铅:修订间差异
小 Cewbot: 修正維基語法 2: 修正不正確的 HTML tag 如 </br> → <br /> |
无编辑摘要 |
||
| 第365行: | 第365行: | ||
=== 二级生产=== |
=== 二级生产=== |
||
{{Further|电池回收#铅酸电池}} |
{{Further|电池回收#铅酸电池}} |
||
除非回收的铅有明显的氧化,否则在初级生产中必要的冶炼过程在二级生产中会被跳过。{{sfn|Thornton|Rautiu|Brush|2001|p=56}}{{le|ISASMELT}}过程是可作为延伸初级生产的新冶炼方法。废铅酸蓄电池(含有硫酸铅和铅的各种氧化物)的硫酸铅可以用碱去除,然后在熔炉中和煤炭反应,生成不纯的铅。{{sfn|Thornton|Rautiu|Brush|2001|p=57}}二级铅的提炼和初级铅类似,但某些提炼过程可以根据回收材料和潜在污染跳过。{{sfn|Thornton|Rautiu|Brush|2001|p=57}}在铅的回收来源中最重要的是铅酸蓄电池,而铅水管和电缆也是重要的来源。{{sfn|Thornton|Rautiu|Brush|2001|p=56}} |
除非回收的铅有明显的氧化,否则在初级生产中必要的冶炼过程在二级生产中会被跳过。{{sfn|Thornton|Rautiu|Brush|2001|p=56}}{{le|ISASMELT}}过程是可作为延伸初级生产的新冶炼方法。废铅酸蓄电池(含有硫酸铅和铅的各种氧化物)的硫酸铅可以用碱去除,然后在熔炉中和煤炭反应,生成不纯的铅。{{sfn|Thornton|Rautiu|Brush|2001|p=57}}二级铅的提炼和初级铅类似,但某些提炼过程可以根据回收材料和潜在污染跳过。{{sfn|Thornton|Rautiu|Brush|2001|p=57}}在铅的回收来源中最重要的是铅酸蓄电池,而铅水管和电缆涂层也是重要的来源。{{sfn|Thornton|Rautiu|Brush|2001|p=56}} |
||
== 用途 == |
== 用途 == |
||
[[File:Lead shielding.jpg|thumb|left|用于屏蔽辐射的铅砖(含4%锑的合金){{sfn|Street|Alexander|1998|p=181}}|alt=一堆黑砖]] |
|||
[[File:Lead electrolytic and 1cm3 cube.jpg|thumb|300px|right|金屬鉛]] |
|||
实际上,铅笔并不是用铅做的,但因为古时候把[[石墨]]误认为是铅,所以铅笔一词在各语言中流传使用而未修正。{{sfn|Evans|1908|pp=133–179}} |
|||
=== 单质 === |
|||
* 鉛的表面能在空氣中產生[[鹼式碳酸鉛]]薄膜,阻止內部氧化。 |
|||
金属铅的高密度、低熔点、展性和惰性使其可用于许多用途。虽然有很多金属在某些方面比铅好,但它们通常较罕见,也更难从矿石中提取。不过,铅的毒性使它在某些用途中被逐步淘汰。{{sfn|Baird|Cann|2012|pp=537–538, 543–547}} |
|||
* 製造[[鉛磚]]或[[鉛衣]]以防護[[X-射線]]及其他[[放射線]]。 |
|||
* 用於製造[[合金]]。等量的鉛和[[錫]]組成的[[焊條]]可用於銲接金屬和電子電路。 |
|||
自中世纪以来,铅就被用于生产子弹。铅很便宜,而且它的低熔点意味着可以用最少的技术设备铸造小型武器弹药和霰弹枪弹。此外,铅的密度比大部分常见金属高,可以更好地保持速度。今天,铅(会加入其它金属来变硬)仍然是子弹的主要材料,{{sfn|Ramage|1980|p=8}}但有人担心用于狩猎的铅弹会破坏环境。{{efn|加利福尼亚州因此在2015年7月开始禁止使用铅弹打猎。{{sfn|California Department of Fish and Wildlife}}}} |
|||
* 製造印刷用[[活字]]。 |
|||
* 用作[[鉛酸蓄電池]]的電極(目前鉛的用途有八成是用來製造電池,主要製造汽車電池)。<ref>世界資源真相和你想的不一樣;作者:資源問題研究會</ref> |
|||
铅也有许多用处应用了其高密度和抗腐蚀性。铅的高密度使它可用作帆船龙骨的{{le|压舱物|ballast}},从而抵消风对帆的倾斜效应。{{sfn|Parker|2005|pp=194–195}}它也用于[[水肺潜水]]的{{le|配重系统|diving weighting system}}中,以抵消潜水员的浮力。{{sfn|Krestovnikoff|Halls|2006|p=70}}1993年,[[比萨斜塔]]的底部就用了600吨的铅来使其稳定。{{sfn|Street|Alexander|1998|p=182}}铅的抗腐蚀性使其可用于保护深海电缆。{{sfn|Jensen|2013|p=136}} |
|||
* 鉛和[[銻]]、[[鎘]]、[[鉍]]製成的低熔點[[伍德合金]],可用於[[保險絲]]、消防自動灑水器等。 |
|||
* 可用於製造[[子彈]]的彈頭。 |
|||
[[File:Parc de Versailles, Bassin de Flore, Jean-Baptiste Tuby (1672-79) 07.jpg|right|thumb|alt=黄色的雕像|17世纪的镀金铅雕像]] |
|||
* 用於製造[[顏料]]和[[油漆]],例如[[鉛白]]、鉛黃、鉛橙和紅色漆油(紅色的鉛漆油因為有毒,所以能用作船底塗漆,以阻止[[藤壺屬|藤壺]]等[[海洋生物]]附在船上減慢船速)等。 |
|||
在建筑工业中,铅也有许多用途。铅片可用于屋顶、{{le|覆层|Cladding (construction)}}、{{le|落水管|rain gutter}}和[[女儿墙]]。{{sfn|Think Lead research}}{{sfn|Weatherings to Parapets}}铅至今仍用于制造雕像和雕塑,以及它们的骨架。{{sfn|Putnam|2003|p=216}}铅在以前也用于[[轮胎平衡|平衡轮胎]],但因为环境原因也被其它材料替代了。{{sfn|United States Geological Survey|2017|p=97}} |
|||
* 可作為陶器[[釉|釉料]]的成份,用於[[屋頂]]、防水蓋片及彩繪玻璃窗。<ref>{{Cite web | url = http://db1x.sinica.edu.tw/caat/caat_rptcaatc.php?_op=%3FSUBJECT_ID%3A300011022 | title = 藝術與建築索引典—鉛 | accessdate =2010-10-20| archive-url = https://web.archive.org/web/20111217195417/http://db1x.sinica.edu.tw/caat/caat_rptcaatc.php?_op=%3FSUBJECT_ID%3A300011022# | archive-date = 2011-12-17 | dead-url = yes }}</ref> |
|||
铅可以添加到如青铜和[[黄铜]]等铜合金中来润滑和增加{{le|可加工性|machinability}}。由于铅不溶于铜,所以它会在合金的缺陷(例如[[晶粒边界]])中形成固体小球。在低浓度下,铅除了起到润滑剂的作用外,这些小球还会阻止{{le|切屑|swarf}}生成,因此增加了可加工性。铅含量较高的铜合金可用于制造[[轴承]]。铅用于润滑,而铜则提供承重支撑。{{sfn|Copper Development Association}} |
|||
铅的密度和原子序都很高,而且容易成型,这使得它可用于屏蔽声音、震动和辐射。{{sfn|Rich|1994|p=101}}铅没有[[自然频率]],{{sfn|Rich|1994|p=101}}因此铅片可用于录音室的隔音。{{sfn|Guruswamy|2000|p=31}}{{le|管风琴声管|Organ pipe}}通常是由铅合金制造的,这些合金会加入不等量的锡来控制音调。{{sfn|Audsley|1965|pp=250–251}}{{sfn|Palmieri|2006|pp=412–413}}由于其密度和[[衰减系数]]都很高,{{sfn|Thornton|Rautiu|Brush|2001|p=7}}铅可用作[[核物理学]]和[[X光]]室的[[电离辐射|辐射]]屏蔽材料。{{sfn|National Council on Radiation Protection and Measurements|2004|p=16}}熔融的铅可用作[[铅冷快中子反应堆]]的[[冷却剂]]。{{sfn|Tuček|Carlsson|Wider|2006|p=1590}} |
|||
=== 电池=== |
|||
在21世纪初,铅最大的用处就是制造[[铅酸蓄电池]]。电池中的铅并没有与人体直接接触,因此毒性问题较少,{{efn|铅酸蓄电池对普通使用者的潜在危害都和铅的毒性无关。{{sfn|Concordia University|2016}}}}但在生产铅酸蓄电池的工厂工作的人可能会暴露于含铅粉尘下。{{sfn|Toxicological Profile for Lead|2007|pp=5–6}}在铅酸蓄电池里,铅、二氧化铅和硫酸的反应可以产生稳定的[[电压]]。{{efn|参见这个参考资料{{sfn|Progressive Dynamics, Inc.}}来得到铅酸蓄电池工作原理的详细资料。}}包含铅酸蓄电池的[[超级电容器]]可用于调节频率、太阳能和风能等应用中。{{sfn|Olinsky-Paul|2013}}这些电池虽然能量密度和充放电效率比[[锂离子电池]]低,但比锂离子电池便宜。{{sfn|Gulbinska|2014}} |
|||
=== 电缆涂层 === |
|||
铅可以制造高压电电缆的外壳,以防止水进到里面,但因为毒性而被逐渐淘汰。{{sfn|Rich|1994|pp=133–134}}它也用于电器的[[焊料]],但因为会影响环境而被某些国家淘汰。{{sfn|Zhao|2008|p=440}}铅是博物馆{{le|Oddy测试|Oddy test}}使用的三种金属之一,可用于探测有机酸、醛和酸性气体。{{sfn|Beiner|Lavi|Seri|Rossin|2015}}{{sfn|Szczepanowska|2013|pp=84–85}} |
|||
=== 化合物=== |
|||
[[File:Crystal_glass.jpg|thumb|[[铅玻璃]]|left]] |
|||
[[File:PbCrO4 and PbCrO4•PbO.jpg|thumb|[[铬酸铅]](黄色)和[[四氧化三铅]](红色)]] |
|||
铅酸蓄电池除了是金属铅最大的用处以外,也是铅化合物最大的用处。它的充放电反应涉及了[[硫酸铅]]和[[二氧化铅]]: |
|||
:{{chem|Pb}}(s) + {{chem|PbO|2}}(s) + 2{{chem|H|2|SO|4}}(aq) → 2{{chem|PbSO|4}}(s) + 2{{chem|H|2|O}}(l) |
|||
含铅颜料可以使[[釉]]和玻璃涂上红色和黄色。{{sfn|Burleson|2001|p=23}}虽然含铅颜料在欧洲和北美已被淘汰,但像是中国{{sfn|Insight Explorer|IPEN|2016}}、印度{{sfn|Singh|2017}}和印尼{{sfn|Ismawati|Primanti|Brosché|Clark|2013|p=2}}的发展中国家仍在使用含铅颜料。四乙酸铅和二氧化铅是有机化学的氧化剂。[[铅玻璃]]中含有12–28%的[[氧化铅]],其光学性质和可以屏蔽辐射的性质{{sfn|Amstock|1997|pp=116–119}}可用于老式电视机和电脑荧幕的[[阴极射线管]]。碲化铅和硒化铅可用于[[太阳能光伏]]和[[红外线]]探测器。{{sfn|Rogalski|2010|pp=485–541}} |
|||
== 致病性 == |
== 致病性 == |
||
| 第483行: | 第503行: | ||
{{Refbegin |30em}} |
{{Refbegin |30em}} |
||
*{{cite book |last=Alsfasser |first=R. |title=Moderne anorganische Chemie |year=2007 |url=https://books.google.com/books?id=HwY4be5bH_sC&q=%5BPb4%5D4-+zintl |trans-title=Modern inorganic chemistry |publisher=Walter de Gruyter |isbn=978-3-11-019060-1 |language=de|ref=harv}} |
*{{cite book |last=Alsfasser |first=R. |title=Moderne anorganische Chemie |year=2007 |url=https://books.google.com/books?id=HwY4be5bH_sC&q=%5BPb4%5D4-+zintl |trans-title=Modern inorganic chemistry |publisher=Walter de Gruyter |isbn=978-3-11-019060-1 |language=de|ref=harv}} |
||
*{{cite book |last=Amstock |first=J. S. |title=Handbook of Glass in Construction |date=1997 |isbn=978-0-07-001619-4 |publisher=[[McGraw-Hill Professional]] |url=https://books.google.com/books?id=apWvKnKKrvsC|ref=harv}} |
|||
*{{cite web |url=https://eos.org/scientific-press/human-activity-has-polluted-european-air-for-2000-years |author=American Geophysical Union |title=Human Activity Has Polluted European Air for 2000 Years |publisher=Eos Science News |year=2017 |author-link=American Geophysical Union |access-date=4 July 2017 |archive-date=27 June 2017 |archive-url=https://web.archive.org/web/20170627215417/https://eos.org/scientific-press/human-activity-has-polluted-european-air-for-2000-years |url-status=dead|ref=harv}} |
*{{cite web |url=https://eos.org/scientific-press/human-activity-has-polluted-european-air-for-2000-years |author=American Geophysical Union |title=Human Activity Has Polluted European Air for 2000 Years |publisher=Eos Science News |year=2017 |author-link=American Geophysical Union |access-date=4 July 2017 |archive-date=27 June 2017 |archive-url=https://web.archive.org/web/20170627215417/https://eos.org/scientific-press/human-activity-has-polluted-european-air-for-2000-years |url-status=dead|ref=harv}} |
||
* {{cite journal |last=Anderson |first=J. |title=Malleability and ductility of metals |year=1869 |pages=341–43 |journal=[[Scientific American]] |volume=21 |issue=22 |doi=10.1038/scientificamerican11271869-341 |url=https://zenodo.org/record/1429682|ref=harv}} |
* {{cite journal |last=Anderson |first=J. |title=Malleability and ductility of metals |year=1869 |pages=341–43 |journal=[[Scientific American]] |volume=21 |issue=22 |doi=10.1038/scientificamerican11271869-341 |url=https://zenodo.org/record/1429682|ref=harv}} |
||
*{{cite journal |last1=Ashikari |first1=M. |title=The memory of the women's white faces: Japaneseness and the ideal image of women |date=2003 |pages=55–79 |doi=10.1080/0955580032000077739 |journal=Japan Forum |volume=15 |issue=1|s2cid=144510689|ref=harv}} |
*{{cite journal |last1=Ashikari |first1=M. |title=The memory of the women's white faces: Japaneseness and the ideal image of women |date=2003 |pages=55–79 |doi=10.1080/0955580032000077739 |journal=Japan Forum |volume=15 |issue=1|s2cid=144510689|ref=harv}} |
||
*{{cite book |last=Audsley |first=G. A.|title=The Art of Organ Building |year=1965 |url=https://books.google.com/books?id=I0h525OVoTgC |isbn=978-0-486-21315-6 |volume=2 |publisher=Courier |ref=harv}} |
|||
*{{cite book |last1=Baird |first1=C. |last2=Cann |first2=N. |title=Environmental Chemistry |date=2012 |publisher=W. H. Freeman and Company |isbn=978-1-4292-7704-4 |edition=5th|ref=harv}} |
|||
*{{cite journal |last1=Becker |first1=M. |last2=Förster |first2=C. |last3=Franzen |first3=C. |last4=Hartrath |first4=J. |last5=Kirsten |first5=E. |last6=Knuth |first6=J. |last7=Klinkhammer |first7=K. W. |last8=Sharma |first8=A. |last9=H. |first9=D. |title=Persistent radicals of trivalent tin and lead |year=2008 |pages=9965–78 |doi=10.1021/ic801198p |pmid=18823115 |journal=[[Inorganic Chemistry (journal)|Inorganic Chemistry]] |volume=47 |issue=21 |display-authors=3|ref=harv}} |
*{{cite journal |last1=Becker |first1=M. |last2=Förster |first2=C. |last3=Franzen |first3=C. |last4=Hartrath |first4=J. |last5=Kirsten |first5=E. |last6=Knuth |first6=J. |last7=Klinkhammer |first7=K. W. |last8=Sharma |first8=A. |last9=H. |first9=D. |title=Persistent radicals of trivalent tin and lead |year=2008 |pages=9965–78 |doi=10.1021/ic801198p |pmid=18823115 |journal=[[Inorganic Chemistry (journal)|Inorganic Chemistry]] |volume=47 |issue=21 |display-authors=3|ref=harv}} |
||
*{{cite journal |last1=Beeman |first1=J. W. |last2=Bellini |first2=F. |last3=Cardani |first3=L. |last4=Casali |first4=N. |title=New experimental limits on the ''α'' decays of lead isotopes |year=2013 |journal=European Physical Journal A |volume=49 |issue=50 |pages=50 |display-authors=3|doi=10.1140/epja/i2013-13050-7|arxiv=1212.2422 |bibcode=2013EPJA...49...50B |s2cid=119280082|ref=harv}} |
*{{cite journal |last1=Beeman |first1=J. W. |last2=Bellini |first2=F. |last3=Cardani |first3=L. |last4=Casali |first4=N. |title=New experimental limits on the ''α'' decays of lead isotopes |year=2013 |journal=European Physical Journal A |volume=49 |issue=50 |pages=50 |display-authors=3|doi=10.1140/epja/i2013-13050-7|arxiv=1212.2422 |bibcode=2013EPJA...49...50B |s2cid=119280082|ref=harv}} |
||
* {{cite journal |last1=Beiner |first1=G. G. |last2=Lavi |first2=M. |last3=Seri |first3=H. |display-authors=3 |last4=Rossin |first4=Anna |last5=Lev |first5=Ovadia |last6=Gun |first6=Jenny |last7=Rabinovich |first7=Rivka |title=Oddy Tests: Adding the Analytical Dimension |journal=Collection Forum |volume=29 |issue=1–2 |year=2015 |pages=22–36 |issn=0831-4985 |doi=10.14351/0831-4985-29.1.22|doi-access=free|ref=harv}} |
|||
*{{Cite book|last1=Bharara|first1=M. S.|last2=Atwood|first2=D. A.|title=Lead: Inorganic Chemistry|year=2006|doi=10.1002/0470862106.ia118|chapter=Lead: Inorganic ChemistryBased in part on the article Lead: Inorganic Chemistry by Philip G. Harrison which appeared in theEncyclopedia of Inorganic Chemistry, First Edition|isbn=978-0470860786|ref=harv}} |
*{{Cite book|last1=Bharara|first1=M. S.|last2=Atwood|first2=D. A.|title=Lead: Inorganic Chemistry|year=2006|doi=10.1002/0470862106.ia118|chapter=Lead: Inorganic ChemistryBased in part on the article Lead: Inorganic Chemistry by Philip G. Harrison which appeared in theEncyclopedia of Inorganic Chemistry, First Edition|isbn=978-0470860786|ref=harv}} |
||
*{{cite book |last1=Bisel |first1=S. C. |last2=Bisel |first2=J. F. |chapter=Health and nutrition at Herculaneum |title=The Natural History of Pompeii |year=2002 |pages=451–75 |publisher=[[Cambridge University Press]] |isbn=978-0-521-80054-9 |editor-first1=W. F. |editor-last1=Jashemski |editor-first2=F. G. |editor-last2=Meyer |chapter-url=https://books.google.com/books?id=3xfjyTqqR7IC|ref=harv}} |
*{{cite book |last1=Bisel |first1=S. C. |last2=Bisel |first2=J. F. |chapter=Health and nutrition at Herculaneum |title=The Natural History of Pompeii |year=2002 |pages=451–75 |publisher=[[Cambridge University Press]] |isbn=978-0-521-80054-9 |editor-first1=W. F. |editor-last1=Jashemski |editor-first2=F. G. |editor-last2=Meyer |chapter-url=https://books.google.com/books?id=3xfjyTqqR7IC|ref=harv}} |
||
| 第496行: | 第520行: | ||
*{{cite book |last1=Bunker |first1=B. C. |last2=Casey |first2=W. H. |title=The Aqueous Chemistry of Oxides |date=2016 |publisher=[[Oxford University Press]] |isbn=978-0-19-938425-9 |url=https://books.google.com/books?id=BfhGCwAAQBAJ&pg=PA89|ref=harv}} |
*{{cite book |last1=Bunker |first1=B. C. |last2=Casey |first2=W. H. |title=The Aqueous Chemistry of Oxides |date=2016 |publisher=[[Oxford University Press]] |isbn=978-0-19-938425-9 |url=https://books.google.com/books?id=BfhGCwAAQBAJ&pg=PA89|ref=harv}} |
||
*{{cite journal |last1=Burbidge |first1=E. M. |author-link1=玛格丽特·伯比奇 |last2=Burbidge |first2=G. R. |author-link2=傑佛瑞·伯比奇|last3=Fowler |first3=W. A. |author-link3=威廉·福勒 |last4=Hoyle |first4=F. |author-link4=弗雷德·霍伊尔 |title=Synthesis of the Elements in Stars |year=1957 |pages=547–654 |display-authors=3 |journal=[[Reviews of Modern Physics]] |volume=29 |issue=4 |bibcode=1957RvMP...29..547B |doi=10.1103/RevModPhys.29.547 |url=https://www.pmf.unizg.hr/_download/repository/burbidge_RMP_29_547_1957.pdf |doi-access=free|ref=harv}} |
*{{cite journal |last1=Burbidge |first1=E. M. |author-link1=玛格丽特·伯比奇 |last2=Burbidge |first2=G. R. |author-link2=傑佛瑞·伯比奇|last3=Fowler |first3=W. A. |author-link3=威廉·福勒 |last4=Hoyle |first4=F. |author-link4=弗雷德·霍伊尔 |title=Synthesis of the Elements in Stars |year=1957 |pages=547–654 |display-authors=3 |journal=[[Reviews of Modern Physics]] |volume=29 |issue=4 |bibcode=1957RvMP...29..547B |doi=10.1103/RevModPhys.29.547 |url=https://www.pmf.unizg.hr/_download/repository/burbidge_RMP_29_547_1957.pdf |doi-access=free|ref=harv}} |
||
*{{cite book |first=M. |last=Burleson |publisher=Sterling|title=The Ceramic Glaze Handbook: Materials, Techniques, Formulas |year=2001 |url=https://books.google.com/books?id=PiJEAhMxLQgC |isbn=9781579904395|ref=harv}} |
|||
* {{cite web |url=https://www.wildlife.ca.gov/hunting/nonlead-ammunition |title=Nonlead Ammunition in California |website=www.wildlife.ca.gov |author=California Department of Fish and Wildlife |access-date=17 May 2017|ref=harv}} |
|||
*{{Cite book|last=Calvo Rebollar|first=Miguel|year=2019|title=Construyendo la Tabla Periódica |publisher=Prames|isbn=978-84-8321-908-9|location=Zaragoza, Spain|ref=harv}} |
*{{Cite book|last=Calvo Rebollar|first=Miguel|year=2019|title=Construyendo la Tabla Periódica |publisher=Prames|isbn=978-84-8321-908-9|location=Zaragoza, Spain|ref=harv}} |
||
*{{cite book|last1=Cangelosi|first1=V. M.|last2=Pecoraro|first2=V. L.|editor-last=Roduner|editor-first=E.|year=2015|title=Nanoscopic Materials: Size-Dependent Phenomena and Growth Principles|chapter=Lead|chapter-url=https://books.google.com/books?id=kGsoDwAAQBAJ&pg=PA875|publisher=Royal Society of Chemistry|isbn=978-1-78262-494-3|pages=843–875|ref=harv}} |
*{{cite book|last1=Cangelosi|first1=V. M.|last2=Pecoraro|first2=V. L.|editor-last=Roduner|editor-first=E.|year=2015|title=Nanoscopic Materials: Size-Dependent Phenomena and Growth Principles|chapter=Lead|chapter-url=https://books.google.com/books?id=kGsoDwAAQBAJ&pg=PA875|publisher=Royal Society of Chemistry|isbn=978-1-78262-494-3|pages=843–875|ref=harv}} |
||
| 第503行: | 第529行: | ||
*{{cite book|last1=Considine|first1=D. M.|last2=Considine|first2=G. D.|title=Van Nostrand's Scientific Encyclopedia|url=https://books.google.com/books?id=t4jjBwAAQBAJ&pg=PA2970|date=2013|publisher=Springer Science & Business Media|isbn=978-1-4757-6918-0|ref=harv}} |
*{{cite book|last1=Considine|first1=D. M.|last2=Considine|first2=G. D.|title=Van Nostrand's Scientific Encyclopedia|url=https://books.google.com/books?id=t4jjBwAAQBAJ&pg=PA2970|date=2013|publisher=Springer Science & Business Media|isbn=978-1-4757-6918-0|ref=harv}} |
||
*{{cite book|author1=Committee on Evaluation of EPA Guidelines for Exposure to Naturally Occurring Radioactive Materials|author2=Commission on Life Sciences|author3=Division on Earth and Life Studies|author4=National Research Council|title=Evaluation of Guidelines for Exposures to Technologically Enhanced Naturally Occurring Radioactive Materials|url=https://books.google.com/books?id=8uhuAgAAQBAJ&pg=PA26|date=1999|publisher=National Academies Press|isbn=978-0-309-58070-0|pages=26, 30–32|ref=harv}} |
*{{cite book|author1=Committee on Evaluation of EPA Guidelines for Exposure to Naturally Occurring Radioactive Materials|author2=Commission on Life Sciences|author3=Division on Earth and Life Studies|author4=National Research Council|title=Evaluation of Guidelines for Exposures to Technologically Enhanced Naturally Occurring Radioactive Materials|url=https://books.google.com/books?id=8uhuAgAAQBAJ&pg=PA26|date=1999|publisher=National Academies Press|isbn=978-0-309-58070-0|pages=26, 30–32|ref=harv}} |
||
*{{cite report |author=[[Concordia University]] |title=Lead acid batteries |url=https://www.concordia.ca/content/dam/concordia/services/safety/docs/EHS-DOC-146_LeadAcidBatteries.pdf |year=2016 |access-date=17 February 2019 |ref=harv}} |
|||
* {{cite web |author=Copper Development Association |title=Leaded Coppers |url=http://www.copper.org/resources/properties/microstructure/cu_leaded.html |website=copper.org |access-date=10 July 2016|ref=harv}} |
|||
*{{cite book |last1=Cotnoir |first1=B. |title=The Weiser Concise Guide to Alchemy |date=2006 |publisher=Weiser Books |isbn=978-1-57863-379-1 |url=https://books.google.com/books?id=BPWDrnEJ7UsC|ref=harv}} |
*{{cite book |last1=Cotnoir |first1=B. |title=The Weiser Concise Guide to Alchemy |date=2006 |publisher=Weiser Books |isbn=978-1-57863-379-1 |url=https://books.google.com/books?id=BPWDrnEJ7UsC|ref=harv}} |
||
*{{cite book |last=Cox |first=P. A. |date=1997 |title=The Elements: Their Origin, Abundance and Distribution |url=https://archive.org/details/elementstheirori0000coxp |url-access=registration |publisher=Oxford University Press |isbn=978-0-19-855298-7|ref=harv}} |
*{{cite book |last=Cox |first=P. A. |date=1997 |title=The Elements: Their Origin, Abundance and Distribution |url=https://archive.org/details/elementstheirori0000coxp |url-access=registration |publisher=Oxford University Press |isbn=978-0-19-855298-7|ref=harv}} |
||
| 第509行: | 第537行: | ||
*{{cite book |first1=A. |last1=Davidson |first2=J. |last2=Ryman |first3=C. A. |last3=Sutherland |first4=E. F. |last4=Milner |first5=R. C. |last5=Kerby |first6=H. |last6=Teindl |first7=A. |last7=Melin |first8=H. M. |last8=Bolt |chapter=Lead |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2014 |doi=10.1002/14356007.a15_193.pub3 |display-authors=3 |isbn=978-3-527-30673-2|ref=harv}} |
*{{cite book |first1=A. |last1=Davidson |first2=J. |last2=Ryman |first3=C. A. |last3=Sutherland |first4=E. F. |last4=Milner |first5=R. C. |last5=Kerby |first6=H. |last6=Teindl |first7=A. |last7=Melin |first8=H. M. |last8=Bolt |chapter=Lead |title=Ullmann's Encyclopedia of Industrial Chemistry |year=2014 |doi=10.1002/14356007.a15_193.pub3 |display-authors=3 |isbn=978-3-527-30673-2|ref=harv}} |
||
*{{cite book |last=Emsley |first=J. |author-link=John Emsley |title=Nature's Building Blocks: An A-Z Guide to the Elements |year=2011 |publisher=Oxford University Press |isbn=978-0-19-960563-7|ref=harv}} |
*{{cite book |last=Emsley |first=J. |author-link=John Emsley |title=Nature's Building Blocks: An A-Z Guide to the Elements |year=2011 |publisher=Oxford University Press |isbn=978-0-19-960563-7|ref=harv}} |
||
*{{cite journal |last1=Evans |first1=J. W. |title=V.— The meanings and synonyms of plumbago |date=1908 |pages=133–79 |doi=10.1111/j.1467-968X.1908.tb00513.x |journal=Transactions of the Philological Society|volume=26 |issue=2 |url=https://zenodo.org/record/2111957|ref=harv}} |
|||
*{{cite journal |last=Fiorini |first=E. |title=2.000 years-old Roman Lead for physics |date=2010 |pages=7–8 |url=http://www.aspera-eu.org/images/stories/news/PAPER_VERSIONS/asperanewsletter0610.pdf |journal=ASPERA |access-date=29 October 2016 |archive-date=26 April 2018 |archive-url=https://web.archive.org/web/20180426184739/http://www.aspera-eu.org/images/stories/news/PAPER_VERSIONS/asperanewsletter0610.pdf |url-status=dead|ref=harv}} |
*{{cite journal |last=Fiorini |first=E. |title=2.000 years-old Roman Lead for physics |date=2010 |pages=7–8 |url=http://www.aspera-eu.org/images/stories/news/PAPER_VERSIONS/asperanewsletter0610.pdf |journal=ASPERA |access-date=29 October 2016 |archive-date=26 April 2018 |archive-url=https://web.archive.org/web/20180426184739/http://www.aspera-eu.org/images/stories/news/PAPER_VERSIONS/asperanewsletter0610.pdf |url-status=dead|ref=harv}} |
||
*{{cite journal |last=de Callataÿ |first=F. |title=The Graeco-Roman economy in the super long-run: Lead, copper, and shipwrecks |date=2005 |pages=361–72 |journal=Journal of Roman Archaeology|volume=18 |doi=10.1017/S104775940000742X|s2cid=232346123|ref=harv}} |
*{{cite journal |last=de Callataÿ |first=F. |title=The Graeco-Roman economy in the super long-run: Lead, copper, and shipwrecks |date=2005 |pages=361–72 |journal=Journal of Roman Archaeology|volume=18 |doi=10.1017/S104775940000742X|s2cid=232346123|ref=harv}} |
||
*{{cite report |last=Guberman |first=D. E. |chapter=Lead |title=2014 Minerals Yearbook |year=2016 |chapter-url=https://minerals.usgs.gov/minerals/pubs/commodity/lead/myb1-2014-lead.pdf |publisher=[[United States Geological Survey]] |access-date=8 May 2017|ref=harv}} |
|||
*{{cite book |last=Guruswamy |first=S. |title=Engineering properties and applications of lead alloys |date=2000 |url=https://books.google.com/books?id=TtGmjOv9CUAC |isbn=978-0-8247-8247-4 |publisher=Marcel Dekker|ref=harv}} |
|||
*{{cite journal |last1=de Marcillac |first1=Pierre |last2=Coron |first2=Noël |last3=Dambier |first3=Gérard |last4=Leblanc |first4=Jacques |last5=Moalic |first5=Jean-Pierre |title=Experimental detection of α-particles from the radioactive decay of natural bismuth |date=2003 |pages=876–78 |display-authors=3 |journal=[[Nature (journal)|Nature]] |volume=422 |pmid=12712201 |doi=10.1038/nature01541 |issue=6934 |bibcode=2003Natur.422..876D|s2cid=4415582|ref=harv}} |
