. Dopamine modulates the reward experiences elicited by music. Proceedings of the National Academy of Sciences of the United States of America. 2019, 116 (9): 3793–98. Bibcode:2019PNAS..116.3793F. PMC 6397525
 . PMID 30670642. doi:10.1073/pnas.1811878116
. PMID 30670642. doi:10.1073/pnas.1811878116  .
. Listening to pleasurable music is often accompanied by measurable bodily reactions such as goose bumps or shivers down the spine, commonly called "chills" or "frissons." ... Overall, our results straightforwardly revealed that pharmacological interventions bidirectionally modulated the reward responses elicited by music. In particular, we found that risperidone impaired participants' ability to experience musical pleasure, whereas levodopa enhanced it. ... Here, in contrast, studying responses to abstract rewards in human subjects, we show that manipulation of dopaminergic transmission affects both the pleasure (i.e., amount of time reporting chills and emotional arousal measured by EDA) and the motivational components of musical reward (money willing to spend). These findings suggest that dopaminergic signaling is a sine qua non condition not only for motivational responses, as has been shown with primary and secondary rewards, but also for hedonic reactions to music. This result supports recent findings showing that dopamine also mediates the perceived pleasantness attained by other types of abstract rewards (37) and challenges previous findings in animal models on primary rewards, such as food (42, 43).
- ^ 66.0 66.1 Goupil L, Aucouturier JJ. Musical pleasure and musical emotions. Proceedings of the National Academy of Sciences of the United States of America. 2019-02, 116 (9): 3364–66. Bibcode:2019PNAS..116.3364G. PMC 6397567
 . PMID 30770455. doi:10.1073/pnas.1900369116
. PMID 30770455. doi:10.1073/pnas.1900369116  .
. In a pharmacological study published in PNAS, Ferreri et al. (1) present evidence that enhancing or inhibiting dopamine signaling using levodopa or risperidone modulates the pleasure experienced while listening to music. ... In a final salvo to establish not only the correlational but also the causal implication of dopamine in musical pleasure, the authors have turned to directly manipulating dopaminergic signaling in the striatum, first by applying excitatory and inhibitory transcranial magnetic stimulation over their participants' left dorsolateral prefrontal cortex, a region known to modulate striatal function (5), and finally, in the current study, by administrating pharmaceutical agents able to alter dopamine synaptic availability (1), both of which influenced perceived pleasure, physiological measures of arousal, and the monetary value assigned to music in the predicted direction. ... While the question of the musical expression of emotion has a long history of investigation, including in PNAS (6), and the 1990s psychophysiological strand of research had already established that musical pleasure could activate the autonomic nervous system (7), the authors' demonstration of the implication of the reward system in musical emotions was taken as inaugural proof that these were veridical emotions whose study has full legitimacy to inform the neurobiology of our everyday cognitive, social, and affective functions (8). Incidentally, this line of work, culminating in the article by Ferreri et al. (1), has plausibly done more to attract research funding for the field of music sciences than any other in this community.
The evidence of Ferreri et al. (1) provides the latest support for a compelling neurobiological model in which musical pleasure arises from the interaction of ancient reward/valuation systems (striatal–limbic–paralimbic) with more phylogenetically advanced perception/predictions systems (temporofrontal). - ^ Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998-05, 393 (6682): 266–268. Bibcode:1998Natur.393..266K. PMID 9607763. S2CID 205000565. doi:10.1038/30498.
- ^ von der Heiden JM, Braun B, Müller KW, Egloff B. The Association Between Video Gaming and Psychological Functioning. Frontiers in Psychology. 2019, 10: 1731. PMC 6676913
 . PMID 31402891. doi:10.3389/fpsyg.2019.01731
. PMID 31402891. doi:10.3389/fpsyg.2019.01731  .
.
- ^ 69.0 69.1 Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function (PDF). Physiological Reviews. 1998-01, 78 (1): 189–225. PMID 9457173. S2CID 223462. doi:10.1152/physrev.1998.78.1.189. (原始内容 (PDF)存档于2019-03-02).
- ^ 70.0 70.1 Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE. The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders. Current Neuropharmacology. 2011-06, 9 (2): 278–88. PMC 3131719
 . PMID 22131937. doi:10.2174/157015911795596612.
. PMID 22131937. doi:10.2174/157015911795596612.
- ^ 71.0 71.1 Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S. The immunoregulatory role of dopamine: an update. Brain, Behavior, and Immunity. 2010-05, 24 (4): 525–28. PMC 2856781
 . PMID 19896530. doi:10.1016/j.bbi.2009.10.015.
