苯丙胺
此條目可參照英語維基百科相應條目來擴充,此條目在對應語言版為高品質條目。 |
此條目翻譯品質不佳,原文在en:Amphetamine。 |
 | |
 | |
| 臨床資料 | |
|---|---|
| 讀音 | |
| 其他名稱 | α-甲基苯乙胺 |
| AHFS/Drugs.com | amphetamine |
| 依賴性 | 生理依賴: 無 心理依賴: 中等 |
| 成癮性 | 中等 |
| 給藥途徑 | 醫用:口服給藥、鼻腔給藥、靜脈注射[1] 非醫療用(Recreational): 口服給藥、鼻腔給藥, 吹入劑、栓劑、靜脈注射 |
| ATC碼 | |
| 法律規範狀態 | |
| 法律規範 |
|
| 藥物動力學數據 | |
| 生物利用度 | 口服 75–100%[2] |
| 血漿蛋白結合率 | 15–40%[3] |
| 藥物代謝 | Amphetamine only: CYP2D6,[4]Dopamine β-hydroxylase,[14][15][16]含黃素的單加氧酶[14][17][18] |
| 代謝產物 | 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-羥基苯基丙酮, 苯甲酸, 馬尿酸, 苯丙醇胺, 苯基丙酮[4][5][6] |
| 藥效起始時間 | 短效型(IR 立即釋放藥物) 使用後(dosing):30–60 ;分鐘內[7] 長效型 (XR 緩緩釋放藥物) 使用後(dosing):1.5–2 ;小時內[8][9][10] |
| 生物半衰期 | D-amph:9–11 ;小時[4][11] L-amph:11–14 ;小時[4][11] 視PH值而定:8–31 ;小時[12] |
| 作用時間 | 短效型(IR 立即釋放藥物) 使用後(dosing):3–7 小時內[8][13] 長效型(XR 緩緩釋放藥物) 使用後(dosing): 12 ;小時內[8][10][13] |
| 排泄途徑 | 主要透過腎臟; 視PH值而定 範圍:1–75%[4] |
| 識別資訊 | |
| |
| CAS編號 | 300-62-9 |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB配體ID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.543 |
| 化學資訊 | |
| 化學式 | C9H13N |
| 摩爾質量 | 135.20622 g/mol[19] |
| 3D模型(JSmol) | |
| 密度 | 0.9±0.1 g/cm3 at 25 °C[20] |
| 熔點 | 11.3 °C(52.3 °F) (預測)[22] |
| 沸點 | 203 °C(397 °F) 為 760 毫米汞柱[21] |
| |
| |
安非他明(英語:amphetamine, alpha-methylphenethylamine)(化學式:C₆H₅CH₂CH(CH₃)NH₂,為中樞神經刺激劑,用來治療注意力不足過動症、嗜睡症、和肥胖症。英語源通稱「安非他明」擷取自α-甲基苯乙胺的英文讀法「alpha‑methylphenethylamine」之縮讀。安非他明是在1887年發現的,以兩種對映異構體的形式存在[I],分別是左旋安非他明和右旋安非他明。準確來說,安非他明指的是特定的化學物質,是外消旋的純胺類型態[24][25],這個物質等同於安非他明的兩個對映異構體:左旋安非他明和右旋安非他明等量之純胺類型態。不過,安非他明一詞已用來表示任何比例安非他明對映異構體的組合,或是任一種異構體[26][25][27]。
以往曾用安非他明來治療鼻塞和抑鬱。在非醫療用途上,安非他明也有用作體能增強藥物、益智藥、春藥及欣快藥物。安非他明在許多國家為合法的處方藥。然而,私自散佈和囤積安非他明被視為非法行為,因為安非他明被用於非醫療用途的助興可能性極高,而且有明顯健康上的危害[25][28][24]。