*{{cite journal |last1=de Marcillac |first1=Pierre |last2=Coron |first2=Noël |last3=Dambier |first3=Gérard |last4=Leblanc |first4=Jacques |last5=Moalic |first5=Jean-Pierre |title=Experimental detection of α-particles from the radioactive decay of natural bismuth |date=2003 |pages=876–78 |display-authors=3 |journal=[[Nature (journal)|Nature]] |volume=422 |pmid=12712201 |doi=10.1038/nature01541 |issue=6934 |bibcode=2003Natur.422..876D|s2cid=4415582|ref=harv}} |
||
*{{cite book |last=Donnelly |first=J. |title=Deep Blue |url=https://books.google.com/books?id=SKtbAwAAQBAJ |year=2014 |publisher=Hachette Children's Group |isbn=978-1-4449-2119-9 |ref=harv}} |
*{{cite book |last=Donnelly |first=J. |title=Deep Blue |url=https://books.google.com/books?id=SKtbAwAAQBAJ |year=2014 |publisher=Hachette Children's Group |isbn=978-1-4449-2119-9 |ref=harv}} |
||
| 第522行: | 第553行: | ||
*{{cite journal |last=Gilfillan |first=S. C. |title=Lead poisoning and the fall of Rome |date=1965 |pages=53–60 |journal=Journal of Occupational Medicine |volume=7 |number=2 |issn=0096-1736 |pmid=14261844|ref=harv}} |
*{{cite journal |last=Gilfillan |first=S. C. |title=Lead poisoning and the fall of Rome |date=1965 |pages=53–60 |journal=Journal of Occupational Medicine |volume=7 |number=2 |issn=0096-1736 |pmid=14261844|ref=harv}} |
||
*{{cite book |last1=Gill |first1=T. |author2=Libraries Board of South Australia |title=The history and topography of Glen Osmond, with map and illustrations |url=https://books.google.com/books?id=oSMQAAAAYAAJ |year=1974 |publisher=Libraries Board of South Australia |isbn=9780724300358|ref=harv}} |
*{{cite book |last1=Gill |first1=T. |author2=Libraries Board of South Australia |title=The history and topography of Glen Osmond, with map and illustrations |url=https://books.google.com/books?id=oSMQAAAAYAAJ |year=1974 |publisher=Libraries Board of South Australia |isbn=9780724300358|ref=harv}} |
||
*{{cite report |last1=Graedel |first1=T. E. |title=Metal stocks in Society – Scientific Synthesis |year=2010 |url=http://www.unep.fr/shared/publications/pdf/DTIx1264xPA-Metal%20stocks%20in%20society.pdf |isbn=978-92-807-3082-1 |publisher=International Resource Panel |access-date=18 April 2017 |page=17 |display-authors=etal<!--only mentions the lead author; others are not named--> |archive-date=26 April 2018 |archive-url=https://web.archive.org/web/20180426184751/http://www.unep.fr/shared/publications/pdf/DTIx1264xPA-Metal%20stocks%20in%20society.pdf |url-status=dead|ref=harv}} |
|||
* {{cite book |last1=Greenwood |first1=N. N. |last2=Earnshaw |first2=A. |title=Chemistry of the Elements |year=1998 |edition=2nd |publisher=Butterworth-Heinemann |isbn=978-0-7506-3365-9 |url=https://books.google.com/books?id=EvTI-ouH3SsC&q=chemistry+of+the+elements |ref=harv}} |
* {{cite book |last1=Greenwood |first1=N. N. |last2=Earnshaw |first2=A. |title=Chemistry of the Elements |year=1998 |edition=2nd |publisher=Butterworth-Heinemann |isbn=978-0-7506-3365-9 |url=https://books.google.com/books?id=EvTI-ouH3SsC&q=chemistry+of+the+elements |ref=harv}} |
||
*{{cite web |last=Grout |first=J. |title=Lead poisoning and Rome |date=2017 |url=http://penelope.uchicago.edu/~grout/encyclopaedia_romana/wine/leadpoisoning.html |website=Encyclopaedia Romana |access-date=15 February 2017 |ref=harv}} |
*{{cite web |last=Grout |first=J. |title=Lead poisoning and Rome |date=2017 |url=http://penelope.uchicago.edu/~grout/encyclopaedia_romana/wine/leadpoisoning.html |website=Encyclopaedia Romana |access-date=15 February 2017 |ref=harv}} |
||
*{{cite book |last=Gulbinska |first=M. K. |title=Lithium-ion Battery Materials and Engineering: Current Topics and Problems from the Manufacturing Perspective |url=https://books.google.com/books?id=ZDRxBAAAQBAJ |year=2014 |publisher=[[Springer Science+Business Media|Springer]] |isbn=978-1-4471-6548-4 |page=96|ref=harv}} |
|||
*{{cite book |last=Hadlington |first=T. J. |title=On the Catalytic Efficacy of Low-Oxidation State Group 14 Complexes |url=https://books.google.com/books?id=KQshDgAAQBAJ |year=2017 |publisher=Springer |isbn=978-3-319-51807-7|ref=harv}} |
*{{cite book |last=Hadlington |first=T. J. |title=On the Catalytic Efficacy of Low-Oxidation State Group 14 Complexes |url=https://books.google.com/books?id=KQshDgAAQBAJ |year=2017 |publisher=Springer |isbn=978-3-319-51807-7|ref=harv}} |
||
*{{cite book |last1=Harbison |first1=R. D. |last2=Bourgeois |first2=M. M. |last3=Johnson |first3=G. T. |title=Hamilton and Hardy's Industrial Toxicology |date=2015 |publisher=John Wiley & Sons |isbn=978-0-470-92973-5|ref=harv}} |
*{{cite book |last1=Harbison |first1=R. D. |last2=Bourgeois |first2=M. M. |last3=Johnson |first3=G. T. |title=Hamilton and Hardy's Industrial Toxicology |date=2015 |publisher=John Wiley & Sons |isbn=978-0-470-92973-5|ref=harv}} |
||
| 第532行: | 第565行: | ||
*{{cite book |last=Hunt |first=A. |title=Dictionary of Chemistry |url=https://books.google.com/books?id=P_lRAwAAQBAJ |year=2014 |publisher=[[Routledge]] |isbn=978-1-135-94178-9|ref=harv}} |
*{{cite book |last=Hunt |first=A. |title=Dictionary of Chemistry |url=https://books.google.com/books?id=P_lRAwAAQBAJ |year=2014 |publisher=[[Routledge]] |isbn=978-1-135-94178-9|ref=harv}} |
||
*{{cite web |author=IAEA - Nuclear Data Section |title=Livechart - Table of Nuclides - Nuclear structure and decay data |year=2017 |website=www-nds.iaea.org |publisher=[[International Atomic Energy Agency]] |url=https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html |access-date=31 March 2017|ref=harv}} |
*{{cite web |author=IAEA - Nuclear Data Section |title=Livechart - Table of Nuclides - Nuclear structure and decay data |year=2017 |website=www-nds.iaea.org |publisher=[[International Atomic Energy Agency]] |url=https://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html |access-date=31 March 2017|ref=harv}} |
||
*{{cite report |author1=Insight Explorer |author2=IPEN |date=2016 |title=New Study Finds Lead Levels in a Majority of Paints Exceed Chinese Regulation and Should Not be on Store Shelves |url=http://www.ipen.org/sites/default/files/documents/PR_New_Study_Finds_Lead_Levels_in_a_Majority_of_Paints_Exceed_Chinese_Regulation_and_Should_Not_be_on_Store_Shelves_EN_27_Jan._2016.pdf |access-date=3 May 2018 |ref=harv}} |
|||
*{{cite report|first1=Yuyun|last1=Ismawati|first2=Andita|last2=Primanti|first3=Sara|last3=Brosché|first4=Scott|last4=Clark|first5=Jack|last5=Weinberg|first6=Valerie|last6=Denney|date=2013|title=Timbal dalam Cat Enamel Rumah Tangga di Indonesia|url=https://ipen.org/pdfs/ina_bf_final_report_rev_2_2013_08-v2-id.pdf|access-date=26 December 2018|publisher=BaliFokus & IPEN|language=id|ref=harv}} |
|||
*{{cite book |last=Jensen |first=C. F. |title=Online Location of Faults on AC Cables in Underground Transmission |year=2013 |publisher=Springer |isbn=978-3-319-05397-4|ref=harv}} |
|||
*{{cite book |last=Kaupp |first=M. |chapter=Chemical bonding of main-group elements |title=The Chemical Bond: Chemical Bonding Across the Periodic Table |pages=1–24 |year=2014 |publisher=John Wiley & Sons |doi=10.1002/9783527664658.ch1 |editor-last=Frenking |editor-first=G. |editor-last2=Shaik |editor-first2=S. |chapter-url=https://application.wiley-vch.de/books/sample/3527333150_c01.pdf |isbn=9783527664658 |s2cid=17979350 |url=https://semanticscholar.org/paper/ebec75488be0e4720dec372219aef32404432921|ref=harv}} |
*{{cite book |last=Kaupp |first=M. |chapter=Chemical bonding of main-group elements |title=The Chemical Bond: Chemical Bonding Across the Periodic Table |pages=1–24 |year=2014 |publisher=John Wiley & Sons |doi=10.1002/9783527664658.ch1 |editor-last=Frenking |editor-first=G. |editor-last2=Shaik |editor-first2=S. |chapter-url=https://application.wiley-vch.de/books/sample/3527333150_c01.pdf |isbn=9783527664658 |s2cid=17979350 |url=https://semanticscholar.org/paper/ebec75488be0e4720dec372219aef32404432921|ref=harv}} |
||
*{{cite book |last=Kellett |first=C. |title=Poison and Poisoning: A Compendium of Cases, Catastrophes and Crimes |year=2012 |url=https://books.google.com/books?id=Wa0hBgAAQBAJ |publisher=Accent Press |isbn=978-1-909335-05-9 |ref=harv}} |
*{{cite book |last=Kellett |first=C. |title=Poison and Poisoning: A Compendium of Cases, Catastrophes and Crimes |year=2012 |url=https://books.google.com/books?id=Wa0hBgAAQBAJ |publisher=Accent Press |isbn=978-1-909335-05-9 |ref=harv}} |
||
*{{cite book |last=King |first=R. B. |title=Inorganic Chemistry of Main Group Elements |date=1995 |publisher=VCH Publishers |isbn=978-1-56081-679-9|ref=harv}} |
*{{cite book |last=King |first=R. B. |title=Inorganic Chemistry of Main Group Elements |date=1995 |publisher=VCH Publishers |isbn=978-1-56081-679-9|ref=harv}} |
||
*{{cite book |last1=Konu |first1=J. |last2=Chivers |first2=T. |chapter=Stable Radicals of the Heavy p-Block Elements |editor-last=Hicks |editor-first=R. G. |title=Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds |chapter-url=https://books.google.com/books?id=WuwW8QDuCKIC |year=2011 |publisher=John Wiley & Sons |isbn=978-0-470-77083-2 |doi=10.1002/9780470666975.ch10|ref=harv}} |
*{{cite book |last1=Konu |first1=J. |last2=Chivers |first2=T. |chapter=Stable Radicals of the Heavy p-Block Elements |editor-last=Hicks |editor-first=R. G. |title=Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds |chapter-url=https://books.google.com/books?id=WuwW8QDuCKIC |year=2011 |publisher=John Wiley & Sons |isbn=978-0-470-77083-2 |doi=10.1002/9780470666975.ch10|ref=harv}} |
||
*{{cite book |last1=Krestovnikoff |first1=M. |last2=Halls |first2=M. |title=Scuba Diving |year=2006 |url=https://archive.org/details/scubadiving0000hall |url-access=registration |publisher=[[Dorling Kindersley]] |isbn=978-0-7566-4063-7|ref=harv}} |
|||
*{{cite book |last1=Langmuir |first1=C. H. |last2=Broecker |first2=W. S. |title=How to Build a Habitable Planet: The Story of Earth from the Big Bang to Humankind |url=https://books.google.com/books?id=EnlnHlmRsqAC |year=2012 |publisher=[[Princeton University Press]] |isbn=978-0-691-14006-3 |ref=harv}} |
*{{cite book |last1=Langmuir |first1=C. H. |last2=Broecker |first2=W. S. |title=How to Build a Habitable Planet: The Story of Earth from the Big Bang to Humankind |url=https://books.google.com/books?id=EnlnHlmRsqAC |year=2012 |publisher=[[Princeton University Press]] |isbn=978-0-691-14006-3 |ref=harv}} |
||
*{{cite web |url=http://www.britishmuseum.org/research/search_the_collection_database/search_object_details.aspx?objectid=399876&partid=1&output=Terms%2F!!%2FOR%2F!!%2F1204%2F!%2F%2F!%2FClassical+Greek%2F!%2F%2F!!%2F%2F!!!%2F&orig=%2Fresearch%2Fsearch_the_collection_database%2Fadvanced_search.aspx¤tPage=7&numpages=10 |publisher=[[大英博物馆|The British Museum]] |title=Lead sling bullet; almond shape; a winged thunderbolt on one side and on the other, in high relief, the inscription DEXAI "Catch!" |access-date=30 April 2012 |ref=CITEREFLead sling bullet |archive-date=14 February 2012 |archive-url=https://web.archive.org/web/20120214235913/http://www.britishmuseum.org/research/search_the_collection_database/search_object_details.aspx?objectid=399876&partid=1&output=Terms%2f!!%2fOR%2f!!%2f1204%2f!%2f%2f!%2fClassical+Greek%2f!%2f%2f!!%2f%2f!!!%2f&orig=%2fresearch%2fsearch_the_collection_database%2fadvanced_search.aspx¤tPage=7&numpages=10 |url-status=dead}} |
*{{cite web |url=http://www.britishmuseum.org/research/search_the_collection_database/search_object_details.aspx?objectid=399876&partid=1&output=Terms%2F!!%2FOR%2F!!%2F1204%2F!%2F%2F!%2FClassical+Greek%2F!%2F%2F!!%2F%2F!!!%2F&orig=%2Fresearch%2Fsearch_the_collection_database%2Fadvanced_search.aspx¤tPage=7&numpages=10 |publisher=[[大英博物馆|The British Museum]] |title=Lead sling bullet; almond shape; a winged thunderbolt on one side and on the other, in high relief, the inscription DEXAI "Catch!" |access-date=30 April 2012 |ref=CITEREFLead sling bullet |archive-date=14 February 2012 |archive-url=https://web.archive.org/web/20120214235913/http://www.britishmuseum.org/research/search_the_collection_database/search_object_details.aspx?objectid=399876&partid=1&output=Terms%2f!!%2fOR%2f!!%2f1204%2f!%2f%2f!%2fClassical+Greek%2f!%2f%2f!!%2f%2f!!!%2f&orig=%2fresearch%2fsearch_the_collection_database%2fadvanced_search.aspx¤tPage=7&numpages=10 |url-status=dead}} |
||
| 第548行: | 第585行: | ||
*{{cite journal |last1=Mosseri |first1=S. |last2=Henglein |first2=A. |last3=Janata |first3=E. |title=Trivalent lead as an intermediate in the oxidation of lead(II) and the reduction of lead(IV) species |year=1990 |pages=2722–26 |doi=10.1021/j100369a089 |journal=Journal of Physical Chemistry |volume=94 |issue=6|ref=harv}} |
*{{cite journal |last1=Mosseri |first1=S. |last2=Henglein |first2=A. |last3=Janata |first3=E. |title=Trivalent lead as an intermediate in the oxidation of lead(II) and the reduction of lead(IV) species |year=1990 |pages=2722–26 |doi=10.1021/j100369a089 |journal=Journal of Physical Chemistry |volume=94 |issue=6|ref=harv}} |
||
*{{cite journal |last1=Nakashima |first1=T. |last2=Hayashi |first2=H. |last3=Tashiro |first3=H. |last4=Matsushita |first4=T. |title=Gender and hierarchical differences in lead-contaminated Japanese bone from the Edo period |year=1998 |pages=55–60 |doi=10.1539/joh.40.55 |display-authors=3 |journal=Journal of Occupational Health |volume=40 |issue=1|doi-access=free|ref=harv}} |
*{{cite journal |last1=Nakashima |first1=T. |last2=Hayashi |first2=H. |last3=Tashiro |first3=H. |last4=Matsushita |first4=T. |title=Gender and hierarchical differences in lead-contaminated Japanese bone from the Edo period |year=1998 |pages=55–60 |doi=10.1539/joh.40.55 |display-authors=3 |journal=Journal of Occupational Health |volume=40 |issue=1|doi-access=free|ref=harv}} |
||
*{{cite book |author=National Council on Radiation Protection and Measurements |title=Structural Shielding Design for Medical X-ray Imaging Facilities |date=2004 |url=https://books.google.com/books?id=DKu4YDjEluoC |isbn=978-0-929600-83-3 |ref=harv}} |
|||
* {{cite book |last=Norman |first=N. C. |title=Periodicity and the s- and p-Block Elements |date=1996 |publisher=Oxford University Press |isbn=978-0-19-855961-0|ref=harv}} |
* {{cite book |last=Norman |first=N. C. |title=Periodicity and the s- and p-Block Elements |date=1996 |publisher=Oxford University Press |isbn=978-0-19-855961-0|ref=harv}} |
||
*{{cite journal |last=Nriagu |first=J. O. |title=Saturnine gout among Roman aristocrats — Did lead poisoning contribute to the fall of the Empire? |year=1983 |pages=660–63 |journal=[[The New England Journal of Medicine]] |volume=308 |issue=11 |doi=10.1056/NEJM198303173081123 |pmid=6338384|ref=harv}} |
*{{cite journal |last=Nriagu |first=J. O. |title=Saturnine gout among Roman aristocrats — Did lead poisoning contribute to the fall of the Empire? |year=1983 |pages=660–63 |journal=[[The New England Journal of Medicine]] |volume=308 |issue=11 |doi=10.1056/NEJM198303173081123 |pmid=6338384|ref=harv}} |
||
*{{Cite web|url=https://www.jewishvirtuallibrary.org/ossuaries-and-sarcophagi|title=Encyclopedia Judaica: Ossuaries and Sarcophagi|website=www.jewishvirtuallibrary.org|access-date=14 July 2018|ref=CITEREFOssuaries and Sarcophagi}} |
*{{Cite web|url=https://www.jewishvirtuallibrary.org/ossuaries-and-sarcophagi|title=Encyclopedia Judaica: Ossuaries and Sarcophagi|website=www.jewishvirtuallibrary.org|access-date=14 July 2018|ref=CITEREFOssuaries and Sarcophagi}} |
||
*{{cite web |last=Olinsky-Paul |year=2013 |first=T. |title=East Penn and Ecoult battery installation case study webinar |url=http://www.cesa.org/assets/Uploads/ESTAP-Ecoult-Battery-Installation-Case-Study-Presentation.pdf |website=Clean Energy States Alliance |access-date=28 February 2017|ref=harv}} |
|||
*{{cite book |title=The Organ |date=2006 |url=https://books.google.com/books?id=cgDJaeFFUPoC |isbn=978-0-415-94174-7 |editor-last=Palmieri |editor-first=R. |publisher=Psychology Press|ref=harv}} |
|||
*{{cite book |title=Crystal Chemistry of Tetrahedral Structures |url=https://books.google.com/books?id=bwQgvsxftTgC |date=1964 |last=Parthé |first=E. |publisher=CRC Press |isbn=978-0-677-00700-7 |ref=harv}} |
*{{cite book |title=Crystal Chemistry of Tetrahedral Structures |url=https://books.google.com/books?id=bwQgvsxftTgC |date=1964 |last=Parthé |first=E. |publisher=CRC Press |isbn=978-0-677-00700-7 |ref=harv}} |
||
*{{cite book |last=Parker |first=R. B. |title=The New Cold-Molded Boatbuilding: From Lofting to Launching |year=2005 |url=https://books.google.com/books?id=VnoW5YpDN7sC |publisher=WoodenBoat Books |isbn=978-0-937822-89-0|ref=harv}} |
|||
*{{cite book |last=Pauling |first=L. |title=General Chemistry |date=1947 |author-link=Linus Pauling |publisher=W. H. Freeman and Company |isbn=978-0-486-65622-9 |url=https://archive.org/details/generalchemistry00paul_0 |ref=harv}} |
*{{cite book |last=Pauling |first=L. |title=General Chemistry |date=1947 |author-link=Linus Pauling |publisher=W. H. Freeman and Company |isbn=978-0-486-65622-9 |url=https://archive.org/details/generalchemistry00paul_0 |ref=harv}} |
||
*{{cite book |last=Polyanskiy |first=N. G. |date=1986 |script-title=ru:Аналитическая химия элементов: Свинец |trans-title=Analytical Chemistry of the Elements: Lead |editor=Fillipova, N. A |publisher=Nauka |language=ru|ref=harv}} |
*{{cite book |last=Polyanskiy |first=N. G. |date=1986 |script-title=ru:Аналитическая химия элементов: Свинец |trans-title=Analytical Chemistry of the Elements: Lead |editor=Fillipova, N. A |publisher=Nauka |language=ru|ref=harv}} |
||
*{{cite web |title=Primary Lead Refining Technical Notes |url=http://www.ldaint.org/technotes2.htm |publisher=LDA International |access-date=7 April 2007 |archive-url=https://web.archive.org/web/20070322191856/http://www.ldaint.org/technotes2.htm |archive-date=22 March 2007 |ref=CITEREFPrimary Lead Refining }} |
*{{cite web |title=Primary Lead Refining Technical Notes |url=http://www.ldaint.org/technotes2.htm |publisher=LDA International |access-date=7 April 2007 |archive-url=https://web.archive.org/web/20070322191856/http://www.ldaint.org/technotes2.htm |archive-date=22 March 2007 |ref=CITEREFPrimary Lead Refining }} |
||
*{{cite web |author=Progressive Dynamics, Inc. |title=How Lead Acid Batteries Work: Battery Basics |url=http://www.progressivedyn.com/battery_basics.html |website=progressivedyn.com |access-date=3 July 2016 |archive-url=https://web.archive.org/web/20181119073137/https://www.progressivedyn.com/service/battery-basics/ |archive-date=19 November 2018|ref=harv}} |
|||
*{{cite book |last1=Putnam |first1=B. |title=The Sculptor's Way: A Guide to Modelling and Sculpture |url=https://books.google.com/books?id=-DC3UDjjPvgC |date=2003 |publisher=Dover Publications|isbn=978-0-486-42313-5|ref=harv}} |
|||
* {{cite journal |last=Pyykkö |first=P. |title=Relativistic effects in structural chemistry |year=1988 |pages=563–94 |journal=[[Chemical Reviews]] |volume=88 |doi=10.1021/cr00085a006 |issue=3|ref=harv}} |
* {{cite journal |last=Pyykkö |first=P. |title=Relativistic effects in structural chemistry |year=1988 |pages=563–94 |journal=[[Chemical Reviews]] |volume=88 |doi=10.1021/cr00085a006 |issue=3|ref=harv}} |
||
*{{cite book |last=Rabinowitz |first=M. B. |chapter=Imputing lead sources from blood lead isotope ratios |editor-last1=Beard |editor-first1=M. E. |editor-last2=Allen Iske |editor-first2=S. D. |title=Lead in Paint, Soil, and Dust: Health Risks, Exposure Studies, Control Measures, Measurement Methods, and Quality Assurance |year=1995 |publisher=ASTM |isbn=978-0-8031-1884-3 |pages=63–75 |chapter-url=https://books.google.com/books?id=-wt0AXfjiUIC |doi=10.1520/stp12967s|ref=harv}} |
*{{cite book |last=Rabinowitz |first=M. B. |chapter=Imputing lead sources from blood lead isotope ratios |editor-last1=Beard |editor-first1=M. E. |editor-last2=Allen Iske |editor-first2=S. D. |title=Lead in Paint, Soil, and Dust: Health Risks, Exposure Studies, Control Measures, Measurement Methods, and Quality Assurance |year=1995 |publisher=ASTM |isbn=978-0-8031-1884-3 |pages=63–75 |chapter-url=https://books.google.com/books?id=-wt0AXfjiUIC |doi=10.1520/stp12967s|ref=harv}} |
||
| 第564行: | 第607行: | ||
*{{cite journal |last1=Retief |first1=F. |last2=Cilliers |first2=L. P. |title=Lead poisoning in ancient Rome |year=2006 |pages=147–64 (149–51) |journal=Acta Theologica |volume=26 |issue=2 |doi=10.4314/actat.v26i2.52570|doi-access=free|ref=harv}} |
*{{cite journal |last1=Retief |first1=F. |last2=Cilliers |first2=L. P. |title=Lead poisoning in ancient Rome |year=2006 |pages=147–64 (149–51) |journal=Acta Theologica |volume=26 |issue=2 |doi=10.4314/actat.v26i2.52570|doi-access=free|ref=harv}} |
||
*{{cite book |last=Rich |first=V. |title=The International Lead Trade |year=1994 |publisher=Woodhead Publishing |isbn=978-0-85709-994-5 |url=https://books.google.com/books?id=mfPJCgAAQBAJ|ref=harv}} |
*{{cite book |last=Rich |first=V. |title=The International Lead Trade |year=1994 |publisher=Woodhead Publishing |isbn=978-0-85709-994-5 |url=https://books.google.com/books?id=mfPJCgAAQBAJ|ref=harv}} |
||
*{{cite book |last=Rogalski |first=A. |title=Infrared Detectors |edition=2nd |year=2010 |url=https://books.google.com/books?id=0VUJSafhaK0C |access-date=19 November 2016 |publisher=CRC Press |isbn=978-1-4200-7671-4|ref=harv}} |
|||
*{{cite book |last=Röhr |first=C. |chapter=Binare Zintl-Phasen |trans-chapter=Binary Zintl Phases |title=Intermetallische Phasen |trans-title=Intermettallic Phases |publisher=[[弗里堡大學|Universitat Freiburg]] |language=de |chapter-url=http://ruby.chemie.uni-freiburg.de/Vorlesung/intermetallische_6_2.html |access-date=18 February 2017 |year=2017|ref=harv}} |
*{{cite book |last=Röhr |first=C. |chapter=Binare Zintl-Phasen |trans-chapter=Binary Zintl Phases |title=Intermetallische Phasen |trans-title=Intermettallic Phases |publisher=[[弗里堡大學|Universitat Freiburg]] |language=de |chapter-url=http://ruby.chemie.uni-freiburg.de/Vorlesung/intermetallische_6_2.html |access-date=18 February 2017 |year=2017|ref=harv}} |
||
*{{cite web |url=https://ocw.mit.edu/courses/earth-atmospheric-and-planetary-sciences/12-744-marine-isotope-chemistry-fall-2012/nuclear-systematics/MIT12_744F12_Lec4.pdf |title=Radioactive Decay Series |series=Nuclear Systematics |publisher=[[MIT OpenCourseWare]] |date=2012 |access-date=28 April 2018|ref=CITEREFRadioactive Decay Series2012}} |