. PMID 19896530. doi:10.1016/j.bbi.2009.10.015.
- ^ Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Experimental Biology and Medicine. 2003-02, 228 (2): 134–42. PMID 12563019. S2CID 10896819. doi:10.1177/153537020322800202.
- ^ Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, Fernández BE. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World Journal of Nephrology. 2015-05-06, 4 (2): 196–212. PMC 4419129
 . PMID 25949933. doi:10.5527/wjn.v4.i2.196
. PMID 25949933. doi:10.5527/wjn.v4.i2.196  .
.
- ^ Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001-09, 38 (3): 297–302. PMID 11566894. doi:10.1161/hy0901.096422
 .
.
- ^ 75.0 75.1 75.2 75.3 75.4 Rubí B, Maechler P. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology. 2010-12, 151 (12): 5570–81. PMID 21047943. doi:10.1210/en.2010-0745
 .
.
- ^ WHO Model List of Essential Medicines (PDF). World Health Organization. 2013-10 [2015-09-24]. (原始内容存档 (PDF)于2014-02-10).
- ^ Noori S, Friedlich P, Seri I. Pharmacology Review Developmentally Regulated Cardiovascular, Renal, and Neuroendocrine Effects of Dopamine. NeoReviews. 2003, 4 (10): e283–e288 [2015-09-24]. S2CID 71902752. doi:10.1542/neo.4-10-e283. (原始内容存档于2018-09-19).
- ^ 78.0 78.1 Bhatt-Mehta V, Nahata MC. Dopamine and dobutamine in pediatric therapy. Pharmacotherapy. 1989, 9 (5): 303–14. PMID 2682552. S2CID 25614283. doi:10.1002/j.1875-9114.1989.tb04142.x.
- ^ Moses S. Dopamine. Family Practice Notebook. [2016-02-01]. (原始内容存档于2016-02-01).
- ^ Katritsis DG, Gersh BJ, Camm AJ. Clinical Cardiology: Current Practice Guidelines. OUP Oxford. 2013 [2023-10-13]. ISBN 978-0-19-150851-6. (原始内容存档于2023-01-12).
Dopamine binds to beta-1, beta-2, alpha-1 and dopaminergic receptors
- ^ 81.0 81.1 81.2 Bronwen JB, Knights KM. Pharmacology for Health Professionals 2nd. Elsevier Australia. 2009: 192. ISBN 978-0-7295-3929-6.
- ^ De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock (PDF). The New England Journal of Medicine. 2010-03, 362 (9): 779–89. PMID 20200382. S2CID 2208904. doi:10.1056/NEJMoa0907118. (原始内容 (PDF)存档于2019-02-28).
- ^ Karthik S, Lisbon A. Low-dose dopamine in the intensive care unit. Seminars in Dialysis. 2006, 19 (6): 465–71. PMID 17150046. S2CID 22538344. doi:10.1111/j.1525-139X.2006.00208.x.
- ^ Lewis RJ. Sax's Dangerous Properties of Industrial Materials 11th. Hoboken, NJ: Wiley & Sons. 2004: 1552. ISBN 978-0-471-47662-7.
- ^ Standaert DG, Walsh RR. Pharmacology of dopaminergic neurotransmission. Tashjian AH, Armstrong EJ, Golan DE (编). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. 2011: 186–206. ISBN 978-1-4511-1805-6.
- ^ Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, Suhara T. Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[β-11C]DOPA. Life Sciences. 2006-07-17, 79 (8): 730–36. PMID 16580023. doi:10.1016/j.lfs.2006.02.017.
- ^ Mobbs CV, Hof PR. Handbook of the neuroscience of aging. Amsterdam: Elsevier/Academic Press. 2009. ISBN 978-0-12-374898-0. OCLC 299710911.
- ^ Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiology of Aging. 2000, 21 (5): 683–68. PMID 11016537. S2CID 40871554. doi:10.1016/S0197-4580(00)00149-4.
- ^ Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998-09, 30 (1): 56–61. PMID 9704881. S2CID 31445572. doi:10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J.
- ^ Wong DF, Wagner HN, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984-12, 226 (4681): 1393–96. Bibcode:1984Sci...226.1393W. PMID 6334363. S2CID 24278577. doi:10.1126/science.6334363.
- ^ Wang E, Snyder SD. Handbook of the aging brain. San Diego, California: Academic Press. 1998. ISBN 978-0-12-734610-6. OCLC 636693117.
- ^ Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Frontiers in Neurology. 2015-03-12, 6: 52. PMC 4357260
 . PMID 25814977. doi:10.3389/fneur.2015.00052
. PMID 25814977. doi:10.3389/fneur.2015.00052  .