首個藥用安非他明的藥品名稱為Benzedrine。當今藥用安非他明[參 1]以下列幾種形式存在:外消旋安非他明[參 2]、阿得拉爾 [II]、右旋安非他明,或前驅藥物甲磺酸賴氨酸安非他明。安非他明藉着自身作用於兒茶酚胺神經傳導元素:正腎上腺素及多巴胺的特點來活化痕量胺受體,進而增加單胺類神經遞質和神經遞質在腦內的活動。[1][11][30]
在醫療用的劑量範圍內,安非他明能帶來情緒以及執行功能的變化,例如:欣快感的增強、性慾的改變、清醒度的提升、大腦執行功能的進化。安非他明所改變的生理反應包含:減少反應時間、降低疲勞、以及肌耐力的增強。若攝取劑量遠超過醫療用的劑量範圍,將會導致大腦執行功能受損以及橫紋肌溶解症,攝取過份超越醫療用劑量範圍的安非他明可引發嚴重的藥物成癮,服用遠超醫療用劑量範圍的安非他明會引起精神疾病(例如:妄想、偏執)。然而長期攝取醫療劑量範圍的安非他明並不會引起上述疾病。那些為享樂而攝入的安非他明通常會遠超過醫療用劑量範圍,且伴隨着非常嚴重甚至致命的副作用。[11][31][32][33]。
安非他明屬於苯乙胺衍生物。安非他明的衍生物也歸在同一分類中[參 3]的分類中[III],比如說:安非他酮[參 4]、卡西酮、MDMA、和甲基苯丙胺[參 5]。安非他明也與人體內可自然生成的兩個屬於痕量胺的神經傳導物質——特別是苯乙胺和N-甲基苯乙胺——有關。苯乙胺只比安非他明少了一個甲基,而N-甲基苯乙胺則是安非他明的結構異構(差異只在於甲基位置的不同)。[34][35][36]
用途
[編輯]醫療
[編輯]安非他明可以治療注意力不足過動症(ADHD)、嗜睡症(一種睡眠疾病)、和肥胖症。有時候安非他明會以仿單標示外使用的方式處方來治療頑固性憂鬱症和頑固性強迫症[1][11][37][38]。在動物試驗中,已知非常高劑量的安非他明會造成某些動物的多巴胺系統和神經系統的受損。[39][40]但是,在人體試驗中,注意力不足過動症患者在接受安非他明的治療後,則發現安非他明可促進大腦的發育及神經的成長。[41][42][43]
回顧許多核磁共振照影(英語:Nuclear Magnetic Resonance Imaging)的研究後發現,長期以安非他明治療注意力不足過動症患者能顯著降低患者大腦結構及大腦執行功能上的異常。並且改善大腦中數個部位,例如:基底神經節的右尾狀核[41][42][43]。
眾多臨床研究的系統性及統合性回顧已確立長期使用安非他明治療注意力不足過動症的療效及安全。[44][45][46][47]
持續長達兩年的隨機對照試驗[參 6][a]結果顯示:長期使用安非他明治療注意力不足過動症,是有效且安全的[44][47]。
兩個系統性/統合性回顧的結果顯示長期且持續地使用中樞神經興奮劑治療注意力不足過動症能有效地減少注意力不足過動症的核心徵狀(核心徵狀即為:過動、衝動和分心/無法專心)、增進生活品質、提升學業成就、廣泛地強化大腦的執行功能。[IV]這些執行功能分別與下列項目有關:學業、反社會行為、駕駛習慣、藥物濫用、肥胖、職業、日常活動、自尊心、服務使用(例如:學習、職業、健康、財金、和法律等)、社交功能[45][47]。
一篇系統性/統合性回顧標誌了一個重要發現:一個為期九個月的隨機雙盲試驗中,持續以安非他明治療的ADHD患者,其智力商數平均增加4.5單位[註 1],且在專注力、衝動、過動的改善皆呈現持續進步的態勢。[44]另一篇系統性/統合性回顧則指出:根據迄今為止為時最長的數個臨床追蹤研究[參 7],可以得到一個結論:即便從兒童時期開始以中樞神經興奮劑治療直到老年,中樞神經興奮劑都能持續有效地控制ADHD的徵狀並且減少物質濫用的風險。[47] 研究表明,ADHD與大腦的執行功能受損有關。而這些受損的執行功能分別與大腦中部分的神經傳導系統有關[參 8][48];又此部分受損的神經傳導系統和中腦皮質激素-多巴胺[參 9]的傳導及藍斑核[參 10]和前額葉[參 11]中的正腎上腺素[參 12]的傳導相關。[48]