*{{cite web |url=https://ocw.mit.edu/courses/earth-atmospheric-and-planetary-sciences/12-744-marine-isotope-chemistry-fall-2012/nuclear-systematics/MIT12_744F12_Lec4.pdf |title=Radioactive Decay Series |series=Nuclear Systematics |publisher=[[MIT OpenCourseWare]] |date=2012 |access-date=28 April 2018|ref=CITEREFRadioactive Decay Series2012}} |
||
| 第569行: | 第613行: | ||
*{{cite journal |last=Scarborough |first=J. |title=The myth of lead poisoning among the Romans: An essay review |date=1984 |pages=469–475 |journal=Journal of the History of Medicine and Allied Sciences|volume=39 |issue=4 |doi=10.1093/jhmas/39.4.469 |pmid=6389691|ref=harv}} |
*{{cite journal |last=Scarborough |first=J. |title=The myth of lead poisoning among the Romans: An essay review |date=1984 |pages=469–475 |journal=Journal of the History of Medicine and Allied Sciences|volume=39 |issue=4 |doi=10.1093/jhmas/39.4.469 |pmid=6389691|ref=harv}} |
||
*{{cite journal |last=Silverman |first=M. S. |title=High-pressure (70-k) synthesis of new crystalline lead dichalcogenides |date=1966 |pages=2067–69 |journal=[[Inorganic Chemistry (journal)|Inorganic Chemistry]] |volume=5 |issue=11 |doi=10.1021/ic50045a056|ref=harv}} |
*{{cite journal |last=Silverman |first=M. S. |title=High-pressure (70-k) synthesis of new crystalline lead dichalcogenides |date=1966 |pages=2067–69 |journal=[[Inorganic Chemistry (journal)|Inorganic Chemistry]] |volume=5 |issue=11 |doi=10.1021/ic50045a056|ref=harv}} |
||
*{{cite news |last=Singh |first=P. |date=2017 |title=Over 73% of paints found to have excessive lead: Study |url=https://timesofindia.indiatimes.com/life-style/health-fitness/health-news/over-73-of-paints-found-to-have-excessive-lead-study/articleshow/61334283.cms |newspaper=[[Times of India]] |access-date=3 May 2018 |ref=harv}} |
|||
*{{cite journal|last1=Slater|first1=J. C.|title=Atomic Radii in Crystals|journal=The Journal of Chemical Physics|volume=41|issue=10|year=1964|pages=3199–3204|issn=0021-9606|doi=10.1063/1.1725697|bibcode=1964JChPh..41.3199S|ref=harv}} |
*{{cite journal|last1=Slater|first1=J. C.|title=Atomic Radii in Crystals|journal=The Journal of Chemical Physics|volume=41|issue=10|year=1964|pages=3199–3204|issn=0021-9606|doi=10.1063/1.1725697|bibcode=1964JChPh..41.3199S|ref=harv}} |
||
*{{cite journal |last1=Sinha |first1=S. P. |last2=Shelly |first2=<!--empty; same in source--> |last3=Sharma |first3=V. |last4=Meenakshi |first4=<!--empty; same in source--> |last5=Srivastava |first5=S. |last6=Srivastava |first6=M. M. |title=Neurotoxic effects of lead exposure among printing press workers |year=1993 |doi=10.1007/BF00192162 |pmid=8400649 |pages=490–93|journal=Bulletin of Environmental Contamination and Toxicology |volume=51 |issue=4 |s2cid=26631583 |display-authors=3|ref=harv}} |
*{{cite journal |last1=Sinha |first1=S. P. |last2=Shelly |first2=<!--empty; same in source--> |last3=Sharma |first3=V. |last4=Meenakshi |first4=<!--empty; same in source--> |last5=Srivastava |first5=S. |last6=Srivastava |first6=M. M. |title=Neurotoxic effects of lead exposure among printing press workers |year=1993 |doi=10.1007/BF00192162 |pmid=8400649 |pages=490–93|journal=Bulletin of Environmental Contamination and Toxicology |volume=51 |issue=4 |s2cid=26631583 |display-authors=3|ref=harv}} |
||
*{{cite journal |last1=Smirnov |first1=A. Yu. |last2=Borisevich |first2=V. D. |last3=Sulaberidze |first3=A. |title=Evaluation of specific cost of obtainment of lead-208 isotope by gas centrifuges using various raw materials |year=2012 |pages=373–78 |journal=Theoretical Foundations of Chemical Engineering |volume=46 |issue=4 |doi=10.1134/s0040579512040161|s2cid=98821122|ref=harv}} |
*{{cite journal |last1=Smirnov |first1=A. Yu. |last2=Borisevich |first2=V. D. |last3=Sulaberidze |first3=A. |title=Evaluation of specific cost of obtainment of lead-208 isotope by gas centrifuges using various raw materials |year=2012 |pages=373–78 |journal=Theoretical Foundations of Chemical Engineering |volume=46 |issue=4 |doi=10.1134/s0040579512040161|s2cid=98821122|ref=harv}} |
||
*{{cite journal |last1=Stabenow |first1=F. |last2=Saak |first2=W. |last3=Weidenbruch |first3=M. |title=Tris(triphenylplumbyl)plumbate: An anion with three stretched lead–lead bonds |year=2003 |pages=2342–2343 |doi=10.1039/B305217F |pmid=14518905 |journal=Chemical Communications|issue=18|ref=harv}} |
*{{cite journal |last1=Stabenow |first1=F. |last2=Saak |first2=W. |last3=Weidenbruch |first3=M. |title=Tris(triphenylplumbyl)plumbate: An anion with three stretched lead–lead bonds |year=2003 |pages=2342–2343 |doi=10.1039/B305217F |pmid=14518905 |journal=Chemical Communications|issue=18|ref=harv}} |
||
*{{cite book |last1=Street |first1=A. |last2=Alexander |first2=W. |title=Metals in the Service of Man |edition=11th |url=https://books.google.com/books?id=cOCTPwAACAAJ |year=1998 |publisher=[[Penguin Books]] |isbn=978-0-14-025776-2|ref=harv}} |
|||
*{{cite journal |last=Stone |first=R. |year=1997 |title=An Element of Stability |journal=Science |volume=278 |issue=5338 |pages=571–572 |doi=10.1126/science.278.5338.571|bibcode=1997Sci...278..571S |s2cid=117946028|ref=harv}} |
*{{cite journal |last=Stone |first=R. |year=1997 |title=An Element of Stability |journal=Science |volume=278 |issue=5338 |pages=571–572 |doi=10.1126/science.278.5338.571|bibcode=1997Sci...278..571S |s2cid=117946028|ref=harv}} |
||
*{{cite book |last=Szczepanowska |first=H. M. |title=Conservation of Cultural Heritage: Key Principles and Approaches |url=https://books.google.com/books?id=yu9_LZ1AD_gC |year=2013 |publisher=Routledge |isbn=978-0-415-67474-4|ref=harv}} |
|||
*{{cite journal |last1=Tétreault |first1=J. |last2=Sirois |first2=J. |last3=Stamatopoulou |first3=E. |title=Studies of lead corrosion in acetic acid environments |date=1998 |pages=17–32 |journal=Studies in Conservation |volume=43 |issue=1 |doi=10.2307/1506633 |jstor=1506633|ref=harv}} |
*{{cite journal |last1=Tétreault |first1=J. |last2=Sirois |first2=J. |last3=Stamatopoulou |first3=E. |title=Studies of lead corrosion in acetic acid environments |date=1998 |pages=17–32 |journal=Studies in Conservation |volume=43 |issue=1 |doi=10.2307/1506633 |jstor=1506633|ref=harv}} |
||
*{{cite journal |last1=Takahashi |first1=K. |last2=Boyd |first2=R. N. |last3=Mathews |first3=G. J. |last4=Yokoi |first4=K. |title=Bound-state beta decay of highly ionized atoms |date=1987 |url=http://www.ca.infn.it/~oldeman/resneu/p1522_1.pdf |display-authors=3 |access-date=27 August 2013 |oclc=1639677 |volume=36 |issue=4 |pages=1522–1528 |journal=[[Physical Review C]] |archive-url=https://web.archive.org/web/20141021195204/http://www.ca.infn.it/~oldeman/resneu/p1522_1.pdf |archive-date=21 October 2014 |url-status=dead |doi=10.1103/physrevc.36.1522 |pmid=9954244 |bibcode=1987PhRvC..36.1522T|ref=harv}} |
*{{cite journal |last1=Takahashi |first1=K. |last2=Boyd |first2=R. N. |last3=Mathews |first3=G. J. |last4=Yokoi |first4=K. |title=Bound-state beta decay of highly ionized atoms |date=1987 |url=http://www.ca.infn.it/~oldeman/resneu/p1522_1.pdf |display-authors=3 |access-date=27 August 2013 |oclc=1639677 |volume=36 |issue=4 |pages=1522–1528 |journal=[[Physical Review C]] |archive-url=https://web.archive.org/web/20141021195204/http://www.ca.infn.it/~oldeman/resneu/p1522_1.pdf |archive-date=21 October 2014 |url-status=dead |doi=10.1103/physrevc.36.1522 |pmid=9954244 |bibcode=1987PhRvC..36.1522T|ref=harv}} |
||
| 第579行: | 第626行: | ||
*{{cite book |last1=Thornton |first1=I. |last2=Rautiu |first2=R. |last3=Brush |first3=S. M. |title=Lead: The Facts |date=2001 |url=http://www.ila-lead.org/UserFiles/File/factbook/leadTheFacts.pdf |publisher=International Lead Association |access-date=5 February 2017 |isbn=978-0-9542496-0-1|ref=harv }} |
*{{cite book |last1=Thornton |first1=I. |last2=Rautiu |first2=R. |last3=Brush |first3=S. M. |title=Lead: The Facts |date=2001 |url=http://www.ila-lead.org/UserFiles/File/factbook/leadTheFacts.pdf |publisher=International Lead Association |access-date=5 February 2017 |isbn=978-0-9542496-0-1|ref=harv }} |
||
*{{cite web |title=Toxicological Profile for Lead |date=2007 |url=https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf |work=Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine |archive-url=https://web.archive.org/web/20170701213753/https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf |archive-date=1 July 2017 |ref=CITEREFToxicological Profile for Lead2007 }} |
*{{cite web |title=Toxicological Profile for Lead |date=2007 |url=https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf |work=Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine |archive-url=https://web.archive.org/web/20170701213753/https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf |archive-date=1 July 2017 |ref=CITEREFToxicological Profile for Lead2007 }} |
||
*{{cite journal |last1=Tuček |first1=K. |last2=Carlsson |first2=J. |last3=Wider |first3=H. |title=Comparison of sodium and lead-cooled fast reactors regarding reactor physics aspects, severe safety and economical issues |date=2006 |pages=1589–98 |doi=10.1016/j.nucengdes.2006.04.019 |url=http://www.ecolo.org/documents/documents_in_english/SFRvsLFR-05.pdf |journal=Nuclear Engineering and Design |volume=236 |issue=14–16|ref=harv}} |
|||
*{{cite web |author=<!--Not stated--> |title=Think Lead research summary |url=http://leadsheet.co.uk/wp-content/uploads/2016/06/lsa-research-summary.pdf |publisher=The Lead Sheet Association |access-date=20 February 2017 |ref=CITEREFThink Lead research |archive-date=20 February 2017 |archive-url=https://web.archive.org/web/20170220173232/http://leadsheet.co.uk/wp-content/uploads/2016/06/lsa-research-summary.pdf |url-status=dead }} |
|||
*{{cite journal |last1=Thürmer |first1=K. |last2=Williams |first2=E. |last3=Reutt-Robey |first3=J. |title=Autocatalytic oxidation of lead crystallite surfaces |date=2002 |pages=2033–35 |doi=10.1126/science.297.5589.2033 |journal=Science |volume=297 |issue=5589 |pmid=12242437 |bibcode=2002Sci...297.2033T |s2cid=6166273 |url=https://semanticscholar.org/paper/79518d3315bfb93d2cae634655ec1b030ce285fc|ref=harv}} |
*{{cite journal |last1=Thürmer |first1=K. |last2=Williams |first2=E. |last3=Reutt-Robey |first3=J. |title=Autocatalytic oxidation of lead crystallite surfaces |date=2002 |pages=2033–35 |doi=10.1126/science.297.5589.2033 |journal=Science |volume=297 |issue=5589 |pmid=12242437 |bibcode=2002Sci...297.2033T |s2cid=6166273 |url=https://semanticscholar.org/paper/79518d3315bfb93d2cae634655ec1b030ce285fc|ref=harv}} |
||
*{{cite web |last=Tolliday |first=B. |title=Significant growth in lead usage underlines its importance to the global economy |date=2014 |url=http://www.ila-lead.org/news/lead-in-the-news/2012-11-30/significant-growth-in-lead-usage-underlines-its--importance-to-the-global-economy- |publisher=International Lead Association|access-date=28 February 2017 |quote=Global demand for lead has more than doubled since the early 1990s and almost 90% of use is now in lead-acid batteries |archive-date=28 February 2017 |archive-url=https://web.archive.org/web/20170228171821/http://www.ila-lead.org/news/lead-in-the-news/2012-11-30/significant-growth-in-lead-usage-underlines-its--importance-to-the-global-economy- |url-status=dead|ref=harv}} |
|||
*{{cite news |title=Toronto museum explores history of contraceptives |year=2003 |url=http://www.abc.net.au/news/2003-08-13/toronto-museum-explores-history-of-contraceptives/1463884 |website=ABC News |access-date=13 February 2016 |ref=CITEREFToronto museum explores2003 }} |
*{{cite news |title=Toronto museum explores history of contraceptives |year=2003 |url=http://www.abc.net.au/news/2003-08-13/toronto-museum-explores-history-of-contraceptives/1463884 |website=ABC News |access-date=13 February 2016 |ref=CITEREFToronto museum explores2003 }} |
||
* {{cite book |last=Tungate |first=M. |title=Branded Beauty: How Marketing Changed the Way We Look |url=https://books.google.com/books?id=jQA_B84aVhYC&pg=PA14 |year=2011 |publisher=Kogan Page Publishers |isbn=978-0-7494-6182-9|ref=harv}} |
* {{cite book |last=Tungate |first=M. |title=Branded Beauty: How Marketing Changed the Way We Look |url=https://books.google.com/books?id=jQA_B84aVhYC&pg=PA14 |year=2011 |publisher=Kogan Page Publishers |isbn=978-0-7494-6182-9|ref=harv}} |
||
*{{cite book |author=United States Geological Survey |title=Geological Survey Professional Paper |url=https://books.google.com/books?id=_LdUAAAAYAAJ |year=1973 |publisher=[[United States Government Publishing Office]] |page=314 |author-link=United States Geological Survey|ref=harv}} |
*{{cite book |author=United States Geological Survey |title=Geological Survey Professional Paper |url=https://books.google.com/books?id=_LdUAAAAYAAJ |year=1973 |publisher=[[United States Government Publishing Office]] |page=314 |author-link=United States Geological Survey|ref=harv}} |
||
| ⚫ | * {{cite web |author=[[University of California, Berkeley|University of California]] Nuclear Forensic Search Project |title=Decay Chains |url=http://metadata.berkeley.edu/nuclear-forensics/Decay%20Chains.html |access-date=23 November 2015 |website=Nuclear Forensics: A Scientific Search Problem|ref=harv}} |
||
*{{cite report |author=United States Geological Survey |title=Lead |year=2005 |url=http://minerals.usgs.gov/minerals/pubs/commodity/lead/lead_mcs05.pdf |access-date=20 February 2016 |ref=harv}} |
*{{cite report |author=United States Geological Survey |title=Lead |year=2005 |url=http://minerals.usgs.gov/minerals/pubs/commodity/lead/lead_mcs05.pdf |access-date=20 February 2016 |ref=harv}} |
||
*{{cite web |author=United States Geological Survey |title=Lead |year=2017 |series=Mineral Commodities Summaries |url=https://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2017-lead.pdf |access-date=8 May 2017|ref=harv}} |
*{{cite web |author=United States Geological Survey |title=Lead |year=2017 |series=Mineral Commodities Summaries |url=https://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2017-lead.pdf |access-date=8 May 2017|ref=harv}} |
||
| ⚫ | *{{cite web |author=[[University of California, Berkeley|University of California]] Nuclear Forensic Search Project |title=Decay Chains |url=http://metadata.berkeley.edu/nuclear-forensics/Decay%20Chains.html |access-date=23 November 2015 |website=Nuclear Forensics: A Scientific Search Problem|ref=harv}} |
||
*{{cite report |last1=Vogel |first1=N. A. |last2=Achilles |first2=R. |title=The Preservation and Repair of Historic Stained and Leaded Glass |year=2013 |url=https://www.nps.gov/tps/how-to-preserve/preservedocs/preservation-briefs/33Preserve-Brief-StainedGlass.pdf |publisher=[[United States Department of the Interior]] |access-date=30 October 2016 |ref=harv}} |
*{{cite report |last1=Vogel |first1=N. A. |last2=Achilles |first2=R. |title=The Preservation and Repair of Historic Stained and Leaded Glass |year=2013 |url=https://www.nps.gov/tps/how-to-preserve/preservedocs/preservation-briefs/33Preserve-Brief-StainedGlass.pdf |publisher=[[United States Department of the Interior]] |access-date=30 October 2016 |ref=harv}} |
||
*{{cite journal |last=Waldron |first=H. A. |title=Lead and lead poisoning in antiquity |date=1985 |pages=107–08 |journal=Medical History |volume=29 |issue=1 |pmc=1139494|doi=10.1017/S0025727300043878|ref=harv}} |
*{{cite journal |last=Waldron |first=H. A. |title=Lead and lead poisoning in antiquity |date=1985 |pages=107–08 |journal=Medical History |volume=29 |issue=1 |pmc=1139494|doi=10.1017/S0025727300043878|ref=harv}} |
||
| 第593行: | 第643行: | ||
*{{cite journal |last=Winder |first=C. |title=The history of lead — Part 3 |year=1993b |url=http://lead.org.au/lanv2n3/lanv2n3-22.html |access-date=12 February 2016 |url-status=dead |archive-url=https://web.archive.org/web/20070831200744/http://lead.org.au/lanv2n3/lanv2n3-22.html |archive-date=31 August 2007 |journal=LEAD Action News |volume=2 |issue=3 |issn=1324-6011|ref=harv}} |
*{{cite journal |last=Winder |first=C. |title=The history of lead — Part 3 |year=1993b |url=http://lead.org.au/lanv2n3/lanv2n3-22.html |access-date=12 February 2016 |url-status=dead |archive-url=https://web.archive.org/web/20070831200744/http://lead.org.au/lanv2n3/lanv2n3-22.html |archive-date=31 August 2007 |journal=LEAD Action News |volume=2 |issue=3 |issn=1324-6011|ref=harv}} |
||
*{{cite book |last=Windholz |first=M. |title=Merck Index of Chemicals and Drugs |date=1976 |id=Monograph 8393 |edition=9th |publisher=[[Merck & Co.]] |isbn=978-0-911910-26-1|title-link=Merck Index|ref=harv}} |
*{{cite book |last=Windholz |first=M. |title=Merck Index of Chemicals and Drugs |date=1976 |id=Monograph 8393 |edition=9th |publisher=[[Merck & Co.]] |isbn=978-0-911910-26-1|title-link=Merck Index|ref=harv}} |
||
*{{cite web |author=<!--Not stated--> |title=Weatherings to Parapets and Cornices |url=http://leadsheet.co.uk/home/lsa-pocket-guide/weatherings-to-parapets-and-cornices/ |publisher=The Lead Sheet Association |access-date=20 February 2017 |ref=CITEREFWeatherings to Parapets |archive-date=20 February 2017 |archive-url=https://web.archive.org/web/20170220172917/http://leadsheet.co.uk/home/lsa-pocket-guide/weatherings-to-parapets-and-cornices/ |url-status=dead }} |
|||
*{{cite journal |last1=Webb |first1=G. W. |last2=Marsiglio |first2=F. |last3=Hirsch |first3=J. E. |title=Superconductivity in the elements, alloys and simple compounds |journal=Physica C: Superconductivity and Its Applications |volume=514 |year=2015 |pages=17–27 |doi=10.1016/j.physc.2015.02.037|arxiv=1502.04724 |bibcode=2015PhyC..514...17W |s2cid=119290828 |ref=harv}} |
*{{cite journal |last1=Webb |first1=G. W. |last2=Marsiglio |first2=F. |last3=Hirsch |first3=J. E. |title=Superconductivity in the elements, alloys and simple compounds |journal=Physica C: Superconductivity and Its Applications |volume=514 |year=2015 |pages=17–27 |doi=10.1016/j.physc.2015.02.037|arxiv=1502.04724 |bibcode=2015PhyC..514...17W |s2cid=119290828 |ref=harv}} |
||
*{{cite web |author=World Nuclear Association |title=Nuclear Radiation and Health Effects |year=2015 |url=http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Nuclear-Radiation-and-Health-Effects/ |access-date=12 November 2015|ref=harv}} |
*{{cite web |author=World Nuclear Association |title=Nuclear Radiation and Health Effects |year=2015 |url=http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Nuclear-Radiation-and-Health-Effects/ |access-date=12 November 2015|ref=harv}} |
||
| 第602行: | 第653行: | ||
*{{cite book |last1=Yu |first1=L. |last2=Yu |first2=H. |title=Chinese Coins: Money in History and Society |year=2004 |url=https://books.google.com/books?id=QfWQB0peEWYC |publisher=Long River Press |isbn=978-1-59265-017-0|ref=harv}} |
*{{cite book |last1=Yu |first1=L. |last2=Yu |first2=H. |title=Chinese Coins: Money in History and Society |year=2004 |url=https://books.google.com/books?id=QfWQB0peEWYC |publisher=Long River Press |isbn=978-1-59265-017-0|ref=harv}} |
||
*{{cite journal |last1=Zhang |first1=X. |last2=Yang |first2=L. |last3=Li |first3=Y. |last4=Li |first4=H. |last5=Wang |first5=W. |last6=Ye |first6=B. |title=Impacts of lead/zinc mining and smelting on the environment and human health in China |year=2012 |pages=2261–73 |display-authors=3 |journal=Environmental Monitoring and Assessment |volume=184 |issue=4 |doi=10.1007/s10661-011-2115-6 |pmid=21573711|s2cid=20372810|ref=harv}} |
*{{cite journal |last1=Zhang |first1=X. |last2=Yang |first2=L. |last3=Li |first3=Y. |last4=Li |first4=H. |last5=Wang |first5=W. |last6=Ye |first6=B. |title=Impacts of lead/zinc mining and smelting on the environment and human health in China |year=2012 |pages=2261–73 |display-authors=3 |journal=Environmental Monitoring and Assessment |volume=184 |issue=4 |doi=10.1007/s10661-011-2115-6 |pmid=21573711|s2cid=20372810|ref=harv}} |
||
*{{cite book |last=Zhao |first=F. |title=Information Technology Entrepreneurship and Innovation |url=https://books.google.com/books?id=aXm9AQAAQBAJ |year=2008 |publisher=IGI Global |isbn=978-1-59904-902-1 |page=440|ref=harv}} |
|||
*{{cite book |last1=Zuckerman |first1=J. J. |last2=Hagen |first2=A. P. |title=Inorganic Reactions and Methods, the Formation of Bonds to Halogens |date=1989 |publisher=John Wiley & Sons |isbn=978-0-471-18656-4|ref=harv}} |
*{{cite book |last1=Zuckerman |first1=J. J. |last2=Hagen |first2=A. P. |title=Inorganic Reactions and Methods, the Formation of Bonds to Halogens |date=1989 |publisher=John Wiley & Sons |isbn=978-0-471-18656-4|ref=harv}} |
||
*{{cite journal |last=Zýka |first=J. |title=Analytical study of the basic properties of lead tetraacetate as oxidizing agent |year=1966 |pages=569–81 |journal=Pure and Applied Chemistry |volume=13 |issue=4 |doi=10.1351/pac196613040569 |s2cid=96821219 |url=http://www.sciencemadness.org/talk/files.php?pid=242223&aid=18041 |access-date=2 March 2017|ref=harv}} |
*{{cite journal |last=Zýka |first=J. |title=Analytical study of the basic properties of lead tetraacetate as oxidizing agent |year=1966 |pages=569–81 |journal=Pure and Applied Chemistry |volume=13 |issue=4 |doi=10.1351/pac196613040569 |s2cid=96821219 |url=http://www.sciencemadness.org/talk/files.php?pid=242223&aid=18041 |access-date=2 March 2017|ref=harv}} |
||
2022年10月11日 (二) 15:56的版本
| 本条目近期正在擴充或大幅編修。 若本条目已數日無大修改,請移除本模板。 致添加本模板的編者:如須在短時間內大幅修改,請務必在編輯期間用 {{inuse}}或{{inuse2}}來取代本模板。本条目由Nucleus hydro elemon(贡献·日志)於19個月前最后编辑。 |
铅(拼音:qiān,注音:ㄑㄧㄢ,粤拼:jyun4;英語:Lead),是一種化學元素,其化學符號为Pb[3](源于拉丁語:Plumbum),原子序數为82,原子量為207.2 u。铅是比大多數常見材料密度更高的重金屬。它是柔軟、可鍛鑄、熔點较低的金属。剛切割出來的鉛呈略帶藍色的銀色,但暴露在空氣中後會失去光澤,变成暗灰色。鉛是原子序最高的穩定元素,其三個穩定同位素是較重元素的主要衰變鏈的終點。
鉛是一種較不易反應的後過渡金屬。它的弱金屬性由其兩性性質所展現:铅傾向形成共價鍵,而它和它的氧化物與酸、鹼都会反應。和其它碳族元素的+4氧化态不同,铅在其化合物中通常以+2氧化態出现,但在有機鉛化合物中则和其它碳族元素一样以+4氧化态出现。鉛傾向與自身結合成鏈狀和多面體結構,这点与其它碳族元素类似。
鉛很容易從其礦石中提取出來,这使得近东人很早就发现了它。方鉛礦是鉛的主要礦石,而这种矿石通常帶有銀。对银的需求使得古罗马开始广泛提取和使用铅。鉛的產量在羅馬帝國衰落後下降,直到工業革命才達到相比的水平。铅合金因为可以容易地铸造印刷机的活字,所以在印刷机的发展中发挥了至关重要的作用。[4]在2014年,鉛的全球年產量約為1000萬噸,其中一半以上來自回收。鉛的高密度、低熔點、延展性和對氧化的相對惰性使其有用。這些特性加上其相對豐富含量和低成本,使其廣泛用於建築、管路系統、鉛酸蓄電池、彈頭和散彈彈丸、砝碼、銲料、白镴、易熔合金、含鉛油漆、含鉛汽油和輻射屏蔽。
鉛的毒性在19世纪后期广为人知,因此在許多應用中逐步被淘汰。鉛是會在軟組織和骨骼中積累的神經毒素,會損害神經系統並干擾生物酶的功能,導致神經系統疾病。此外,铅还会影响健康、心血管和肾脏系统。
物理性质
纯铅是亮银色,带有一丝蓝色的金属,[5]在湿气下失去光泽。铅有高密度、延展性和因为钝化造成的抗腐蚀性。[6]