.
- ^ Marino F, Cosentino M. Multiple sclerosis: Repurposing dopaminergic drugs for MS—the evidence mounts. Nature Reviews. Neurology. 2016-04, 12 (4): 191–92. PMID 27020558. S2CID 26319461. doi:10.1038/nrneurol.2016.33.
- ^ Dickson DV. Neuropathology of movement disorders. Tolosa E, Jankovic JJ (编). Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. 2007: 271–83. ISBN 978-0-7817-7881-7.
- ^ 95.0 95.1 Tuite PJ, Krawczewski K. Parkinsonism: a review-of-systems approach to diagnosis. Seminars in Neurology. 2007-04, 27 (2): 113–22. PMID 17390256. S2CID 260319916. doi:10.1055/s-2007-971174.
- ^ Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. 2011-12, 61 (7): 1109–22. PMC 3139704
 . PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010.
. PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010.
- ^ Ceravolo R, Frosini D, Rossi C, Bonuccelli U. Spectrum of addictions in Parkinson's disease: from dopamine dysregulation syndrome to impulse control disorders. Journal of Neurology. 2010-11, 257 (Suppl 2): S276–83. PMID 21080189. S2CID 19277026. doi:10.1007/s00415-010-5715-0.
- ^ 98.0 98.1 98.2 98.3 98.4 Ghodse H. Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment 4th. Cambridge University Press. 2010: 87–92. ISBN 978-1-139-48567-8.
- ^ Siciliano CA, Jones SR. Cocaine Potency at the Dopamine Transporter Tracks Discrete Motivational States During Cocaine Self-Administration. Neuropsychopharmacology. 2017-08, 42 (9): 1893–1904. PMC 5520781
 . PMID 28139678. doi:10.1038/npp.2017.24.
. PMID 28139678. doi:10.1038/npp.2017.24.
- ^ Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006, 20 (9): 713–38. PMID 16953648. S2CID 39535277. doi:10.2165/00023210-200620090-00002.
- ^ 101.0 101.1 Freye E. Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. 2009. ISBN 978-90-481-2448-0.
- ^ Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. Journal of Neurochemistry. 2011-01, 116 (2): 164–76. PMC 3005101
 . PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
. PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
- ^ Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clinical Pharmacokinetics. 1999-12, 37 (6): 457–70. PMID 10628897. S2CID 397390. doi:10.2165/00003088-199937060-00002.
- ^ Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012-10, 9 (4): 739–52. PMC 3480574
 . PMID 23065655. doi:10.1007/s13311-012-0150-9.
. PMID 23065655. doi:10.1007/s13311-012-0150-9.
- ^ Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows (PDF). Nature Reviews. Neuroscience. 2015-05, 16 (5): 305–12 [2023-10-22]. PMID 25873042. S2CID 205511111. doi:10.1038/nrn3939. (原始内容存档 (PDF)于2019-09-23).
- ^ 106.0 106.1 Sinha R. The clinical neurobiology of drug craving. Current Opinion in Neurobiology. 2013-08, 23 (4): 649–54. PMC 3735834
 . PMID 23764204. doi:10.1016/j.conb.2013.05.001.
. PMID 23764204. doi:10.1016/j.conb.2013.05.001.
- ^ Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014-01, 76 (Pt B): 235–49. PMC 3818510
 . PMID 23688927. doi:10.1016/j.neuropharm.2013.05.007.
. PMID 23688927. doi:10.1016/j.neuropharm.2013.05.007.
- ^ Nestler EJ. Transcriptional mechanisms of drug addiction. Clinical Psychopharmacology and Neuroscience. 2012-12, 10 (3): 136–43. PMC 3569166
 . PMID 23430970. doi:10.9758/cpn.2012.10.3.136.
. PMID 23430970. doi:10.9758/cpn.2012.10.3.136.
- ^ 109.0 109.1 109.2 109.3 Healy D. The Creation of Psychopharmacology. Harvard University Press. 2004: 37–73. ISBN 978-0-674-01599-9.
- ^ Brunton L. Goodman and Gilman's The Pharmacological Basis of Therapeutics 12th. McGraw Hill. 1990: 417–55.
- ^ 111.0 111.1 111.2 Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia Bulletin. 2009-05, 35 (3): 549–62. PMC 2669582
 . PMID 19325164. doi:10.1093/schbul/sbp006.
. PMID 19325164. doi:10.1093/schbul/sbp006.