中樞神經興奮劑,例如:methylphenidate和安非他明對於治療ADHD都是有效的,因為中樞神經興奮劑刺激了上述神經系統中的神經傳導物質活動。[31][48][49]
至少超過80%的ADHD患者在使用中樞神經興奮劑治療後,其ADHD的徵狀可以獲得改善。[50]
使用中樞神經興奮劑治療的ADHD患者相較之下,普遍與同儕及家庭成員的關係較佳並且在學校擁有較好的表現。興奮劑能使ADHD患者較不易分心、衝動、且擁有較長的專注力時間和範圍。[51][52]
根據考科藍協作組織[參 13]所提供的文獻回顧結果[V]指出:使用中樞神經興奮劑治療的ADHD患者即便其徵狀改善,相較於使用非中樞神經興奮劑,仍因副作用而有較高的停藥率。[54][55]
回顧結果也發現,中樞神經興奮劑並不會惡化抽動綜合症的徵狀,例如:妥瑞氏症,除非服用dextroamphetamine[b]的劑量過高才有可能在部分妥瑞氏症合併注意力不足過動症患者身上觀察到抽動綜合症的徵狀惡化。[56]
中樞神經興奮劑只要依照醫師指示用藥,都是相當安全的。[57][58][59][59][60]中樞神經興奮劑,例如:利他能與專思達,可能導致:心悸、頭痛、胃痛、喪失食慾、失眠、因相對專注而變得冷淡(面無表情)等副作用,因此6歲以下的兒童不適宜服用。(副作用產生與否因人而異)[61]
隨着時間推進與各方的努力,中樞神經興奮劑的相關副作用已可藉由包括但不限於劑量調整、服藥時間、飯前飯後服用、服藥頻率等服藥模式之改變以及改變藥物組合等方式獲得相當程度的減少。[59][62][63][64][65]
提昇表現
[編輯]- 認知方面
2015年,一篇系統性回顧[參 14]和一篇統合分析/整合分析[參 15]回顧了數篇優秀的臨床試驗[參 16]報告後發現,低劑量(醫療用劑量)的安非他明能適度但不強烈地促進一個人的認知功能,包含工作記憶、長期的情節記憶、抑制控制以及在一些方面的注意力。[66] [67]安非他明強化認知功能的效果已知是部分透過間接活化在大腦前額葉的多巴胺受體D1和腎上腺素受體 α2。[31] [66]一篇2014年的系統性回顧發現,低劑量(醫療用劑量)的安非他明能促進和穩固記憶鞏固的過程,進而提升一個人回憶的能力。[68]低劑量(醫療用劑量)的安非他明也可增加大腦皮層(質)區的效率,這能讓一個人的工作記憶獲得進步。[31][69]安非他明和其他用於治療ADHD的中樞神經刺激劑能透過提升task saliency來增加一個人去做事情的動機、並強化一個人的警覺心(清醒度),因而能刺激一個人開始做「以目標為導向」的行為。[31][70][71]中樞神經興奮劑(例如:安非他明)能提升一個人在困難且枯燥的任務中的表現。[31][71][72]超過醫療用劑量範圍(包含其誤差範圍及容許最大上限)的安非他明劑量將不利於工作記憶和其他的認知功能。[31][71]
- 生理
雖然安非他明可以提升速度、耐力(延遲疲勞的發生)、肌耐力、身體素質和警覺心並減少心理反應時間。[32][73][74][75]然而,「非因醫療需求使用安非他明」在各種運動場合都是被嚴格禁止的。[76][77]
安非他明藉由抑制多巴胺在中樞神經系統中的回收及外流來促進耐力和反應時間的提升。[74][75][78]安非他明和其他作用於多巴胺系統的藥物一樣,都能增加在固定施力(levels of perceived exertion)下的動力(能)輸出。這是因為安非他明能奪取(override)體溫的「安全開關」的控制權並將身體核心溫度的上限提高以取得在體溫安全上限提高前被身體保留的能量。[75][79][80]於醫療用劑量範圍(包含其誤差範圍),安非他明的副作用不至於影響運動員的運動表現;[32][74]然而,當攝取的劑量過多時,安非他明可能會引起嚴重的後果,例如:橫紋肌溶解症和高熱(體溫過高)。[33][81][74]
醫療上的禁忌