铅的最密堆积结构和高原子量造成了11.34 g/cm3的高密度,[7]比铁(7.87 g/cm3)、铜(8.93 g/cm3)和锌(7.14 g/cm3)等常见金属的密度高。[8]一些如钨和金(都是19.3 g/cm3)的金属有更高的密度,而已知密度最大的金属锇的密度为22.59 g/cm3,几乎是铅的两倍。[9]
铅很软,莫氏硬度仅为1.5,可以用指甲刮痕。[10]它有良好的展性和略微的延性。[11][a]铅的体积模量为45.8 GPa。作为比较,铝的体积模量为75.2 GPa,铜为137.8 GPa,低碳钢则为160–169 GPa。[12]铅的强度较低,为12–17 MPa(铝的强度是它的六倍,铜为十倍,低碳钢则为十五倍),但可以通过加入少量铜或锑强化。[13]
相较于其它金属,铅的熔点很低,只有327.5 °C(621.5 °F)[14]。[7][b]它的沸点是所有碳族元素中最低的,为1,749 °C(3,180 °F)。[14]铅在20 °C下的电阻率为192 nΩ·m,比其它工业金属(铜为15.43 nΩ·m,金为20.51 nΩ·m,铝为24.15 nΩ·m)大了一个数量级。[16]铅在7.19 K以下会转变成超导体。[17]这个超导临界温度是第一类超导体中最高的,也是单质超导体中第三高的。[18]
原子性质
铅有82个电子,电子排布为 [Xe]4f145d106s26p2。照理来说,元素周期表越往下,元素的外层电子离原子核越远,较低轨道的屏蔽效应越强,电离能就越低,但铅的第一和第二电离能的总和(移除两个6p电子所需的能量)比上面的锡略高。这个现象是由镧系收缩导致的。和元素周期律预测的相反,铅的前四个电离能的总和比锡高。[19]在重元素中变得显著的相对论效应的影响也促成了这种现象。[c]相对论效应导致了惰性电子对效应,使得铅的6s电子难以参与键合,因此铅晶体中最近原子之间的距离异常的长。[21]
较轻的碳族元素由于其s轨道和p轨道的能量足够近,可以杂化成四个 sp3轨道,因此都可以形成稳定或亚稳定的四配位钻石结构同素异形体。不过,铅因为惰性电子对效应而导致s轨道和p轨道的能量差距变大,而这个大差距使得铅的晶格能无法弥补杂化轨道所需的能量。[22]因此,铅不形成钻石结构,而是使p电子离域,形成金属键和面心立方结构。[23]其它有相似大小[24]的二价金属钙和锶也会形成这种结构。[25][d]
同位素
铅有四种自然存在的穩定同位素:鉛-204、鉛-206、鉛-207和鉛-208[27]以及痕量的五种短寿命放射性同位素。[28]铅的质子数82是幻数,因此核壳层模型认为铅的原子核很稳定。[29]铅-208的中子数126是另一个幻数,这使得它更加稳定。[29]
铅是最重的稳定元素,而铅-208是所有元素中最重的稳定同位素(以前认为原子序83的铋是最重的稳定元素,但在2003年发现它唯一的原生核素铋-209有极弱的放射性[e])。铅的四种稳定同位素理论上都可以α衰变成汞的同位素并释放能量,但至今仍未观察到衰变。它们的半衰期预测在1035至10189年之间。[32]
铅-206、铅-207和铅-208分別是铀-238、铀-235和钍-232衰变的最终产物。[33]它们的衰变链分别叫做鈾衰變鏈、錒衰變鏈和釷衰變鏈。[34]铅同位素的丰度会因为铀或钍的存在而可能有很大的变化。举个例子,铅-208正常的同位素丰度为52%,但在钍矿中的丰度可高达90%。[35]因此,铅的标准原子质量只能精确到十分位。[36]随着时间流逝,铅-206和铅-207的数量会因为铀的衰变而增加,但铅-204不会增加,因此它们的比例可用于测年。随着铀衰变成铅,它们的比例也会改变,这也是铀铅测年法可以使用的基础。[37]铅-207会发生核磁共振,可用于研究其溶液和固态化合物,[38][39]甚至包括人体。[40]