- ^ Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. (PDF). CNS Spectr. 2018, 23 (3): 187–91. PMID 29954475. S2CID 49599226. doi:10.1017/S1092852918001013. (原始内容存档 (PDF)于2020-04-29).
- ^ 113.0 113.1 Malenka RC, Nestler EJ, Hyman SE. Chapters 10 and 13. Sydor A, Brown RY (编). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 266, 318–23. ISBN 978-0-07-148127-4.
- ^ Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biological Psychiatry. 2011-06, 69 (12): e101–11. PMC 3012746
 . PMID 20875636. doi:10.1016/j.biopsych.2010.06.023.
. PMID 20875636. doi:10.1016/j.biopsych.2010.06.023.
- ^ Spencer RC, Devilbiss DM, Berridge CW. The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex. Biological Psychiatry. 2015-06, 77 (11): 940–50. PMC 4377121
 . PMID 25499957. doi:10.1016/j.biopsych.2014.09.013.
. PMID 25499957. doi:10.1016/j.biopsych.2014.09.013.
- ^ Ilieva IP, Hook CJ, Farah MJ. Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis. Journal of Cognitive Neuroscience. 2015-06, 27 (6): 1069–89 [2023-10-19]. PMID 25591060. S2CID 15788121. doi:10.1162/jocn_a_00776. (原始内容存档于2018-09-19).
- ^ 117.0 117.1 Wood PB. Role of central dopamine in pain and analgesia. Expert Review of Neurotherapeutics. 2008-05, 8 (5): 781–97. PMID 18457535. S2CID 24325199. doi:10.1586/14737175.8.5.781.
- ^ 118.0 118.1 118.2 Flake ZA, Scalley RD, Bailey AG. Practical selection of antiemetics. American Family Physician. 2004-03, 69 (5): 1169–74 [2023-10-26]. PMID 15023018. (原始内容存档于2016-03-24).
- ^ Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014, 311 (16): 1670–83. PMID 24756517. doi:10.1001/jama.2014.3654.
- ^ Roshchina VV. Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells. Lyte M, Primrose PE (编). Microbial Endocrinology. New York: Springer. 2010: 17–52. ISBN 978-1-4419-5576-0.
- ^ Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role?. Trends in Genetics. 2004-07, 20 (7): 292–99. PMID 15219393. doi:10.1016/j.tig.2004.05.007.
- ^ 122.0 122.1 122.2 122.3 122.4 Barron AB, Søvik E, Cornish JL. The roles of dopamine and related compounds in reward-seeking behavior across animal phyla. Frontiers in Behavioral Neuroscience. 2010, 4: 163. PMC 2967375
 . PMID 21048897. doi:10.3389/fnbeh.2010.00163
. PMID 21048897. doi:10.3389/fnbeh.2010.00163  .
.
- ^ Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, et al. Isolation of Araguspongine M, a new stereoisomer of an Araguspongine/Xestospongin alkaloid, and dopamine from the marine sponge Neopetrosia exigua collected in Palau. Marine Drugs. 2004, 2 (4): 154–63. PMC 3783253
 . doi:10.3390/md204154
. doi:10.3390/md204154  .
.
- ^ Kass-Simon G, Pierobon P. Cnidarian chemical neurotransmission, an updated overview. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2007-01, 146 (1): 9–25. PMID 17101286. doi:10.1016/j.cbpa.2006.09.008.
- ^ Cottrell GA. Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species. British Journal of Pharmacology and Chemotherapy. 1967-01, 29 (1): 63–69. PMC 1557178
 . PMID 19108240. doi:10.1111/j.1476-5381.1967.tb01939.x.
. PMID 19108240. doi:10.1111/j.1476-5381.1967.tb01939.x.
- ^ Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007-08, 55 (4): 662–76. PMID 17698017. S2CID 2092645. doi:10.1016/j.neuron.2007.07.023
 .
.
- ^ Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research. Brain Research Reviews. 1991-09-01, 16 (3): 223–44. PMID 1665095. S2CID 10775295. doi:10.1016/0165-0173(91)90007-U.
- ^ Takikawa Y, Kawagoe R, Hikosaka O. A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping (PDF). Journal of Neurophysiology. 2004-10, 92 (4): 2520–29. PMID 15163669. S2CID 12534057. doi:10.1152/jn.00238.2004. (原始内容 (PDF)存档于2019-03-02).
- ^ Yamagata N, Ichinose T, Aso Y, Plaçais PY, Friedrich AB, Sima RJ, et al. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proceedings of the National Academy of Sciences of the United States of America. 2015-01, 112 (2): 578–83. Bibcode:2015PNAS..112..578Y. PMC 4299218
 . PMID 25548178. doi:10.1073/pnas.1421930112
. PMID 25548178. doi:10.1073/pnas.1421930112  .