[編輯]根據國際化學品安全規劃署(IPCS, International Programme on Chemical Safety)和美國食品藥物管理局(USFDA),[VI]安非他明不建議處方給有藥物濫用、心血管疾病、對於各種刺激嚴重反應過度、和嚴重焦慮歷史的人。[VII][83][84]安非他明也不被建議處方給正經歷動脈血管硬化(血管硬化)、中度到重度高血壓、青光眼(眼壓過高)、或甲狀腺機能亢進(身體在體內製造出過量的甲狀腺賀爾蒙/激素)的人。[83][84][85]曾對中樞神經刺激劑有藥物過敏的人以及正在服用單胺氧化酶抑制劑(MAOI)或單胺氧化酶抑制劑類藥物(MAOIs),可能不適合使用安非他明。即便曾有合併使用安非他明和單胺氧化酶抑制劑後仍一切平安的案例。[83][84][86][87]
IPCS和美國食品藥物管理局也同意患有神經性厭食症、雙極性情感疾患、憂鬱、高血壓、狂躁、精神分裂症、雷諾氏綜合症、癲癇發作、抽動綜合症、妥瑞氏症、和有甲狀腺問題、肝腎問題的人在使用安非他明時應密切追蹤上述疾病的變化。[83][84]
人體試驗證明,醫療用劑量下的安非他明並不會導致胎兒或新生兒畸形。然而超越醫療用劑量甚多的安非他明確實會增加胎兒或新生兒畸形的機會。[84]
研究觀察發現,安非他明會進入母親的母乳中,因此建議母親不要在使用安非他明藥物的期間內授乳。[83][84]
由於安非他明可能影響食慾繼而導致可反轉的身高及體重的成長遲緩,[VIII],因此建議兒童或青少年在用藥期間定期測量自己的身高及體重。[83]
副作用
[編輯]安非他明的副作用以及其發生率和嚴重度大致上與使用的劑量呈正相關。[33][89][73]成分為安非他明的藥品,諸如:阿得拉爾、Dexedrine、和安非他明的等價物質(generic equivalents)目前皆已獲得美國食品藥物管理署許可用於長期性的治療。[89][29]
攝取大幅超出醫療劑量的安非他明將大幅增加嚴重副作用出現的風險。[73]
生理
[編輯]在治療劑量下,生理副作用會因年齡或個人情況而有所不同[89]。心血管方面的副作用包含:迷走-血管反射導致的高血壓或是低血壓、雷諾氏症(因小動脈收縮而導致流往手腳的血流減少)、以及心搏過速(tachycardia)。[89][73][88]
男性性方面而言,副作用可能包含:勃起障礙、頻繁勃起、或是勃起時間過長。[89]
消化方面的副作用可能包含:腹痛、喪失胃口、反胃以及體重降低。[89][90]其他潛在的副作用包含:視力模糊、口乾、磨牙、流鼻血、多汗症、藥物性鼻炎(藥物導致的鼻塞)、癲癇閾值/觸發門檻降低,以及抽搐[來源組 1]。在一般的治療劑量下鮮少發生危險副作用[73]。
安非他明刺激延腦的呼吸中樞,使得呼吸變得較快速且較深。[73]正常人在治療劑量下,此作用通常難以察覺;然而,此作用在呼吸已經受損的病人身上有可能變得明顯。[73]
安非他明會使膀胱括約肌收縮,而導致解尿困難[73]。此效果可以應用在遺尿或是失去膀胱控制能力的病人身上。[73]
安非它命在胃腸道的作用是難以預測的[73]。安非他明可能會減少胃腸活動力(內容物通過腸胃道的速率)[73];然而,安非他明亦可能在胃腸道的平滑肌處於鬆弛狀態時,增加其活動力。[73]
安非他明有輕微的止痛作用且可以增強鴉片類物質的止痛作用。[73]
美國食藥署2011年委任的研究發現:不論是小孩或是成人,「安非他明(於醫療情境下使用)」和「其他用於治療ADHD的中樞神經興奮劑」均和重大的心血管疾病(猝死、心臟病發、中風)無關[來源組 2];然而,當病患已有心血管方面的疾病時,禁用此藥。[來源組 3]
心理
[編輯]在醫療用劑量範圍,最常見的副作用為:警覺心的增強、(對未來或即將發生的不愉快之事的)憂慮/擔心/恐懼、理解力提升、專注力的提升、主動性/自主決斷行事的能力的提升、自信心的提升、社交能力的提升;情緒陰晴不定、失眠或清醒、和疲勞感的減退。[89][73]