除了稳定同位素以外,铅还有一些痕量同位素。其中一种痕量同位素是铅-210,虽然它的半衰期只有22.2年,[27]但在自然界中仍以铀-238的衰变产物少量存在。铅-211、铅-212和铅-214也都分别存在于铀-235、钍-232和铀-238的衰变链中,因此这三种铅同位素都存在于自然界。极痕量的铅-209可由铀-235的衰变产物镭-223罕见的簇衰变而成,或是在镎-237(由铀的中子捕获而成)的衰变中产生。通过测量铅-210和铅-206(它们都存在于同一条衰变链中)的比例,可以得出样本的年龄。[41]
人们已发现43种铅的同位素,原子量在178–220之间。[27]其中,铅-205是最稳定的放射性同位素,半衰期1.73×107年。[f]第二稳定的同位素是铅-202,半衰期为52,500年,比所有铅的天然放射性同位素长。[27]
化学性质

块状的铅在湿气下会形成含有各种成分的保护层。这层保护层主要是碳酸铅,[43][44][45]而在城市或海洋则可能产生硫酸铅或氯化铅。[46]这层保护层使得块状铅对空气呈惰性。[46]和大部分金属一样,铅粉会自燃,[47]并产生蓝白色的火焰。[48]
氟气在室温下就能和铅反应,形成氟化铅。氯气需要加热才能发生反应,而且产生的氯化铅会使铅的反应性下降。[46]熔融的铅会和氧族元素反应,生成对应的化合物。[49]
金属铅可以抵御硫酸和磷酸,但不能抵御盐酸和硝酸。[50]乙酸等有机酸在氧气存在下可以溶解铅。[46]浓碱也可以溶解铅,生成亚铅酸盐。[51]
无机化合物
铅有两种常见的氧化态+4和+2。碳族元素通常以四价存在。二价的碳和硅很罕见,二价锗较少见,二价锡较重要(但仍比四价锡少见),而二价铅化合物要比四价铅化合物多。[46]这个现象是由惰性电子对效应导致的,结果就是铅的6s轨道收缩,使它在离子化合物中变得惰性。惰性电子对效应在共价化合物(例如有机铅化合物)中没那么明显。在这些化合物中,6s和6p轨道的大小接近,可以发生sp3杂化,因此铅在这些化合物中和碳一样主要以四价存在。[52]
铅(II)和铅(IV)的电负性差距很大(分别是1.87和2.23),这个差距表明碳族元素+4氧化态的稳定性随着族往下而下降。作为比较,锡(II)和锡(IV)的电负性差距不大,分别是1.80和1.96。[53]
铅(II)