.
- ^ 130.0 130.1 Waddell S. Reinforcement signalling in Drosophila; dopamine does it all after all. Current Opinion in Neurobiology. 2013-06, 23 (3): 324–29. PMC 3887340
 . PMID 23391527. doi:10.1016/j.conb.2013.01.005.
. PMID 23391527. doi:10.1016/j.conb.2013.01.005.
- ^ 131.0 131.1 131.2 131.3 131.4 Kulma A, Szopa J. Catecholamines are active compounds in plants. Plant Science. 2007, 172 (3): 433–40. doi:10.1016/j.plantsci.2006.10.013.
- ^ 132.0 132.1 Ingle PK. L-DOPA bearing plants (PDF). Natural Product Radiance. 2003, 2: 126–33 [2015-09-24]. (原始内容存档 (PDF)于2014-03-02).
- ^ Wichers HJ, Visser JF, Huizing HJ, Pras N. Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2,4-D and NaCl on these compounds. Plant Cell, Tissue and Organ Culture. 1993, 33 (3): 259–64. S2CID 44814336. doi:10.1007/BF02319010.
- ^ Longo R, Castellani A, Sberze P, Tibolla M. Distribution of L-dopa and related amino acids in Vicia. Phytochemistry. 1974, 13 (1): 167–71. Bibcode:1974PChem..13..167L. doi:10.1016/S0031-9422(00)91287-1.
- ^ Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA. Dopamine functions as an antiherbivore defense in the temperate green alga Ulvaria obscura. Oecologia. 2006-06, 148 (2): 304–11. Bibcode:2006Oecol.148..304V. PMID 16489461. S2CID 5029574. doi:10.1007/s00442-006-0378-3.
- ^ 136.0 136.1 136.2 Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell & Melanoma Research. 2009-10, 22 (5): 563–79. PMID 19627559. doi:10.1111/j.1755-148X.2009.00610.x
 .
.
- ^ Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease. Progress in Neurobiology. 2005-02, 75 (2): 109–24. PMID 15784302. S2CID 503902. doi:10.1016/j.pneurobio.2005.02.001.
- ^ Andrews RS, Pridham JB. Melanins from DOPA-containing plants. Phytochemistry. 1967, 6 (1): 13–18. Bibcode:1967PChem...6...13A. doi:10.1016/0031-9422(67)85002-7.
- ^ Beldade P, Brakefield PM. The genetics and evo-devo of butterfly wing patterns. Nature Reviews. Genetics. 2002-06, 3 (6): 442–52. PMID 12042771. S2CID 17417235. doi:10.1038/nrg818.
- ^ Fahn S. The history of dopamine and levodopa in the treatment of Parkinson's disease. Movement Disorders. 2008, 23 (Suppl 3): S497–508. PMID 18781671. S2CID 45572523. doi:10.1002/mds.22028.
- ^ Benes FM. Carlsson and the discovery of dopamine. Trends in Pharmacological Sciences. 2001-01, 22 (1): 46–47. PMID 11165672. doi:10.1016/S0165-6147(00)01607-2.
- ^ Barondes SH. Better Than Prozac
 . New York: Oxford University Press. 2003: 21–22, 39–40. ISBN 978-0-19-515130-5.
. New York: Oxford University Press. 2003: 21–22, 39–40. ISBN 978-0-19-515130-5.
- ^ Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007-10, 318 (5849): 426–30. Bibcode:2007Sci...318..426L. PMC 2601629
 . PMID 17947576. doi:10.1126/science.1147241.
. PMID 17947576. doi:10.1126/science.1147241.
- ^ 144.0 144.1 Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW. Perspectives on poly(dopamine). Chemical Science. 2013, 4 (10): 3796. doi:10.1039/C3SC51501J.
- ^ 145.0 145.1 Lynge ME, van der Westen R, Postma A, Städler B. Polydopamine—a nature-inspired polymer coating for biomedical science (PDF). Nanoscale. 2011-12, 3 (12): 4916–28. Bibcode:2011Nanos...3.4916L. PMID 22024699. doi:10.1039/c1nr10969c. (原始内容存档于2014-03-07).
外部链接
[编辑]- U.S. National Library of Medicine: Drug Information Portal - Dopamine (页面存档备份,存于互联网档案馆)
- Dopamine: analyte monograph - The Association for Clinical Biochemistry and Laboratory Medicine
- Biochemistry of Parkinson's Disease (页面存档备份,存于互联网档案馆)(英文)