比較少見的副作用包括焦慮、性慾改變、應激性、重複性的或強迫性的行為(repetitive or obsessive behaviors)、靜不下來;[來源組 4]。副作用出現與否因人而異,端視用藥者的個性及精神狀態(mental state)。
安非他明所引起的精神疾病,例如:妄想和偏執,可能出現在重度的使用者身上。[33][89][98]長期攝取醫療劑量的安非他明雖然有可能引起上一段文中所述的疾病,但這是非常罕見的。[33][89][99]根據美國食品藥物管理局所提供的資訊,「目前沒有證據顯示『中樞神經刺激劑』會導致『攻擊性的行為(aggressive behavior)』或『敵意(hostility)』」。[89]
使用醫療劑量的用藥者可能會培養出習慣在某個特定地方用藥的偏好。[54][100][100][101]
法律
[編輯]安非他明在中華民國受毒品危害防制條例管制,若無處方箋,在司法上視為第二級管制藥品,持有、運輸、販賣皆為違法行為。[102]
嚴重過量
[編輯]安非他明過量使用會引起許多徵狀,然而在適當的醫療照護下,不至於死亡。[84][103]
藥物過量徵狀的嚴重度與劑量成正比;與身體對安非他明的藥物耐受性成反比。[73][84]已知每天攝取達到5公克的安非他明(每天最大攝取量的五十倍)會導致身體對安非他明產生藥物耐受性。[84]嚴重過量的安非他明攝取所致的徵狀列於下方;安非他明中毒一旦到達出現全身抽搐和昏迷則必須立刻急救以避免死亡。[33][73]在2013年,安非他明、甲基安非他明和其他列於ICD-10 第五章:精神和行為障礙§使用化學藥物、物質或酒精引起的精神和行為障礙中的安非他明相關物質的過量使用在世界上共導致3788人死亡。(3,425–4,145人死亡、95%信賴區間)。[IX][104]
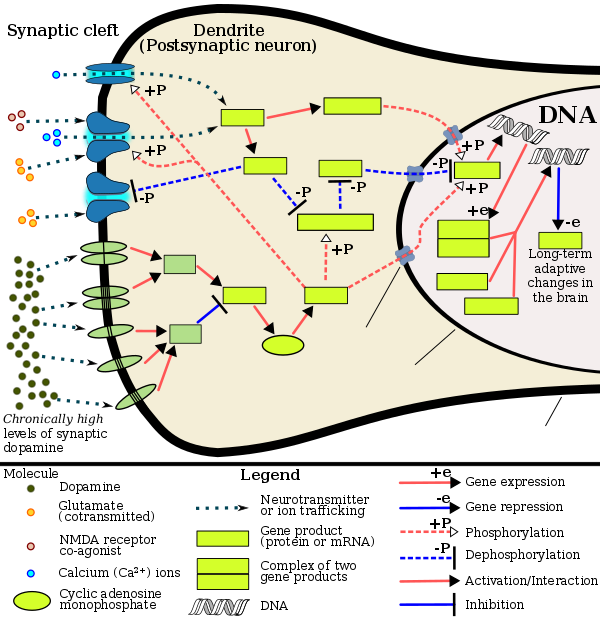
被過度活化達到病態程度的中腦邊緣迴路(一個連接腹側被蓋區和伏隔核的多巴胺通道),在安非他明的成癮中扮演着主要的角色。[105][106]
當一個人經常服用嚴重過量的安非他明,將伴隨安非他明成癮的高度風險,因為持續過量的安非他明會逐漸增加伏隔核的ΔFosB(「成癮」與否的分子開關和主控蛋白)的檔次。[107][108][109]一旦伏隔核的ΔFosB過度表達,這個人的「成癮性行為」[註 2](例如:出現試圖取得安非他明的衝動行為)將開始隨之增加。[107][110]雖然目前沒有治療安非他明成癮的有效藥物,但規律的且每次都有持續一定時間的有氧運動能降低安非他明的成癮風險也是治療安非他明成癮的天然療法。[111][112][來源組 5]運動能提升臨床治療的預後,且可能與認知行為治療(目前已知最有效的安非他明成癮的臨床治療法)相搭配為聯合療法。[111][113][114]
| 生物系統 | 輕度、中度過量[33][73][84] | 過量[來源組 6] |
|---|---|---|
| 心臟血管系統 | ||
| 中樞神經系統 | ||
| 肌肉骨骼系統 |
| |
| 呼吸系統 |
|
|
| 生殖泌尿系統 | ||
| 其他 |
成癮
[編輯]| 「成癮及生理、心理依賴」的相關術語詞彙表[101][108][117][118] | |
|---|---|
| |
長期服用遠超醫療用劑量範圍的安非他明會導致安非他明成癮。然而長期攝取醫療劑量範圍的安非他明並不會引起上述問題。[119][120][121]安非他明濫用(例如:長期攝取嚴重過量的安非他明)會導致大腦對於該劑量產生藥物耐受性。漸漸地,濫用者必須服用更大量的安非他明以換取同樣的效果。[122][123]
分子生物機轉
[編輯]當前關於「長期安非他明濫用所致的成癮」的模型中,已知會改變一些腦部的結構(特別是伏隔核)[124][125][126]。造成腦部結構改變的最重要的轉錄因子(transcription factor)為:ΔFosB、cAMP反應元件結合蛋白(CREB)、和核因子κB(NF-κB)。[X][125]ΔFosB在藥物成癮的發展過程中扮演着至關重要的角色,主要的原因在於其在伏隔核中D1-type中型多棘神經元的過度表達,為「成癮」及「成癮衍生的行為」及「神經元為了適應新常態所做的調適」的充分且必要條件。[XI][107][108][125]