无机铅化合物中的铅通常以+2氧化态存在。就算是像氟气和氯气这样的强氧化剂也只能将铅氧化到PbF2和PbCl2。[46]Pb2+离子通常无色,[54]会部分水解成Pb(OH)+和[Pb4(OH)4]4+(其中的氢氧根是桥接配体),[55][56]还原性不如锡(II)。Pb2+的定性分析通常依赖它和稀盐酸反应生成的氯化铅沉淀。由于氯化铅的溶解度不是特别小,所以在极稀溶液中会往溶液通入硫化氢,通过生成硫化铅来分析Pb2+。[57]
一氧化铅有两种同质异形体,分别是红色的密陀僧 α-PbO和黄色的黄丹 β-PbO,后者只在488 °C以上稳定。密陀僧是最常用的无机铅化合物。[58]因为增加铅(II)溶液的pH会使其水解,所以氢氧化铅并不存在。[59]铅会和较重的氧族元素反应。硫化铅是半导体和光导体,还可以制造极敏感的红外线探测器。另外两种氧族元素化合物——硒化铅和碲化铅也都是光导体。它们的颜色随着族往下而变浅。[60]

包括二砹化铅[61]和像是PbFCl的混合卤化物等二卤化铅都获得了良好的表征。除二氟化铅以外的二卤化铅(特别是碘化铅)在光照下都会分解。[62]许多铅(II)的拟卤化物都是已知的,例如其氰化物、氰酸盐和硫氰酸盐。[60][63]铅(II)可以形成多种卤素配离子,例如[PbCl4]2−、[PbCl6]4−和链状离子[Pb2Cl9]n5n−。[62]
和其它二价重金属的硫酸盐一样,硫酸铅不溶于水。硝酸铅和乙酸铅极易溶于水,因此可用于合成其它铅化合物。[64]
铅(IV)
无机铅(IV)化合物不多,它们仅在氧化性溶液中形成,通常不存在于标准条件下。[65]一氧化铅可以继续被氧化成Pb3O4,它的结构被描述为氧化铅(II,IV)或2PbO·PbO2,是铅最著名的混合价态化合物。二氧化铅是强氧化剂,可以把盐酸氧化成氯气,[66]这是因为反应应该产生的PbCl4不稳定,会分解成PbCl2和Cl2。[67]类似一氧化铅形成亚铅酸盐,二氧化铅也可以形成铅酸盐。二硫化铅[68]和二硒化铅[69]都只在高压下稳定。四氟化铅(黄色晶体)是稳定的,但稳定性比二氟化铅低。四氯化铅(黄色油状液体)在室温下就会分解,四溴化铅更不稳定,而四碘化铅是否存在仍是问题。[70]
其它氧化态
一些铅化合物中的铅不以+4或+2氧化态存在。铅(III)存在于较大的有机铅化合物中,因为Pb3+和其配合物都是自由基而不稳定。[72][73][74]这个不稳定性同样适用于存在于某些自由基中的铅(I)。[75]
许多铅(II)和铅(IV)的混合氧化物是已知的。在空气下加热PbO2会使其在293 °C下分解成Pb12O19,在351 °C下分解成Pb12O17,在374 °C下分解成Pb3O4,最终在605 °C下分解成PbO。Pb2O3和各种非整比氧化物可以在高压下制备。这些氧化物大多呈有缺陷的萤石结构,其中一些应该是由氧占据的地方被空位占据。PbO的晶体结构也可以看作是有缺陷的萤石结构,其中每个交替的氧原子层都被空位取代。[76]
负价铅存在于津特耳相中,例如Ba2Pb中的Pb(−IV)[77]和各种多面体形的簇合物(如三角双锥形的Pb52−离子,其中有两个铅原子的氧化态为-1,另外三个为0[78])。它们可以由在液氨里用钠还原铅而成。[79]
有机铅化合物

碳
氢
铅
和其它碳族元素一样,铅可以成链,但因为Pb–Pb键键能要比C–C键键能低很多而成链倾向较低。[49]铅也可以形成双键和三键。[80]它和碳成键成有机铅化合物,但这些化合物因为Pb–C键较弱[55]而比普通的有机化合物不稳定。[81]这使得有机铅化学的研究要比有机锡化学少。[82]有机铅化合物的铅通常以+4氧化态存在,但也有Pb[CH(SiMe3)2]2和Pb(η5-C5H5)2等例外。[82]
最简单的有机化合物甲烷对应的铅化合物是铅烷,它可以由金属铅和氢原子反应而成。[83]它的衍生物四甲基铅和四乙基铅是最著名的有机铅化合物。这些衍生物比较稳定,四乙基铅在加热[84]或光照下才会分解。[85][g]钠铅合金会和卤代烃反应,生成如四乙基铅的有机铅化合物。[86]四乙酸铅是实验室中重要的氧化剂,可用于有机合成。[87]在四乙基铅仍然可以添加到汽油的时代,它的产量比任何有机金属化合物都要高。[82]其它有机铅化合物都不稳定,[81]大部分有机化合物都没有对应的铅化合物。[83]
起源与存量
| 原子序 | 元素 | 相对丰度 |
|---|---|---|
| 42 | 钼 | 0.798 |
| 46 | 钯 | 0.440 |
| 50 | 锡 | 1.146 |
| 78 | 铂 | 0.417 |
| 80 | 汞 | 0.127 |
| 82 | 铅 | 1 |
| 90 | 钍 | 0.011 |
| 92 | 铀 | 0.003 |
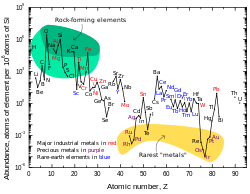
太空
铅在太阳系的丰度是0.121 ppb,[88][h]是铂的两倍半,汞的八倍,金的十七倍。[88]由于更重的元素都会衰变成铅[89],因此宇宙中铅的丰度在不断上升。[90]现在太阳系中铅的丰度要比45亿年前高出0.75%。[91]左边的图表显示虽然铅的原子序较高,但它的丰度要比大部分原子序大于40的元素高。[88]
铅的稳定同位素铅-204、铅-206、铅-207和铅-208大多是由恒星中原子核不断通过s-过程和r-过程捕获中子而成的。[92]在s-过程中(s代表慢),每次中子捕获都需要等待几年或几十年,使得较不稳定的原子核有足够时间发生β衰变。[93]稳定的铊-203捕获一个中子成铊-204,后者会β衰变成铅-204。铅-204捕获另一个中子成半衰期1500万年的铅-205,之后继续捕获中子产生铅-206、铅-207和铅-208,铅-208再捕获中子就会产生会迅速衰变成铋-209的铅-209。铋-209会捕获中子成铋-210,之后β衰变成钋-210,最后α衰变成铅-206,s-过程就在铅-206、铅-207、铅-208和铋-209的循环中结束。[94]
在r-过程中(r代表快),原子核捕获中子的速度要比衰变的速度快。[95]r-过程会在如超新星或两个中子星合并时等有大量中子的环境中发生,中子流通量可达每秒每平方厘米1022个中子。[96]r-过程产生的铅的数量不如s-过程。[97]126个中子在原子核中会排列成完整的壳层,在能量上容纳更多中子会变得更加困难,[98]因此r-过程会在原子核达到126个中子时停止。[99]当中子流减少时,这些原子核会β衰变成锇、铱和铂的稳定同位素。[100]
地球
铅是亲硫元素,代表它经常以硫化物的形式存在。[101]天然金属铅很罕见。[102]大部分铅矿都较轻,因此在形成时仍在地壳中而没有沉下去。这使得铅的地壳丰度较高,为14 ppm,是第38常见的元素。[103][i]
方铅矿(PbS)是最常见的铅矿,通常与锌矿一起出现。[105]大部分铅矿都和方铅矿相关,例如硫锑铅矿 Pb5Sb4S11是由方铅矿衍生而成的混合物硫化物矿物,铅矾 PbSO4是方铅矿的氧化产物,而白铅矿 PbCO3则是方铅矿的分解产物。砷、锡、锑、银、金、铜和铋都是铅矿中常见的杂质。[105]
世界铅资源超过20亿吨,其中有大量矿藏分布在澳大利亚、中国、爱尔兰、墨西哥、秘鲁、葡萄牙、俄罗斯和美国。2016年,全球的铅储量(经济上可行的开采资源)总计8800万吨,其中澳大利亚有3500万吨,中国有1700万吨,俄罗斯则有640万吨。[106]
铅在大气层中的环境背景值不超过0.1 μg/m3,在土壤中的环境背景值为100 mg/kg,在蔬菜中为4 mg/kg,在水中为5 μg/L。[107]
历史
史前
在小亚细亚已发现可追溯到公元前7000–6500年的铅珠,这可能是冶炼金属的第一个例子。[109]那时候铅因为太软且色泽暗淡用处不多。[109]广泛生产铅的主要原因是因为冶炼方铅矿(一种常见的铅矿)可以得到银。[110]古埃及人第一个把铅矿用作化妆品,而这些化妆品后来传播到古希腊。[111]埃及人也把铅用于渔网的沉子、玻璃、搪瓷和装饰品中。[110]新月沃土的许多文明都把铅用作写字的材料、硬币[112]和建筑材料。[110]古中国宫廷会用铅作为兴奋剂、[110]货币[113]和避孕药,[114]印度河流域文明和中部美洲文明[110]用铅做护身符,而东非和南非人则在金属线的拉丝中使用铅。[115]
古典时代
公元前3000年,因为银被广泛用作装饰材料和交易媒介,因此小亚细亚开始开采铅矿。之后,爱琴海诸岛和拉夫里奧也开发了铅矿床。[116]这三个地区直到公元前1200年都在集体主导铅矿的生产。[117]约公元前2000年,腓尼基人开始开采伊比利亚半岛的矿床,而到了公元前1600年,塞浦路斯、希腊和撒丁岛开始开采铅矿。[118]

罗马共和国在欧洲和地中海的扩张以及采矿业的发展使其成为古典时代铅的最大生产者,预测的年输出为八万吨。和前人一样,罗马人的铅大多都是冶炼银的副产物。[108][120]在中欧、不列颠尼亚、巴尔干半岛、希腊、安纳托利亚和西班牙都有铅矿的开采,其中后者占了全球40%的产量。[108]
铅板用于写信,[121]在犹太也有使用铅棺材。[122]自公元前5世纪,铅还被用于制造弹弓弹药。罗马大量使用铅弹药来攻击100至150米内的目标,古迦太基和罗马的佣兵巴利阿里掷石兵就闻名于他们的射程和精准度。[123]

在罗马帝国,铅被用于制造水管。铅因为容易加工、熔点低、能够轻松制造完全防水的焊接接头及抗腐蚀性[124]使得它也广泛用于其它用处,如药品、屋顶、货币和战争。[125][126][127]当时老加图、科鲁迈拉和老普林尼等作家都推荐用铅(或镀铅)容器制备加到红酒和食物里的甜味剂和防腐剂。铅会与之反应生成铅糖(乙酸铅)而使食物变得好吃,而使用铜或青铜容器会因为产生乙酸铜而使食物变苦。[128]
"Wine—An enological specimen bank", 1992[129]
罗马作者维特鲁威报告了铅的健康危害,[130][131]而现代作家认为铅中毒在罗马帝国的衰落中扮演重要角色,[132][133][j]但一些研究者批评了这种说法,例如指出并非所有的腹痛都是由铅中毒引起的。[135][136]考古研究表明罗马的铅水管会增加自来水的铅含量,但这个含量“不太可能真正有害”。[137][138]
和锡、锑的混淆
自青铜器时代以来,冶金学家和工程师都知道锡和铅的差别,但它们的名称在某些语言中相似。罗马人就把铅叫做plumbum nigrum(黑铅),锡则叫做plumbum candidum(亮铅)。在其它语言中也可以看到铅和锡的相关性,例如捷克语的olovo意为铅,但俄文对应的同源字 олово意为锡。[139]此外,铅和锑的性质类似,它们都以硫化物矿物(方铅矿和辉锑矿)存在,而且这些矿物还会一起出现,使得有人混淆了它们。老普林尼就错误的把冶炼辉锑矿的产物写做铅,而实际产物是锑。[140]
中世纪和文艺复兴

在西罗马帝国灭亡后,西欧的铅矿开采下降了,只剩下安达卢斯有相当量的铅出口。[142][143]这时铅的最大生产者在亚洲,尤其是开采量迅速增长的中国和印度。[143]
铅于11和12世纪又开始被用于屋顶和水管,因此在欧洲的产量开始回升。自13世纪起,铅就被用来制造花窗玻璃。[144]在欧洲和阿拉伯炼金术中,铅(炼金术符号![]() )[145]被认为是不纯的卑金属,通过分离、纯化和平衡它的构成本质就可以转化成金。[146]铅还用于掺假红酒。虽然教宗诏书在1498年禁止掺假红酒被用于基督教仪式,但直到18世纪末都有人因为喝这种红酒而中毒。[142][147]铅是印刷机部件的关键材料,这使得制造印刷机的工人经常会因为吸入含铅粉尘而导致铅中毒。[148]因为铅便宜、对铁枪管的伤害更小、密度高(可以更好地保持速度)和熔点低(使得生产更容易),铅也是子弹的主要材料。[149]西欧贵族广泛使用铅粉来使脸变白,这被视为是谦虚的标志。[150][151]这个时尚后来扩展到白色假发和眼线,到了法国大革命才淡出。随着艺妓的出现,18世纪的日本也有类似的时尚,而它直到20世纪才消失。女性的白脸“代表她们作为日本女性的女性美德”,[152]而她们的面部增白剂就是铅。[153]
)[145]被认为是不纯的卑金属,通过分离、纯化和平衡它的构成本质就可以转化成金。[146]铅还用于掺假红酒。虽然教宗诏书在1498年禁止掺假红酒被用于基督教仪式,但直到18世纪末都有人因为喝这种红酒而中毒。[142][147]铅是印刷机部件的关键材料,这使得制造印刷机的工人经常会因为吸入含铅粉尘而导致铅中毒。[148]因为铅便宜、对铁枪管的伤害更小、密度高(可以更好地保持速度)和熔点低(使得生产更容易),铅也是子弹的主要材料。[149]西欧贵族广泛使用铅粉来使脸变白,这被视为是谦虚的标志。[150][151]这个时尚后来扩展到白色假发和眼线,到了法国大革命才淡出。随着艺妓的出现,18世纪的日本也有类似的时尚,而它直到20世纪才消失。女性的白脸“代表她们作为日本女性的女性美德”,[152]而她们的面部增白剂就是铅。[153]
欧洲和亚洲以外的铅
欧洲定居者来到新大陆便很快开始了铅的生产,最早的纪录可追溯到1621年的弗吉尼亚殖民地。[154]在澳大利亚,殖民者于1841年开辟的第一个矿山就是铅矿。[155]在非洲的西非裂谷系[156]和刚果盆地就有铅的开采和冶炼,而铅被用于和欧洲人交易,直到17世纪以来也用于货币。[157]
工业革命

18世纪下半叶,欧美经历了工业革命,这时铅的产量首次超过罗马。[158]原本英国是领先的生产国,但到19世纪中叶,随着英国矿山的枯竭以及德国、西班牙和美国开采铅矿的发展,英国失去了这一地位。[159]1900年,美国主导全球的铅产量,而其它非欧洲国家(加拿大、墨西哥和澳大利亚)也有明显的产量。[160]铅需求的很大一部分来自管道和含铅油漆。[161]当时有很多工人暴露于铅下,铅中毒案例也迅速增多,导致了对摄入铅的影响的研究。铅中毒和痛风有关,英国医师阿弗列德·巴灵·加洛德注意到三分之一的痛风病人都是水管工和画家。在19世纪也有铅对健康的长期影响(包括精神错乱)的研究。1870年代至1880年代,英国颁布了第一条旨在减少工厂铅中毒案例的法律。[161]
现代

人们在19世纪末和20世纪初发现铅对人类构成威胁的更多证据。铅中毒机制得到了更好的理解,并且在美国和欧洲已逐步停止使用。英国于1878年引入强制性工厂检查,并于1898年任命了第一位工厂医疗检查员。这些事件的结果是从1900年到1944年,铅中毒事件减少了25倍。[162]大部分欧洲国家在1930年就禁止了含铅油漆。[163]
人类最近一次大量接触铅是四乙基铅还作为汽油抗震剂的时候。美国和欧盟于2000年逐步淘汰了四乙基铅。[161]
美国和西欧国家在1970年代立法来试图减少空气中的铅污染。[164][165]立法的影响很显著,美国疾病控制与预防中心的研究指出美国在1976–1980年时有77.8%的人有高血铅水平,到了1991–1994年则下降到2.2%。[166]20世纪末最主要的含铅产品是铅酸蓄电池。[167]
西方集团在1960年至1990年的铅出口量增加了31%,[168]而东方集团的全球铅份额从1950年的10%增加到1990年的30%。在1970年代中期和1980年代,苏联是全球最大的铅生产者,而中国的铅产量在20世纪末开始增长。[169]到了2004年,中国的铅产量超越了澳大利亚,成为全球最大的铅生产者。[170]和之前欧洲工业化一样,铅对中国人的健康有负面影响。[171]
生产

铅的产量因为铅酸蓄电池的增加而增加。[172]铅的生产有两大类——从铅矿开采而来的初级铅和从废料回收的二级铅。人们在2014年生产了458万吨的初级铅和564万吨的二级铅,而那年的前三大生产国分别是中国、澳大利亚和美国,[106]那年的前三大二级铅生产国则为中国、美国和印度。[173]根据国际资源委员会2010年的社会金属库存报告,在全球范围内使用、储存、丢弃或消散到环境中的铅总量是人均8公斤,其中已发展国家的人均值(20–15公斤)会比未发展国家(1–4公斤)高。[174]
初级铅和二级铅有类似的生产过程。一些初级生产厂会用废铅来补充他们的业务,而这种趋势在未来可能会增加。如果有足够的技术,通过二次工艺生产的铅与通过初级工艺生产的铅没有区别。来自建筑行业的废铅通常相当干净,无需冶炼即可重新熔化。因此就能源需求而言,二级铅比初级铅便宜50%或更多。[175]
初级生产
大部分铅矿的铅含量较低(富矿也只含3–8%铅),因此需要提取浓缩。[176]铅矿会经过磨碎、分离、铣削、泡沫浮选和干燥,得到的产物的铅含量为30–80%(通常为50–60%),[176]然后转化成(不纯的)金属铅。
初级生产有两个主要方法,分别是涉及在不同容器焙烧和提取的两步法以及在同一个容器焙烧和提取的直接法。直接法较为常见,但两步法仍然重要。[177]
| 国家 | 出口量 (千吨) |
|---|---|
| 2,400 | |
| 500 | |
| 335 | |
| 310 | |
| 250 | |
| 225 | |
| 135 | |
| 80 | |
| 76 | |
| 75 | |
| 41 | |
| 41 | |
| 40 | |
| 40 | |
| 35 | |
| 33 | |
| 33 | |
| 其它国家 | 170 |
两步法
首先,铅的硫化物矿物会在空气中焙烧,硫化铅被氧化成氧化铅:[178]
- 2 PbS(s) + 3 O2(g) → 2 PbO(s) + 2 SO2(g)↑
由于反应物本来就不是纯硫化铅,因此产物也不是纯氧化铅,而是铅与其它金属的各种氧化物、硫酸盐和硅酸盐。[179]这些不纯的氧化铅会在高炉中被焦炭还原成(一样不纯的)铅:[180]
- 2 PbO(s) + C(s) → 2 Pb(s) + CO2(g)↑
这些杂质主要是砷、锑、铋、锌、铜、银和金,它们会在各种高温冶金过程中除去。融化的铅会在反射炉中和空气、水蒸气和硫反应,氧化除银、金和铋以外的杂质。这些氧化产物会浮在液铅上并被除去。[181][182]之后,银和金可以通过在铅中加锌来分离。铅不溶于锌,但银和金都可溶于锌,因此把锌溶液去除也能一起去除银和金。[182][183]最后的铋杂质则可以通过加入金属钙和镁,使铋与其反应并浮在铅上,然后去除。[182]
除了冶金以外,极纯的铅还可以由Betts法生产。不纯的铅做成的阳极和纯铅做成的阴极在氟硅酸铅(PbSiF6)电解质中电解,阳极的铅就会溶于溶液并镀到阴极上,而杂质则会留在溶液中。[182][184]这个过程的费用很高,因此只用于制造极纯的铅条。[185]
直接法
首先,铅的硫化物矿物会在熔炉中融化和氧化,产生氧化铅。碳(以焦炭或煤气的形式)和助焊剂之后会加入到熔融的铅中。氧化铅会被碳还原成金属铅。[177]
如果反应物富含铅,那么有80%的铅可以以铅条的形式取出,而剩下的20%以富含氧化铅的炉渣存在。对于铅含量较低的反应物,所有的铅都会被氧化成炉渣。[177]这些铅含量高(25–40%)的炉渣可以通过燃烧,用电炉还原或两者兼施得到金属铅。[177]
替代方法
人们仍在继续研究更清洁、能耗更低的铅提取方法,但缺点是浪费太多铅或是产物的硫含量太高。电解提炼可能有潜力作为替代生产方法,但这个方法除非电很便宜,否则在经济上不可行。[186]
二级生产
除非回收的铅有明显的氧化,否则在初级生产中必要的冶炼过程在二级生产中会被跳过。[175]ISASMELT过程是可作为延伸初级生产的新冶炼方法。废铅酸蓄电池(含有硫酸铅和铅的各种氧化物)的硫酸铅可以用碱去除,然后在熔炉中和煤炭反应,生成不纯的铅。[187]二级铅的提炼和初级铅类似,但某些提炼过程可以根据回收材料和潜在污染跳过。[187]在铅的回收来源中最重要的是铅酸蓄电池,而铅水管和电缆涂层也是重要的来源。[175]
用途

实际上,铅笔并不是用铅做的,但因为古时候把石墨误认为是铅,所以铅笔一词在各语言中流传使用而未修正。[189]
单质
金属铅的高密度、低熔点、展性和惰性使其可用于许多用途。虽然有很多金属在某些方面比铅好,但它们通常较罕见,也更难从矿石中提取。不过,铅的毒性使它在某些用途中被逐步淘汰。[190]
自中世纪以来,铅就被用于生产子弹。铅很便宜,而且它的低熔点意味着可以用最少的技术设备铸造小型武器弹药和霰弹枪弹。此外,铅的密度比大部分常见金属高,可以更好地保持速度。今天,铅(会加入其它金属来变硬)仍然是子弹的主要材料,[149]但有人担心用于狩猎的铅弹会破坏环境。[k]
铅也有许多用处应用了其高密度和抗腐蚀性。铅的高密度使它可用作帆船龙骨的压舱物,从而抵消风对帆的倾斜效应。[192]它也用于水肺潜水的配重系统中,以抵消潜水员的浮力。[193]1993年,比萨斜塔的底部就用了600吨的铅来使其稳定。[194]铅的抗腐蚀性使其可用于保护深海电缆。[195]

在建筑工业中,铅也有许多用途。铅片可用于屋顶、覆层、落水管和女儿墙。[196][197]铅至今仍用于制造雕像和雕塑,以及它们的骨架。[198]铅在以前也用于平衡轮胎,但因为环境原因也被其它材料替代了。[106]
铅可以添加到如青铜和黄铜等铜合金中来润滑和增加可加工性。由于铅不溶于铜,所以它会在合金的缺陷(例如晶粒边界)中形成固体小球。在低浓度下,铅除了起到润滑剂的作用外,这些小球还会阻止切屑生成,因此增加了可加工性。铅含量较高的铜合金可用于制造轴承。铅用于润滑,而铜则提供承重支撑。[199]
铅的密度和原子序都很高,而且容易成型,这使得它可用于屏蔽声音、震动和辐射。[200]铅没有自然频率,[200]因此铅片可用于录音室的隔音。[201]管风琴声管通常是由铅合金制造的,这些合金会加入不等量的锡来控制音调。[202][203]由于其密度和衰减系数都很高,[204]铅可用作核物理学和X光室的辐射屏蔽材料。[205]熔融的铅可用作铅冷快中子反应堆的冷却剂。[206]
电池
在21世纪初,铅最大的用处就是制造铅酸蓄电池。电池中的铅并没有与人体直接接触,因此毒性问题较少,[l]但在生产铅酸蓄电池的工厂工作的人可能会暴露于含铅粉尘下。[208]在铅酸蓄电池里,铅、二氧化铅和硫酸的反应可以产生稳定的电压。[m]包含铅酸蓄电池的超级电容器可用于调节频率、太阳能和风能等应用中。[210]这些电池虽然能量密度和充放电效率比锂离子电池低,但比锂离子电池便宜。[211]
电缆涂层
铅可以制造高压电电缆的外壳,以防止水进到里面,但因为毒性而被逐渐淘汰。[212]它也用于电器的焊料,但因为会影响环境而被某些国家淘汰。[213]铅是博物馆Oddy测试使用的三种金属之一,可用于探测有机酸、醛和酸性气体。[214][215]
化合物