一旦ΔFosB充分過度表達(sufficiently overexpressed),將誘發越來越嚴重的成癮狀態並伴隨ΔFosB值的持續創新高。[107][108]ΔFosB已被證明與酒精成癮、大麻成癮、可卡因成癮、派醋甲酯成癮、尼古丁成癮、鴉片成癮、phencyclidine成癮、異丙酚、和安非他明的替代性物質成癮、及其他成癮有關。[來源組 7]ΔJunD為一個轉錄因子;而G9a為組織蛋白甲基轉移酶的一種。ΔJunD和G9a直接與伏隔核中的ΔFosB值的升高成反比。[108][125][130]
利用載體讓伏隔核中的ΔJunD充分過度表達,可以使由長期藥物濫用所致的漸進式神經元和行為改變完全停止。(比如說:ΔFosB所致的改變)。[125]ΔFosB也在人們於天然酬賞(natural rewards)中的行為反應調節上扮演重要的腳色。天然酬賞包含:美味的食物、性愛、運動......。[110][125][131]因為天然酬賞以及成癮性藥物皆會激發ΔFosB(這些酬賞讓大腦刺激ΔFosB的增加),長期過度地從事上述行為將可能導致類似的成癮之病理生理(pathological)。[110][125]
ΔFosB是導致「安非他明成癮」、「安非他明引起的性成癮」中最關鍵的致癮因素。「安非他明引起的性成癮」為「安非他明使用」加上「過度的性活動」所引發的「衝動之下的性行為」。[110][132][133]這類的性成癮與多巴胺失調綜合症相關,有時此症會出現在正在服用作用於多巴胺的藥物的人身上。[110][131]
安非他明基因調控(gene regulation)的效果端視劑量與通路(dose- and route-dependent)而定。[126]絕大多數主題為「基因調節(gene regulation)」和「成癮」的研究都是透過動物試驗以及利用靜脈注射的方式對實驗動物注射超高劑量的安非他明來進行。[126]少數幾個透過人體試驗(依照體重來決定醫療用劑量)來進行的研究表明,口服醫療用劑量的安非他明並不會影響基因調控,即便有,也是極為輕微的。這表示安非他明用作醫療用途是十分安全的。[126]
藥物治療
[編輯]截至2014年5月[update]並沒有能夠有效治療安非他明成癮的藥理療法[134][135][136]。2015年到2016年間的論文回顧結果指出:選擇性TAAR1促進劑有非常大的可能在將來被用來治療中樞神經興奮劑的成癮;[137][138]然而,截至2016年2月[update],已知可作為選擇性TAAR1促進劑的物質都屬於試驗性藥物。[137][138]安非他明成癮與伏隔核中的多巴胺接收器們以及位置相同(co-localized)的NMDA 接受器們的活化高度相關;[XII][106]鎂離子藉由封鎖一個接受器-鈣離子通道,來阻斷 NMDA接受器們。[106][139]一篇論文回顧做成結論:根據動物試驗,因成癮而使用中樞神經刺激劑的人,可以發現過量的中樞神經興奮劑顯著降低腦細胞內部的鎂離子活動。[106]利用鎂元素補充劑,能降低安非他明使用者自我服用[c]的機會。然而這不被認為是有效治療安非他明成癮的單一療法(mono-therapy)。[XIII][106]
行為治療
[編輯]認知行為治療是當前治療中樞神經刺激劑成癮的療法。[114]除此之外,運動在生物神經元產生的效果的研究中表明維持每天從事有氧運動(例如:跑步等)的習慣,能避免藥物成癮纏身;本身也是一個對於治療安非他明成癮的有效附加療法。[來源組 5]運動能讓所有疾病的預後都更加樂觀,特別是對於中樞神經刺激劑成癮。[111][113][140]值得一提的是,有氧運動能降低擅自服用中樞神經興奮劑的慾望,降低再次擅自服用中樞神經興奮劑的概率(reinstatment)(i.e., relapse)、降低「試圖取得藥物所做出的舉動(drug-seeking behavior)」、降低多巴胺受體D2在紋狀體中的密度。[110][140]它在病生理學中的腳色是相對於「興奮劑的使用」和「興奮劑的效果」,它會引起紋狀體中DRD2密度的減少。[110]一篇論文回顧提到,藉由改變紋狀體(striatum)中的ΔFosB、c-Fos immunoreactivity或部份的腦內回饋系統來避免藥物成癮在一個人身上的發展。[112]
| 神經可塑性和行為可塑性的形式 | 增強物的種類 | 來源 | |||||
|---|---|---|---|---|---|---|---|
| 鴉片類 | 中樞神經刺激劑 | 高脂肪或高糖食物 | 性交 | 運動與神經元關係 | 環境豐富化 | ||
| 伏隔核中D1-type中的ΔFosB表現 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [110] |
| 行為可塑性 | |||||||
| 攝取量的增加 | 有 | 有 | 有 | [110] | |||
| 中樞神經刺激劑跨越-敏化作用 | 有 | 不適用 | 有 | 有 | 削減 | 削減 | [110] |
| 未經過處方而自行私下攝取中樞神經刺激劑 | ↑ | ↑ | ↓ | ↓ | ↓ | [110] | |
| 強化「在特定地點攝取興奮劑的習慣」 | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [110] |
| 強化「試圖取得該致癮藥物的行為」 | ↑ | ↑ | ↓ | ↓ | [110] | ||
| 神經化學物質的可塑性 | |||||||
| 伏隔核中CREB磷酸化 | ↓ | ↓ | ↓ | ↓ | ↓ | [110] | |
| 伏隔核中對於多巴胺的過敏反應 | 沒有 | 有 | 沒有 | 有 | [110] | ||
| 經過變動的紋狀體多巴胺接收器的訊號發送 | ↓DRD2 , ↑DRD3 | ↑DRD1, ↓DRD2 , ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [110] | |
| 經過變動的紋狀體鴉片樣肽受體的訊號發送 | 未改變,或 ↑μ-鴉片接收器 |
↑μ-鴉片接收器 ↑κ-鴉片接收器 |
↑μ-鴉片接收器 | ↑μ-鴉片接收器 | 未改變 | 未改變 | [110] |
| 發生於紋狀體鴉片肽的改變 | ↑強啡肽 腦啡肽未改變 |
↑強啡肽 | ↓腦啡肽 | ↑強啡肽 | ↑強啡肽 | [110] | |
| 多巴胺通道的神經突觸的可塑性 | |||||||
| 伏隔核中樹突的數量 | ↓ | ↑ | ↑ | [110] | |||
| 伏隔核中樹突棘的密度 | ↓ | ↑ | ↑ | [110] | |||
註解:DRD2 = 多巴胺受體D2;↑ = 上升;↓ = 下降
依賴和戒斷徵狀
[編輯]根據另一篇由考科藍協作組織所做的一篇論文回顧指出當一個長期嚴重攝取安非他明或甲基安非他明的藥物成癮者某天突然停止攝取安非他明或甲基安非他明,那麼根據許多成癮個案的報告顯示,具有時效性(time-limited)的戒斷徵狀將在他們上一次攝取安非他明後的24小時內出現。 在成癮患者停用安非他明後,安非他明的戒斷徵狀的出現率接近九成。這九成都出現至少六個定義在「《精神疾病診斷與統計手冊》安非他明戒斷徵狀」中的徵狀。年紀與劑量和戒斷徵狀的嚴重度呈正相關。安非他明的戒斷徵狀共有兩個階段且總共可能歷時三周或更多。第一階段(撞牆期marked "crush" phase)約持續一周。[141]安非他明的戒斷徵狀可能包含:對於各種刺激極度敏感、躁動不安(irritability)、焦慮、對於安非他明有難以抑制的渴求、煩躁、疲倦、多食症、過動或行動遲緩、缺乏動機、嗜睡、和清醒夢。[141][142]