铅酸蓄电池除了是金属铅最大的用处以外,也是铅化合物最大的用处。它的充放电反应涉及了硫酸铅和二氧化铅:
- Pb(s) + PbO
2(s) + 2H
2SO
4(aq) → 2PbSO
4(s) + 2H
2O(l)
含铅颜料可以使釉和玻璃涂上红色和黄色。[216]虽然含铅颜料在欧洲和北美已被淘汰,但像是中国[217]、印度[218]和印尼[219]的发展中国家仍在使用含铅颜料。四乙酸铅和二氧化铅是有机化学的氧化剂。铅玻璃中含有12–28%的氧化铅,其光学性质和可以屏蔽辐射的性质[220]可用于老式电视机和电脑荧幕的阴极射线管。碲化铅和硒化铅可用于太阳能光伏和红外线探测器。[221]
致病性
铅有毒,尤其破坏儿童的神经系统,可导致血液病和脑病,因此被利用鉛以外的金屬,如:銀、銅、鋅等與錫共同製成的「無鉛焊錫」所替代。长期接触铅和它的盐(尤其是可溶的和强氧化性的PbO2)可以导致肾病和类似绞痛的腹痛。有人认为许多古罗马皇帝有老人痴呆症是由于当时使用铅来造水管(但最大量的來源是铅盐用来作为酒及食物的甜味劑)造成的。而且,人体积蓄铅后很难自行排出,只能通过药物来清除。
由于人們怀疑铅导致儿童智力衰退,兒童體內鉛過多會降低學習能力、記憶力、對神經傳導以及维生素的代謝產生負面影響[222],所以大眾減少使用它。有研究指出,兒童血液每增加10微毫克的鉛,小孩智商就會降低5.5%。美國有研究顯示城市禁用含鉛油漆後,暴力犯罪也減少,鉛的含量與犯罪呈現正相關。部份发达国家不再出售含铅油漆(台灣仍廣泛使用含有高濃度鉛的紅丹漆),亦不再出售含鉛汽油,例如香港,因含鉛汽油經燃燒後能隨空氣周圍散播,嚴重影響健康,尤其是兒童,如不禁用普通人難以避免受害。接觸鉛後,例如鉛造的釣魚用具,應洗手,尤其是進食前。
體內鉛含量過多可能產生的症狀:慢性肌肉或關節疼痛、聽覺視覺功能變差、易有過敏性疾病、注意力不集中或過動、精神障礙或退化
部份玻璃含有鉛,稱為鉛玻璃(例如美國70年代製的玻璃窗,70年代後美國禁止使用鉛來製造玻璃窗),亦不應接觸,應找專業防污染人士拆除,兒童要避免使用使用鉛的玩具和顏料,例如使用鉛製油漆製造的玩具,亦要避免兒童誤食含鉛的細小玩具,因為鉛化學物有甜味,會令兒童不斷吃,同時鉛會欺騙身體令身體以為鉛是必須的元素,讓人繼續不斷吃進肚裡。電氣技術員亦應避免用口來咬開電線因電線的膠皮含鉛,長期接觸下已有人中鉛毒。微量的鉛能損害女性的生育能力。在对生育能力的影响方面,鉛对男性相较于女性的危害较小,不过暴露在高濃度的鉛下亦能減少男性的生育能力。接觸高濃度的鉛能令兒童及成人的腦和腎臟嚴重受損,最終導致死亡。國家亦應注意鉛的回收,例如每架汽車均使用的鉛電池,以免污染土地和地下水,目前美國的汽車鉛電池的回收率為99%,而且地球上的鉛快用完,要避免將來沒有鉛用。[223]
鉛出現在空氣污染、染髮劑、油漆、飲水、一些肥料、工業污染物。
當鉛自廢棄的電子零件中釋放出來,污染地下水以及土壤,將對自然環鏡帶來不良影響;而人類又極容易食用受鉛污染的土壤所栽種的青菜,鉛即會累積在人體進行產生危害,因此目前在產品應用上盡量避免使用鉛(但是食物中自然含有的微量鉛,不會危害人體)。
台灣的醫學研究顯示,對體內鉛含量較高的腎臟病病人注射排鉛劑能減慢腎病惡化的速度,至少能延後洗腎四年,此研究很有機會能有效降低台灣的醫療支出[224]。
使用含铅的陶瓷制品有可能导致中毒,尤其在裝酸性溶液(例如果汁)時,这些溶液可以溶解陶瓷的铅离子令人們把它喝到肚裡。有些相架可能有鉛。
食物和水的管制
- 食物
香港: 2mg/kg till 11/2019, the new version is much lower but haven't mention lots of famous food[225][226]
- 鹽
食品法典委員會 (聯合國及世衛): 1mg/kg[227]
香港: 2mg/kg
中国大陆: 2mg/kg[227]
- 水
香港: 1mg/kg or 1ppm[225]
世界十大鉛消耗國的消耗量
單位:千噸
| 國家 | 1977 | 1982 | 1987 | 1992 |
|---|---|---|---|---|
| 美國 | 988.4 | 1106.1 | 1216.9 | 1246.3 |
| 蘇聯/俄羅斯 | 620.0 | 810.0 | 755.0 | 600.0 |
| 日本 | 245.8 | 354.0 | 378.0 | 422.2 |
| 德國 | 377.9 | 433.2 | 444.2 | 413.5 |
| 英國 | 241.0 | 271.9 | 287.5 | 263.7 |
| 義大利 | 206.1 | 243.0 | 244.0 | 259.0 |
| 法國 | 190.2 | 194.5 | 207.5 | 252.0 |
| 中國 | 200.0 | 215.0 | 256.0 | 250.5 |
| 南韓 | - | 31.5 | 112.4 | 172.8 |
| 西班牙 | 94.7 | 102.7 | 105.8 | 121.5 |
| 十大國消耗量 | 3164.1 | 3761.9 | 4027.3 | 4001.5 |
| 全球消耗量 | 4435.6 | 5236.6 | 5676.5 | 5342.2 |
環境保護
避免使用
美國禁用鉛彈頭的請願
2010年8月3日,生物多樣性研究中心聯同美國鳥類保護協會和其他環保組織提交了一份100頁的請願書,要求美國國家環境保護局(EPA)禁止含鉛彈頭和漁具捕殺野生動物。請願書提到,鉛對人類和數百萬野生雀鳥造成健康風險。8月27日,請願書被駁回。
一般子彈在發射時也會釋放出鉛、造成慢性傷害;但無鉛子彈也會造成健康問題,因此換成無鉛子彈的計划受阻。
參見
注释
- ^ 展性是物质在压力下变形的难度,而延性是物质的拉伸能力。
- ^ 湿手指可以浸入液态铅中而不会造成灼伤。[15]
- ^ 镧系收缩有10%可以归结为相对论效应的影响。[20]
- ^ 钻石结构的锡被称为α锡或灰锡,仅在13.2 °C(55.8 °F)以下稳定。室温下稳定的锡同素异形体是β锡(白锡),它的晶体结构为扭曲的面心立方结构,是灰锡的钻石结构和铅标准的面心立方结构之间的结构,符合元素周期表越往下金属性越强的趋势。[26]
- ^ 铋-209的半衰期为1.9×1019年。[30]一公斤天然铋的放射性活度约为0.003 Bq(每秒衰变的次数)。作为比较,一公斤人体的放射性活度约为65 Bq。[31]
- ^ 铅-205的衰变方式是电子捕获,因此如果它的82个电子都被完全电离,周围也没有电子,它就无法衰变。铅-205会衰变成铊-205,但完全电离的铊-205就不是稳定同位素了,会衰变成有一个电子的铅-205。[42]
- ^ 四苯基铅更加稳定,270 °C才分解。[82]
- ^ 来源中的丰度不是以分比计算,而是以和硅(丰度定义为106)的相对比例计算的。所有元素按这个相对比例计算的总和为2.6682×1010,其中铅占了3.258。
- ^ 元素丰度可能会因为来源的不同而有所改变。[104]
- ^ 儒略·恺撒只生一个孩子和奥古斯都据称不育的事实都被归咎于铅中毒。[134]
- ^ 加利福尼亚州因此在2015年7月开始禁止使用铅弹打猎。[191]
- ^ 铅酸蓄电池对普通使用者的潜在危害都和铅的毒性无关。[207]
- ^ 参见这个参考资料[209]来得到铅酸蓄电池工作原理的详细资料。
参考文献
- ^ Meija et al. 2016.
- ^ Pb(0)存在于铅和一氧化碳反应形成的羰基化合物中,参见Ling, Jiang; Qiang, Xu. Observation of the lead carbonyls PbnCO (n=1–4): Reactions of lead atoms and small clusters with carbon monoxide in solid argon. The Journal of Chemical Physics. 122 (3): 034505. 2005, 122 (3): 34505. Bibcode:2005JChPh.122c4505J. ISSN 0021-9606. PMID 15740207. doi:10.1063/1.1834915.
- ^ 夏征农、陈至立 (编). 《辞海》第六版彩图本. 上海: 上海辞书出版社. 2009年: 第3227页. ISBN 9787532628599.
- ^ Theodore Low De Vinne (1899). The Practice of Typography: A Treatise on the Processes of Type-making, the Point System, the Names, Sizes, Styles, and Prices of Plain Printing Types. Century Company. pp. 9–36.
- ^ Greenwood & Earnshaw 1998,第372頁.
- ^ Greenwood & Earnshaw 1998,第372–373頁.
- ^ 7.0 7.1 Thornton, Rautiu & Brush 2001,第6頁.
- ^ Lide 2005,第12-35, 12-40頁.
- ^ Lide 2005,第4-13, 4-21, 4-33頁.
- ^ Vogel & Achilles 2013,第8頁.
- ^ Anderson 1869,第341–343頁.
- ^ Gale & Totemeier 2003,第15–2–15–3頁.
- ^ Thornton, Rautiu & Brush 2001,第8頁.
- ^ 14.0 14.1 Lide 2005,第12-219頁.
- ^ Willey 1999.
- ^ Lide 2005,第12-45頁.
- ^ Blakemore 1985,第272頁.
- ^ Webb, Marsiglio & Hirsch 2015.
- ^ Lide 2005,第10-179頁.
- ^ Pyykkö 1988,第563–594頁.
- ^ Norman 1996,第36頁.
- ^ Greenwood & Earnshaw 1998,第226–227, 374頁.
- ^ Christensen 2002,第867頁.
- ^ Slater 1964.
- ^ Considine & Considine 2013,第501, 2970頁.
- ^ Parthé 1964,第13頁.
- ^ 27.0 27.1 27.2 27.3 IAEA - Nuclear Data Section 2017.
- ^ University of California Nuclear Forensic Search Project.
- ^ 29.0 29.1 Stone 1997.
- ^ de Marcillac et al. 2003,第876–78頁.
- ^ World Nuclear Association 2015.
- ^ Beeman et al. 2013.
- ^ Radioactive Decay Series 2012.
- ^ Committee on Evaluation of EPA Guidelines for Exposure to Naturally Occurring Radioactive Materials et al. 1999.
- ^ Smirnov, Borisevich & Sulaberidze 2012.
- ^ Greenwood & Earnshaw 1998,第368頁.
- ^ Levin 2009,第40–41頁.
- ^ Webb 2000,第115頁.
- ^ Wrackmeyer & Horchler 1990.
- ^ Cangelosi & Pecoraro 2015.
- ^ Fiorini 2010,第7–8頁.
- ^ Takahashi et al. 1987.
- ^ Thürmer, Williams & Reutt-Robey 2002,第2033–2035頁.
- ^ Tétreault, Sirois & Stamatopoulou 1998,第17–32頁.
- ^ Thornton, Rautiu & Brush 2001,第10–11頁.
- ^ 46.0 46.1 46.2 46.3 46.4 46.5 Greenwood & Earnshaw 1998,第373頁.
- ^ Bretherick 2016,第1442頁.
- ^ Harbison, Bourgeois & Johnson 2015,第132頁.
- ^ 49.0 49.1 Greenwood & Earnshaw 1998,第374頁.
- ^ Thornton, Rautiu & Brush 2001,第11–12頁.
- ^ Polyanskiy 1986,第20頁.
- ^ Kaupp 2014,第9–10頁.
- ^ Dieter & Watson 2009,第509頁.
- ^ Hunt 2014,第215頁.
- ^ 55.0 55.1 King 1995,第43–63頁.
- ^ Bunker & Casey 2016,第89頁.
- ^ Whitten, Gailey & David 1996,第904–905頁.
- ^ Greenwood & Earnshaw 1998,第384頁.
- ^ Greenwood & Earnshaw 1998,第387頁.
- ^ 60.0 60.1 Greenwood & Earnshaw 1998,第389頁.
- ^ Zuckerman & Hagen 1989,第426頁.
- ^ 62.0 62.1 Greenwood & Earnshaw 1998,第382頁.
- ^ Bharara & Atwood 2006,第4頁.
- ^ Greenwood & Earnshaw 1998,第388頁.
- ^ Toxicological Profile for Lead 2007,第277頁.
- ^ Downs & Adams 2017,第1128頁.
- ^ Brescia 2012,第234頁.
- ^ Macintyre 1992,第3775頁.
- ^ Silverman 1966,第2067–2069頁.
- ^ Greenwood & Earnshaw 1998,第381頁.
- ^ Yong, Hoffmann & Fässler 2006,第4774–4778頁.
- ^ Becker et al. 2008,第9965–9978頁.
- ^ Mosseri, Henglein & Janata 1990,第2722–2726頁.
- ^ Konu & Chivers 2011,第391–392頁.
- ^ Hadlington 2017,第59頁.
- ^ Greenwood & Earnshaw 1998,第384–386頁.
- ^ Röhr 2017.
- ^ Alsfasser 2007,第261–263頁.
- ^ Greenwood & Earnshaw 1998,第393頁.
- ^ Stabenow, Saak & Weidenbruch 2003.
- ^ 81.0 81.1 Polyanskiy 1986,第43頁.
- ^ 82.0 82.1 82.2 82.3 Greenwood & Earnshaw 1998,第404頁.
- ^ 83.0 83.1 Wiberg, Wiberg & Holleman 2001,第918頁.
- ^ Toxicological Profile for Lead 2007,第287頁.
- ^ Polyanskiy 1986,第44頁.
- ^ Windholz 1976.
- ^ Zýka 1966,第569頁.
- ^ 88.0 88.1 88.2 88.3 Lodders 2003,第1222–1223頁.
- ^ Lochner, Rohrbach & Cochrane 2005,第12頁.
- ^ Roederer et al. 2009,第1963–1980頁.
- ^ Lodders 2003,第1224頁.
- ^ Burbidge et al. 1957,第608–615頁.
- ^ Burbidge et al. 1957,第551頁.
- ^ Burbidge et al. 1957,第608–609頁.
- ^ Burbidge et al. 1957,第553頁.
- ^ Frebel 2015,第114–115頁.
- ^ Burbidge et al. 1957,第608–610頁.
- ^ Burbidge et al. 1957,第596頁.
- ^ Burbidge et al. 1957,第595頁.
- ^ Burbidge et al. 1957,第582, 609–615頁.
- ^ Langmuir & Broecker 2012,第183–184頁.
- ^ Davidson et al. 2014,第4–5頁.
- ^ Emsley 2011,第286, passim頁.
- ^ Cox 1997,第182頁.
- ^ 105.0 105.1 Davidson et al. 2014,第4頁.
- ^ 106.0 106.1 106.2 106.3 United States Geological Survey 2017,第97頁.
- ^ Rieuwerts 2015,第225頁.
- ^ 108.0 108.1 108.2 Hong et al. 1994,第1841–1843頁.
- ^ 109.0 109.1 Rich 1994,第4頁.
- ^ 110.0 110.1 110.2 110.3 110.4 Winder 1993b.
- ^ History of Cosmetics.
- ^ Chapurukha Kusimba, June 20, 2017. Making Cents of Currency's Ancient Rise. Smithsonian Magazine. [5 November 2021].
- ^ Yu & Yu 2004,第26頁.
- ^ Toronto museum explores 2003.
- ^ Bisson & Vogel 2000,第105頁.
- ^ Wood, Hsu & Bell 2021.
- ^ Rich 1994,第5頁.
- ^ United States Geological Survey 1973.
- ^ Lead sling bullet.
- ^ de Callataÿ 2005,第361–372頁.
- ^ Ceccarelli 2013,第35頁.
- ^ Ossuaries and Sarcophagi.
- ^ Calvo Rebollar 2019,第45頁.
- ^ Rich 1994,第6頁.
- ^ Thornton, Rautiu & Brush 2001,第179–184頁.
- ^ Bisel & Bisel 2002,第459–460頁.
- ^ Retief & Cilliers 2006,第149–151頁.
- ^ Grout 2017.
- ^ Eschnauer & Stoeppler 1992,第58頁.
- ^ Hodge 1981,第486–491頁.
- ^ Marcus Vitruvius Pollio. De architectura. Book 8, 10-11 fulltext. 1914 [c. 15 BC].
- ^ Gilfillan 1965,第53–60頁.
- ^ Nriagu 1983,第660–663頁.
- ^ Frankenburg 2014,第16頁.
- ^ Scarborough 1984.
- ^ Waldron 1985,第107–108頁.
- ^ Reddy & Braun 2010,第1052頁.
- ^ Delile et al. 2014,第6594–6599頁.
- ^ Polyanskiy 1986,第8頁.
- ^ Thomson 1830,第74頁.
- ^ Kellett 2012,第106–107頁.
- ^ 142.0 142.1 Winder 1993a.
- ^ 143.0 143.1 Rich 1994,第7頁.
- ^ Rich 1994,第8頁.
- ^ Ede & Cormack 2016,第54頁.
- ^ Cotnoir 2006,第35頁.
- ^ Samson 1885,第388頁.
- ^ Sinha et al. 1993.
- ^ 149.0 149.1 Ramage 1980,第8頁.
- ^ Tungate 2011,第14頁.
- ^ Donnelly 2014,第171–172頁.
- ^ Ashikari 2003,第65頁.
- ^ Nakashima et al. 1998,第59頁.
- ^ Rabinowitz 1995,第66頁.
- ^ Gill & Libraries Board of South Australia 1974,第69頁.
- ^ Bisson & Vogel 2000,第85頁.
- ^ Bisson & Vogel 2000,第131–132頁.
- ^ Hong et al. 1994,第1841–43頁.
- ^ Lead mining.
- ^ Rich 1994,第11頁.
- ^ 161.0 161.1 161.2 Riva et al. 2012,第11–16頁.
- ^ Hernberg 2000,第246頁.
- ^ Markowitz & Rosner 2000,第37頁.
- ^ More et al. 2017.
- ^ American Geophysical Union 2017.
- ^ Centers for Disease Control and Prevention 1997.
- ^ Rich 1994,第117頁.
- ^ Rich 1994,第17頁.
- ^ Rich 1994,第91–92頁.
- ^ United States Geological Survey 2005.
- ^ Zhang et al. 2012,第2261–2273頁.
- ^ Tolliday 2014.
- ^ Guberman 2016,第42.14–15頁.
- ^ Graedel 2010.
- ^ 175.0 175.1 175.2 Thornton, Rautiu & Brush 2001,第56頁.
- ^ 176.0 176.1 Davidson et al. 2014,第6頁.
- ^ 177.0 177.1 177.2 177.3 Davidson et al. 2014,第17頁.
- ^ Thornton, Rautiu & Brush 2001,第51頁.
- ^ Davidson et al. 2014,第11–12頁.
- ^ Thornton, Rautiu & Brush 2001,第51–52頁.
- ^ Davidson et al. 2014,第25頁.
- ^ 182.0 182.1 182.2 182.3 Primary Lead Refining.
- ^ Pauling 1947.
- ^ Davidson et al. 2014,第34頁.
- ^ Davidson et al. 2014,第23頁.
- ^ Thornton, Rautiu & Brush 2001,第52–53頁.
- ^ 187.0 187.1 Thornton, Rautiu & Brush 2001,第57頁.
- ^ Street & Alexander 1998,第181頁.
- ^ Evans 1908,第133–179頁.
- ^ Baird & Cann 2012,第537–538, 543–547頁.
- ^ California Department of Fish and Wildlife.
- ^ Parker 2005,第194–195頁.
- ^ Krestovnikoff & Halls 2006,第70頁.
- ^ Street & Alexander 1998,第182頁.
- ^ Jensen 2013,第136頁.
- ^ Think Lead research.
- ^ Weatherings to Parapets.
- ^ Putnam 2003,第216頁.
- ^ Copper Development Association.
- ^ 200.0 200.1 Rich 1994,第101頁.
- ^ Guruswamy 2000,第31頁.
- ^ Audsley 1965,第250–251頁.
- ^ Palmieri 2006,第412–413頁.
- ^ Thornton, Rautiu & Brush 2001,第7頁.
- ^ National Council on Radiation Protection and Measurements 2004,第16頁.
- ^ Tuček, Carlsson & Wider 2006,第1590頁.
- ^ Concordia University 2016.
- ^ Toxicological Profile for Lead 2007,第5–6頁.
- ^ Progressive Dynamics, Inc.
- ^ Olinsky-Paul 2013.
- ^ Gulbinska 2014.
- ^ Rich 1994,第133–134頁.
- ^ Zhao 2008,第440頁.
- ^ Beiner et al. 2015.
- ^ Szczepanowska 2013,第84–85頁.
- ^ Burleson 2001,第23頁.
- ^ Insight Explorer & IPEN 2016.
- ^ Singh 2017.
- ^ Ismawati et al. 2013,第2頁.
- ^ Amstock 1997,第116–119頁.
- ^ Rogalski 2010,第485–541頁.
- ^ 體內大掃毒
- ^ 探索頻道科學頻道節目: 鉛的製造 2013-5-26
- ^ 解毒劑排鉛 減緩腎病惡化. [2008-07-25]. (原始内容存档于2008-07-15).
- ^ 225.0 225.1 存档副本. [2020-04-15]. (原始内容存档于2020-04-19).
- ^ 存档副本. [2020-04-15]. (原始内容存档于2020-04-19).
- ^ 227.0 227.1 存档副本. [2020-04-15]. (原始内容存档于2020-04-18).
参考书目
- Alsfasser, R. Moderne anorganische Chemie [Modern inorganic chemistry]. Walter de Gruyter. 2007. ISBN 978-3-11-019060-1 (德语).
- Amstock, J. S. Handbook of Glass in Construction. McGraw-Hill Professional. 1997. ISBN 978-0-07-001619-4.
- American Geophysical Union. Human Activity Has Polluted European Air for 2000 Years. Eos Science News. 2017 [4 July 2017]. (原始内容存档于27 June 2017).
- Anderson, J. Malleability and ductility of metals. Scientific American. 1869, 21 (22): 341–43. doi:10.1038/scientificamerican11271869-341.
- Ashikari, M. The memory of the women's white faces: Japaneseness and the ideal image of women. Japan Forum. 2003, 15 (1): 55–79. S2CID 144510689. doi:10.1080/0955580032000077739.
- Audsley, G. A. The Art of Organ Building 2. Courier. 1965. ISBN 978-0-486-21315-6.
- Baird, C.; Cann, N. Environmental Chemistry 5th. W. H. Freeman and Company. 2012. ISBN 978-1-4292-7704-4.
- Becker, M.; Förster, C.; Franzen, C.; et al. Persistent radicals of trivalent tin and lead. Inorganic Chemistry. 2008, 47 (21): 9965–78. PMID 18823115. doi:10.1021/ic801198p.
- Beeman, J. W.; Bellini, F.; Cardani, L.; et al. New experimental limits on the α decays of lead isotopes. European Physical Journal A. 2013, 49 (50): 50. Bibcode:2013EPJA...49...50B. S2CID 119280082. arXiv:1212.2422
 . doi:10.1140/epja/i2013-13050-7.
. doi:10.1140/epja/i2013-13050-7. - Beiner, G. G.; Lavi, M.; Seri, H.; et al. Oddy Tests: Adding the Analytical Dimension. Collection Forum. 2015, 29 (1–2): 22–36. ISSN 0831-4985. doi:10.14351/0831-4985-29.1.22
 .
. - Bharara, M. S.; Atwood, D. A. Lead: Inorganic ChemistryBased in part on the article Lead: Inorganic Chemistry by Philip G. Harrison which appeared in theEncyclopedia of Inorganic Chemistry, First Edition. Lead: Inorganic Chemistry. 2006. ISBN 978-0470860786. doi:10.1002/0470862106.ia118.
- Bisel, S. C.; Bisel, J. F. Health and nutrition at Herculaneum. Jashemski, W. F.; Meyer, F. G. (编). The Natural History of Pompeii. Cambridge University Press. 2002: 451–75. ISBN 978-0-521-80054-9.
- Bisson, M. S.; Vogel, J. O. Ancient African Metallurgy: The Sociocultural Context. Rowman & Littlefield. 2000. ISBN 978-0-7425-0261-1.
- Blakemore, J. S. Solid State Physics. Cambridge University Press. 1985. ISBN 978-0-521-31391-9.
- Brescia, F. Fundamentals of Chemistry: A Modern Introduction. Elsevier. 2012. ISBN 978-0-323-14231-1.
- Bretherick, L. Bretherick's Handbook of Reactive Chemical Hazards. Elsevier. 2016. ISBN 978-1-4831-6250-8.
- Bunker, B. C.; Casey, W. H. The Aqueous Chemistry of Oxides. Oxford University Press. 2016. ISBN 978-0-19-938425-9.
- Burbidge, E. M.; Burbidge, G. R.; Fowler, W. A.; et al. Synthesis of the Elements in Stars (PDF). Reviews of Modern Physics. 1957, 29 (4): 547–654. Bibcode:1957RvMP...29..547B. doi:10.1103/RevModPhys.29.547
 .
. - Burleson, M. The Ceramic Glaze Handbook: Materials, Techniques, Formulas. Sterling. 2001. ISBN 9781579904395.
- California Department of Fish and Wildlife. Nonlead Ammunition in California. www.wildlife.ca.gov. [17 May 2017].
- Calvo Rebollar, Miguel. Construyendo la Tabla Periódica. Zaragoza, Spain: Prames. 2019. ISBN 978-84-8321-908-9.
- Cangelosi, V. M.; Pecoraro, V. L. Lead. Roduner, E. (编). Nanoscopic Materials: Size-Dependent Phenomena and Growth Principles. Royal Society of Chemistry. 2015: 843–875. ISBN 978-1-78262-494-3.
- Ceccarelli, P. Ancient Greek Letter Writing: A Cultural History (600 BC- 150 BC). OUP Oxford. 2013. ISBN 978-0-19-967559-3.
- Centers for Disease Control and Prevention. Update: blood lead levels--United States, 1991-1994. Morbidity and Mortality Weekly Report. 1997, 46 (7): 141–146. ISSN 0149-2195. PMID 9072671.
- Christensen, N. E. Relativistic Solid State Theory. Schwerdtfeger, P. (编). Relativistic Electronic Structure Theory — Fundamentals. Theoretical and Computational Chemistry 11. Elsevier. 2002: 867–68. ISBN 978-0-08-054046-7. doi:10.1016/s1380-7323(02)80041-3.
- Considine, D. M.; Considine, G. D. Van Nostrand's Scientific Encyclopedia. Springer Science & Business Media. 2013. ISBN 978-1-4757-6918-0.
- Committee on Evaluation of EPA Guidelines for Exposure to Naturally Occurring Radioactive Materials; Commission on Life Sciences; Division on Earth and Life Studies; National Research Council. Evaluation of Guidelines for Exposures to Technologically Enhanced Naturally Occurring Radioactive Materials. National Academies Press. 1999: 26, 30–32. ISBN 978-0-309-58070-0.
- Concordia University. Lead acid batteries (PDF) (报告). 2016 [17 February 2019].
- Copper Development Association. Leaded Coppers. copper.org. [10 July 2016].
- Cotnoir, B. The Weiser Concise Guide to Alchemy. Weiser Books. 2006. ISBN 978-1-57863-379-1.
- Cox, P. A. The Elements: Their Origin, Abundance and Distribution
 . Oxford University Press. 1997. ISBN 978-0-19-855298-7.
. Oxford University Press. 1997. ISBN 978-0-19-855298-7. - Lide, D. R. (编). CRC Handbook of Chemistry and Physics 85th. CRC Press. 2005. ISBN 978-0-8493-0484-2.
- Delile, H.; Blichert-Toft, J.; Goiran, J.-P.; et al. Lead in ancient Rome's city waters. Proceedings of the National Academy of Sciences. 2014, 111 (18): 6594–99. Bibcode:2014PNAS..111.6594D. ISSN 0027-8424. PMC 4020092
 . PMID 24753588. doi:10.1073/pnas.1400097111
. PMID 24753588. doi:10.1073/pnas.1400097111  .
. - Davidson, A.; Ryman, J.; Sutherland, C. A.; et al. Lead. Ullmann's Encyclopedia of Industrial Chemistry. 2014. ISBN 978-3-527-30673-2. doi:10.1002/14356007.a15_193.pub3.
- Emsley, J. Nature's Building Blocks: An A-Z Guide to the Elements. Oxford University Press. 2011. ISBN 978-0-19-960563-7.
- Evans, J. W. V.— The meanings and synonyms of plumbago. Transactions of the Philological Society. 1908, 26 (2): 133–79. doi:10.1111/j.1467-968X.1908.tb00513.x.
- Fiorini, E. 2.000 years-old Roman Lead for physics (PDF). ASPERA. 2010: 7–8 [29 October 2016]. (原始内容 (PDF)存档于26 April 2018).
- de Callataÿ, F. The Graeco-Roman economy in the super long-run: Lead, copper, and shipwrecks. Journal of Roman Archaeology. 2005, 18: 361–72. S2CID 232346123. doi:10.1017/S104775940000742X.
- Guberman, D. E. Lead (PDF). 2014 Minerals Yearbook (报告). United States Geological Survey. 2016 [8 May 2017].
- Guruswamy, S. Engineering properties and applications of lead alloys. Marcel Dekker. 2000. ISBN 978-0-8247-8247-4.
- de Marcillac, Pierre; Coron, Noël; Dambier, Gérard; et al. Experimental detection of α-particles from the radioactive decay of natural bismuth. Nature. 2003, 422 (6934): 876–78. Bibcode:2003Natur.422..876D. PMID 12712201. S2CID 4415582. doi:10.1038/nature01541.
- Donnelly, J. Deep Blue. Hachette Children's Group. 2014. ISBN 978-1-4449-2119-9.
- Dieter, R. K.; Watson, R. T. Transmetalation reactions producing organocopper compounds. Rappoport, Z.; Marek, I. (编). The Chemistry of Organocopper Compounds 1. John Wiley & Sons. 2009: 443–526. ISBN 978-0-470-77296-6.
- Downs, A. J.; Adams, C. J. The Chemistry of Chlorine, Bromine, Iodine and Astatine: Pergamon Texts in Inorganic Chemistry. Elsevier. 2017. ISBN 978-1-4831-5832-7.
- Ede, A.; Cormack, L. B. A History of Science in Society, Volume I: From the Ancient Greeks to the Scientific Revolution, Third Edition. University of Toronto Press. 2016. ISBN 978-1-4426-3503-6.
- Eschnauer, H. R.; Stoeppler, M. Wine—An enological specimen bank. Stoeppler, M. (编). Hazardous Materials in the Environment. Elsevier Science. 1992: 49–72 (58). ISBN 978-0-444-89078-8. doi:10.1016/s0167-9244(08)70103-3.
- Frankenburg, F. R. Brain-Robbers: How Alcohol, Cocaine, Nicotine, and Opiates Have Changed Human History. ABC-CLIO. 2014. ISBN 978-1-4408-2932-1.
- Frebel, A. Searching for the Oldest Stars: Ancient Relics from the Early Universe. Princeton University. 2015. ISBN 978-0-691-16506-6.
- Gale, W. F.; Totemeier, T. C. Smithells Metals Reference Book. Butterworth-Heinemann. 2003. ISBN 978-0-08-048096-1.
- Gilfillan, S. C. Lead poisoning and the fall of Rome. Journal of Occupational Medicine. 1965, 7 (2): 53–60. ISSN 0096-1736. PMID 14261844.
- Gill, T.; Libraries Board of South Australia. The history and topography of Glen Osmond, with map and illustrations. Libraries Board of South Australia. 1974. ISBN 9780724300358.
- Graedel, T. E.; et al. Metal stocks in Society – Scientific Synthesis (PDF) (报告). International Resource Panel: 17. 2010 [18 April 2017]. ISBN 978-92-807-3082-1. (原始内容 (PDF)存档于26 April 2018).
- Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements 2nd. Butterworth-Heinemann. 1998. ISBN 978-0-7506-3365-9.
- Grout, J. Lead poisoning and Rome. Encyclopaedia Romana. 2017 [15 February 2017].
- Gulbinska, M. K. Lithium-ion Battery Materials and Engineering: Current Topics and Problems from the Manufacturing Perspective. Springer. 2014: 96. ISBN 978-1-4471-6548-4.
- Hadlington, T. J. On the Catalytic Efficacy of Low-Oxidation State Group 14 Complexes. Springer. 2017. ISBN 978-3-319-51807-7.
- Harbison, R. D.; Bourgeois, M. M.; Johnson, G. T. Hamilton and Hardy's Industrial Toxicology. John Wiley & Sons. 2015. ISBN 978-0-470-92973-5.
- Hernberg, S. Lead Poisoning in a Historical Perspective (PDF). American Journal of Industrial Medicine. 2000, 38 (3): 244–54 [1 March 2017]. PMID 10940962. doi:10.1002/1097-0274(200009)38:3<244::AID-AJIM3>3.0.CO;2-F. (原始内容 (PDF)存档于21 September 2017).
- A History of Cosmetics from Ancient Times. Cosmetics Info. [18 July 2016]. (原始内容存档于14 July 2016).
- Hodge, T. A. Vitruvius, lead pipes and lead poisoning. American Journal of Archaeology. 1981, 85 (4): 486–91. JSTOR 504874. S2CID 193094209. doi:10.2307/504874.
- Hong, S.; Candelone, J.-P.; Patterson, C. C.; et al. Greenland ice evidence of hemispheric lead pollution two millennia ago by Greek and Roman civilizations (PDF). Science. 1994, 265 (5180): 1841–43. Bibcode:1994Sci...265.1841H. PMID 17797222. S2CID 45080402. doi:10.1126/science.265.5180.1841.
- Hunt, A. Dictionary of Chemistry. Routledge. 2014. ISBN 978-1-135-94178-9.
- IAEA - Nuclear Data Section. Livechart - Table of Nuclides - Nuclear structure and decay data. www-nds.iaea.org. International Atomic Energy Agency. 2017 [31 March 2017].
- Insight Explorer; IPEN. New Study Finds Lead Levels in a Majority of Paints Exceed Chinese Regulation and Should Not be on Store Shelves (PDF) (报告). 2016 [3 May 2018].
- Ismawati, Yuyun; Primanti, Andita; Brosché, Sara; Clark, Scott; Weinberg, Jack; Denney, Valerie. Timbal dalam Cat Enamel Rumah Tangga di Indonesia (PDF) (报告). BaliFokus & IPEN. 2013 [26 December 2018] (印度尼西亚语).
- Jensen, C. F. Online Location of Faults on AC Cables in Underground Transmission. Springer. 2013. ISBN 978-3-319-05397-4.
- Kaupp, M. Chemical bonding of main-group elements (PDF). Frenking, G.; Shaik, S. (编). The Chemical Bond: Chemical Bonding Across the Periodic Table. John Wiley & Sons. 2014: 1–24. ISBN 9783527664658. S2CID 17979350. doi:10.1002/9783527664658.ch1.
- Kellett, C. Poison and Poisoning: A Compendium of Cases, Catastrophes and Crimes. Accent Press. 2012. ISBN 978-1-909335-05-9.
- King, R. B. Inorganic Chemistry of Main Group Elements. VCH Publishers. 1995. ISBN 978-1-56081-679-9.
- Konu, J.; Chivers, T. Stable Radicals of the Heavy p-Block Elements. Hicks, R. G. (编). Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds. John Wiley & Sons. 2011. ISBN 978-0-470-77083-2. doi:10.1002/9780470666975.ch10.
- Krestovnikoff, M.; Halls, M. Scuba Diving
 . Dorling Kindersley. 2006. ISBN 978-0-7566-4063-7.
. Dorling Kindersley. 2006. ISBN 978-0-7566-4063-7. - Langmuir, C. H.; Broecker, W. S. How to Build a Habitable Planet: The Story of Earth from the Big Bang to Humankind. Princeton University Press. 2012. ISBN 978-0-691-14006-3.
- Lead sling bullet; almond shape; a winged thunderbolt on one side and on the other, in high relief, the inscription DEXAI "Catch!". The British Museum. [30 April 2012]. (原始内容存档于14 February 2012).
- Lead mining. The Northern Echo. [16 February 2016].
- Levin, H. L. The Earth Through Time. John Wiley & Sons. 2009. ISBN 978-0-470-38774-0.
- Lochner, J. C.; Rohrbach, G.; Cochrane, K. What is Your Cosmic Connection to the Elements? (PDF). Goddard Space Flight Center. 2005 [2 July 2017]. (原始内容 (PDF)存档于29 December 2016).
- Lodders, K. Solar System abundances and condensation temperatures of the elements (PDF). The Astrophysical Journal. 2003, 591 (2): 1220–47. Bibcode:2003ApJ...591.1220L. ISSN 0004-637X. S2CID 42498829. doi:10.1086/375492.
- Macintyre, J. E. Dictionary of Inorganic Compounds. CRC Press. 1992. ISBN 978-0-412-30120-9.
- Markowitz, G.; Rosner, D. "Cater to the children": the role of the lead industry in a public health tragedy, 1900–55. American Journal of Public Health. 2000, 90 (1): 36–46. PMC 1446124
 . PMID 10630135. doi:10.2105/ajph.90.1.36.
. PMID 10630135. doi:10.2105/ajph.90.1.36. - Meija, Juris; et al. Atomic weights of the elements 2013 (IUPAC Technical Report). Pure and Applied Chemistry. 2016, 88 (3): 265–91. doi:10.1515/pac-2015-0305.
- More, A. F.; Spaulding, N. E.; Bohleber, P.; et al. Next-generation ice core technology reveals true minimum natural levels of lead (Pb) in the atmosphere: Insights from the Black Death (PDF). GeoHealth. 2017, 1 (4): 211–219. ISSN 2471-1403. PMC 7007106
 . PMID 32158988. doi:10.1002/2017GH000064.
. PMID 32158988. doi:10.1002/2017GH000064. - Mosseri, S.; Henglein, A.; Janata, E. Trivalent lead as an intermediate in the oxidation of lead(II) and the reduction of lead(IV) species. Journal of Physical Chemistry. 1990, 94 (6): 2722–26. doi:10.1021/j100369a089.
- Nakashima, T.; Hayashi, H.; Tashiro, H.; et al. Gender and hierarchical differences in lead-contaminated Japanese bone from the Edo period. Journal of Occupational Health. 1998, 40 (1): 55–60. doi:10.1539/joh.40.55
 .
. - National Council on Radiation Protection and Measurements. Structural Shielding Design for Medical X-ray Imaging Facilities. 2004. ISBN 978-0-929600-83-3.
- Norman, N. C. Periodicity and the s- and p-Block Elements. Oxford University Press. 1996. ISBN 978-0-19-855961-0.
- Nriagu, J. O. Saturnine gout among Roman aristocrats — Did lead poisoning contribute to the fall of the Empire?. The New England Journal of Medicine. 1983, 308 (11): 660–63. PMID 6338384. doi:10.1056/NEJM198303173081123.
- Encyclopedia Judaica: Ossuaries and Sarcophagi. www.jewishvirtuallibrary.org. [14 July 2018].
- Olinsky-Paul, T. East Penn and Ecoult battery installation case study webinar (PDF). Clean Energy States Alliance. 2013 [28 February 2017].
- Palmieri, R. (编). The Organ. Psychology Press. 2006. ISBN 978-0-415-94174-7.
- Parthé, E. Crystal Chemistry of Tetrahedral Structures. CRC Press. 1964. ISBN 978-0-677-00700-7.
- Parker, R. B. The New Cold-Molded Boatbuilding: From Lofting to Launching. WoodenBoat Books. 2005. ISBN 978-0-937822-89-0.
- Pauling, L. General Chemistry. W. H. Freeman and Company. 1947. ISBN 978-0-486-65622-9.
- Polyanskiy, N. G. Fillipova, N. A , 编. Аналитическая химия элементов: Свинец [Analytical Chemistry of the Elements: Lead]. Nauka. 1986 (俄语).
- Primary Lead Refining Technical Notes. LDA International. [7 April 2007]. (原始内容存档于22 March 2007).
- Progressive Dynamics, Inc. How Lead Acid Batteries Work: Battery Basics. progressivedyn.com. [3 July 2016]. (原始内容存档于19 November 2018).
- Putnam, B. The Sculptor's Way: A Guide to Modelling and Sculpture. Dover Publications. 2003. ISBN 978-0-486-42313-5.
- Pyykkö, P. Relativistic effects in structural chemistry. Chemical Reviews. 1988, 88 (3): 563–94. doi:10.1021/cr00085a006.
- Rabinowitz, M. B. Imputing lead sources from blood lead isotope ratios. Beard, M. E.; Allen Iske, S. D. (编). Lead in Paint, Soil, and Dust: Health Risks, Exposure Studies, Control Measures, Measurement Methods, and Quality Assurance. ASTM. 1995: 63–75. ISBN 978-0-8031-1884-3. doi:10.1520/stp12967s.
- Ramage, C. K. (编). Lyman Cast Bullet Handbook
 3rd. Lyman Products Corporation. 1980.
3rd. Lyman Products Corporation. 1980. - Reddy, A.; Braun, C. L. Lead and the Romans. Journal of Chemical Education. 2010, 87 (10): 1052–55. Bibcode:2010JChEd..87.1052R. doi:10.1021/ed100631y.
- Roederer, I. U.; Kratz, K.-L.; Frebel, A.; et al. The end of nucleosynthesis: Production of lead and thorium in the early galaxy. The Astrophysical Journal. 2009, 698 (2): 1963–80. Bibcode:2009ApJ...698.1963R. S2CID 14814446. arXiv:0904.3105
 . doi:10.1088/0004-637X/698/2/1963.
. doi:10.1088/0004-637X/698/2/1963. - Rieuwerts, J. The Elements of Environmental Pollution. Routledge. 2015. ISBN 978-0-415-85919-6.
- Riva, M. A.; Lafranconi, A.; d'Orso, M. I.; et al. Lead poisoning: Historical aspects of a paradigmatic "occupational and environmental disease". Safety and Health at Work. 2012, 3 (1): 11–16. PMC 3430923
 . PMID 22953225. doi:10.5491/SHAW.2012.3.1.11.
. PMID 22953225. doi:10.5491/SHAW.2012.3.1.11. - Retief, F.; Cilliers, L. P. Lead poisoning in ancient Rome. Acta Theologica. 2006, 26 (2): 147–64 (149–51). doi:10.4314/actat.v26i2.52570
 .
. - Rich, V. The International Lead Trade. Woodhead Publishing. 1994. ISBN 978-0-85709-994-5.
- Rogalski, A. Infrared Detectors 2nd. CRC Press. 2010 [19 November 2016]. ISBN 978-1-4200-7671-4.
- Röhr, C. Binare Zintl-Phasen [Binary Zintl Phases]. Intermetallische Phasen [Intermettallic Phases]. Universitat Freiburg. 2017 [18 February 2017] (德语).
- Radioactive Decay Series (PDF). Nuclear Systematics. MIT OpenCourseWare. 2012 [28 April 2018].
- Samson, G. W. The divine law as to wines. J. B. Lippincott & Co. 1885.
- Scarborough, J. The myth of lead poisoning among the Romans: An essay review. Journal of the History of Medicine and Allied Sciences. 1984, 39 (4): 469–475. PMID 6389691. doi:10.1093/jhmas/39.4.469.
- Silverman, M. S. High-pressure (70-k) synthesis of new crystalline lead dichalcogenides. Inorganic Chemistry. 1966, 5 (11): 2067–69. doi:10.1021/ic50045a056.
- Singh, P. Over 73% of paints found to have excessive lead: Study. Times of India. 2017 [3 May 2018].
- Slater, J. C. Atomic Radii in Crystals. The Journal of Chemical Physics. 1964, 41 (10): 3199–3204. Bibcode:1964JChPh..41.3199S. ISSN 0021-9606. doi:10.1063/1.1725697.
- Sinha, S. P.; Shelly; Sharma, V.; et al. Neurotoxic effects of lead exposure among printing press workers. Bulletin of Environmental Contamination and Toxicology. 1993, 51 (4): 490–93. PMID 8400649. S2CID 26631583. doi:10.1007/BF00192162.
- Smirnov, A. Yu.; Borisevich, V. D.; Sulaberidze, A. Evaluation of specific cost of obtainment of lead-208 isotope by gas centrifuges using various raw materials. Theoretical Foundations of Chemical Engineering. 2012, 46 (4): 373–78. S2CID 98821122. doi:10.1134/s0040579512040161.
- Stabenow, F.; Saak, W.; Weidenbruch, M. Tris(triphenylplumbyl)plumbate: An anion with three stretched lead–lead bonds. Chemical Communications. 2003, (18): 2342–2343. PMID 14518905. doi:10.1039/B305217F.
- Street, A.; Alexander, W. Metals in the Service of Man 11th. Penguin Books. 1998. ISBN 978-0-14-025776-2.
- Stone, R. An Element of Stability. Science. 1997, 278 (5338): 571–572. Bibcode:1997Sci...278..571S. S2CID 117946028. doi:10.1126/science.278.5338.571.
- Szczepanowska, H. M. Conservation of Cultural Heritage: Key Principles and Approaches. Routledge. 2013. ISBN 978-0-415-67474-4.
- Tétreault, J.; Sirois, J.; Stamatopoulou, E. Studies of lead corrosion in acetic acid environments. Studies in Conservation. 1998, 43 (1): 17–32. JSTOR 1506633. doi:10.2307/1506633.
- Takahashi, K.; Boyd, R. N.; Mathews, G. J.; et al. Bound-state beta decay of highly ionized atoms (PDF). Physical Review C. 1987, 36 (4): 1522–1528 [27 August 2013]. Bibcode:1987PhRvC..36.1522T. OCLC 1639677. PMID 9954244. doi:10.1103/physrevc.36.1522. (原始内容 (PDF)存档于21 October 2014).
- Thomson, T. The History of Chemistry. Henry Colburn and Richard Bentley (publishers). 1830. ISBN 9780405066238.
- Thornton, I.; Rautiu, R.; Brush, S. M. Lead: The Facts (PDF). International Lead Association. 2001 [5 February 2017]. ISBN 978-0-9542496-0-1.
- Toxicological Profile for Lead (PDF). Agency for Toxic Substances and Disease Registry/Division of Toxicology and Environmental Medicine. 2007. (原始内容 (PDF)存档于1 July 2017).
- Tuček, K.; Carlsson, J.; Wider, H. Comparison of sodium and lead-cooled fast reactors regarding reactor physics aspects, severe safety and economical issues (PDF). Nuclear Engineering and Design. 2006, 236 (14–16): 1589–98. doi:10.1016/j.nucengdes.2006.04.019.
- Think Lead research summary (PDF). The Lead Sheet Association. [20 February 2017]. (原始内容 (PDF)存档于20 February 2017).
- Thürmer, K.; Williams, E.; Reutt-Robey, J. Autocatalytic oxidation of lead crystallite surfaces. Science. 2002, 297 (5589): 2033–35. Bibcode:2002Sci...297.2033T. PMID 12242437. S2CID 6166273. doi:10.1126/science.297.5589.2033.
- Tolliday, B. Significant growth in lead usage underlines its importance to the global economy. International Lead Association. 2014 [28 February 2017]. (原始内容存档于28 February 2017).
Global demand for lead has more than doubled since the early 1990s and almost 90% of use is now in lead-acid batteries
- Toronto museum explores history of contraceptives. ABC News. 2003 [13 February 2016].
- Tungate, M. Branded Beauty: How Marketing Changed the Way We Look. Kogan Page Publishers. 2011. ISBN 978-0-7494-6182-9.
- United States Geological Survey. Geological Survey Professional Paper. United States Government Publishing Office. 1973: 314.
- University of California Nuclear Forensic Search Project. Decay Chains. Nuclear Forensics: A Scientific Search Problem. [23 November 2015].
- United States Geological Survey. Lead (PDF) (报告). 2005 [20 February 2016].
- United States Geological Survey. Lead (PDF). Mineral Commodities Summaries. 2017 [8 May 2017].
- Vogel, N. A.; Achilles, R. The Preservation and Repair of Historic Stained and Leaded Glass (PDF) (报告). United States Department of the Interior. 2013 [30 October 2016].
- Waldron, H. A. Lead and lead poisoning in antiquity. Medical History. 1985, 29 (1): 107–08. PMC 1139494
 . doi:10.1017/S0025727300043878.
. doi:10.1017/S0025727300043878. - Wiberg, E.; Wiberg, N.; Holleman, A. F. Inorganic Chemistry. Academic Press. 2001. ISBN 978-0-12-352651-9.
- Willey, D. G. The physics behind four amazing demonstrations — CSI. Skeptical Inquirer. 1999, 23 (6) [6 September 2016].
- Webb, G. A. Nuclear Magnetic Resonance. Royal Society of Chemistry. 2000. ISBN 978-0-85404-327-9.
- Winder, C. The history of lead — Part 3. LEAD Action News. 1993b, 2 (3) [12 February 2016]. ISSN 1324-6011. (原始内容存档于31 August 2007).
- Windholz, M. Merck Index of Chemicals and Drugs 9th. Merck & Co. 1976. ISBN 978-0-911910-26-1. Monograph 8393.
- Weatherings to Parapets and Cornices. The Lead Sheet Association. [20 February 2017]. (原始内容存档于20 February 2017).
- Webb, G. W.; Marsiglio, F.; Hirsch, J. E. Superconductivity in the elements, alloys and simple compounds. Physica C: Superconductivity and Its Applications. 2015, 514: 17–27. Bibcode:2015PhyC..514...17W. S2CID 119290828. arXiv:1502.04724
 . doi:10.1016/j.physc.2015.02.037.
. doi:10.1016/j.physc.2015.02.037. - World Nuclear Association. Nuclear Radiation and Health Effects. 2015 [12 November 2015].
- Wrackmeyer, B.; Horchler, K. 207Pb-NMR Parameters 22. Academic Press. 1990: 249–303. ISBN 978-0-08-058405-8. doi:10.1016/S0066-4103(08)60257-4.
- Whitten, K. W.; Gailey, K. D.; David, R. E. General chemistry with qualitative analysis 3rd. Saunders College. 1996. ISBN 978-0-03-012864-6.
- Winder, C. The history of lead — Part 1. LEAD Action News. 1993a, 2 (1) [5 February 2016]. ISSN 1324-6011. (原始内容存档于31 August 2007).
- Wood, J. R.; Hsu, Y-T.; Bell, C. Sending Laurion Back to the Future: Bronze Age Silver and the Source of Confusion (PDF). Internet Archaeology. 2021, 56 (9). S2CID 236973111. doi:10.11141/ia.56.9.
- Yong, L.; Hoffmann, S. D.; Fässler, T. F. A low-dimensional arrangement of [Pb9]4· clusters in [K(18-crown-6)]2K2Pb9·(en)1.5. Inorganica Chimica Acta. 2006, 359 (15): 4774–78. doi:10.1016/j.ica.2006.04.017.
- Yu, L.; Yu, H. Chinese Coins: Money in History and Society. Long River Press. 2004. ISBN 978-1-59265-017-0.
- Zhang, X.; Yang, L.; Li, Y.; et al. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environmental Monitoring and Assessment. 2012, 184 (4): 2261–73. PMID 21573711. S2CID 20372810. doi:10.1007/s10661-011-2115-6.
- Zhao, F. Information Technology Entrepreneurship and Innovation. IGI Global. 2008: 440. ISBN 978-1-59904-902-1.
- Zuckerman, J. J.; Hagen, A. P. Inorganic Reactions and Methods, the Formation of Bonds to Halogens. John Wiley & Sons. 1989. ISBN 978-0-471-18656-4.
- Zýka, J. Analytical study of the basic properties of lead tetraacetate as oxidizing agent. Pure and Applied Chemistry. 1966, 13 (4): 569–81 [2 March 2017]. S2CID 96821219. doi:10.1351/pac196613040569.
延伸阅读
[在维基数据编辑]
- Astrid, S.; Helmut, S.; Sigel, R. K. O. (编). Lead: Its Effects on Environment and Health. Metal Ions in Life Sciences 17. De Gruyter. 2017. ISBN 978-3-11-044107-9. Table of contents
- Casas, J. S.; Sordo, J. (编). Lead Chemistry, Analytical Aspects. Environmental Impacts and Health Effects. Elsevier. 2006. ISBN 978-0-444-52945-9.
外部連結
- 元素铅在洛斯阿拉莫斯国家实验室的介紹(英文)
- EnvironmentalChemistry.com —— 铅(英文)
- 元素铅在The Periodic Table of Videos(諾丁漢大學)的介紹(英文)
- 元素铅在Peter van der Krogt elements site的介紹(英文)
- WebElements.com – 铅(英文)
| 元素周期表(主族金屬) | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IA 1 |
IIA 2 |
IIIB 3 |
IVB 4 |
VB 5 |
VIB 6 |
VIIB 7 |
VIIIB 8 |
VIIIB 9 |
VIIIB 10 |
IB 11 |
IIB 12 |
IIIA 13 |
IVA 14 |
VA 15 |
VIA 16 |
VIIA 17 |
VIIIA 18 | ||||||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||