這些特徵及徵狀必須非由其他疾病(包含心理疾病)引起,且無法歸因於其他物質的濫用。滿足上述條件,才符合「安非他明戒斷徵狀」綜合症的診斷標準。[141][143]
通過美國食品藥物管理局嚴格審核的安非他明藥品說明書上並未提到任何安非他明在醫療用劑量下突然停用會導致任何安非他明戒斷徵狀的出現。[85][144][145][146]
DSM中,安非他明中毒及戒斷徵狀之標準
[編輯]DSM-5中關於興奮劑中毒的標準如下:
A.最近曾經服用過安非他明類的物質、可卡因或其他興奮劑
B.在服用興奮劑時(或者服用後很快表現出)臨床表現出顯著問題行為或心理變化(如:欣快症或感情遲鈍;群性、社交性的改變;過於警覺;人際交往敏感;焦慮、緊張或者惱怒;刻板行為;判斷力受損)。
C.在服用興奮劑時或服用後即刻表現出以下任意兩種(或以上)徵狀:
- 心動過速或心動過緩
- 瞳孔擴散
- 血壓升高或降低
- 出汗或發冷
- 感到噁心或嘔吐
- 體重降低
- 精神運動性焦躁或精神運動性遲滯
- 肌肉無力、呼吸抑制、胸口疼痛或心律不整
- 精神錯亂、癲癇、運動困難、肌張力障礙或昏迷
包括其他物質中毒的情況在內,其他身體情況不可能出現該發病跡象、徵狀,並且也無法理解為其他的精神問題。
DSM-5中關於興奮劑戒斷徵狀的標準如下:
A.停止服用(或減少服用)長期的安非他明類物質、可卡因或其他興奮劑。
B.在A的情況發生後的幾小時至幾天內,出現煩躁的情緒並伴有以下任意兩種(或以上)的心理變化:
- 疲勞
- 生動而不愉快的夢
- 失眠或嗜睡
- 食慾增大
- 精神運動性焦躁或精神運動性遲滯
B中的徵狀或跡象導致了臨床顯著的壓力,或者在社會、工作等重要方面功能出現受損。
安非他明戒斷徵狀的頻率列表
[編輯]| 徵狀 | 頻率 |
|---|---|
| 無徵狀 | 14% |
| 易怒 | 78% |
| 疼痛和痛苦 | 58% |
| 感到沮喪 | 50% |
| 社交能力受損 | 46% |
| 發抖、出冷汗 | 36% |
| 難以入睡 | 32% |
| 虛脫 | 22% |
| 噁心、嘔吐 | 16% |
| 頭痛 | 14% |
| 難以保持清醒 | 12% |
| 食慾增大 | 12% |
| 便秘 | 10% |
| 食慾縮小 | 8% |
| 腹瀉 | 6% |
| 原因 | 個人嘗試戒毒 | 醫學指導戒毒 |
|---|---|---|
| 對生活整體現狀(犯罪、無聊、金錢)不滿 | 42(89%) | 6(37%) |
| 對心理健康感到擔憂(偏執、憂慮、依賴) | 25(53%) | 3(19%) |
| 家庭原因(父母或配偶的壓力,子女出生) | 24(51%) | 5(31%) |
| 身體健康(動脈注射、血管萎陷、感染) | 17(36%) | 4(25%) |
| 避免入獄 | 0 | 2(12%) |
| 其他原因 | 2(4%) | 0 |
| - | 自我嘗試戒毒(Self detoxication) | 被迫戒毒(Enforced detoxication) |
|---|---|---|
| 服用更多其他藥物 | - | - |
| 大麻(Cannabis) | 22(27%) | 10(59%) |
| 替馬西泮(Temazepam) | 21(26%) | - |
| 酒精 | 17(21%) | 2(12%) |
| 鴉片類物質(Opiates) | 12(15%) | 1(6%) |
| 地西泮(Diazepam) | 4(5%) | - |
| 巴比妥類藥物(Barbiturates) | 3(4%) | 1(6%) |
| 心理學技巧 | - | - |
| 轉移注意力(例如:工作、看電視) | 35(21%) | 1(6%) |
| 不再與藥物成癮的朋友來往 | 31(19%) | - |
| 獲得人們的支持(家庭、社會中的支持團體) | 11(7%) | - |
| 把藥物及針頭丟掉 | 5(3%) | - |
中毒與致病
[編輯]中毒
[編輯]在囓齒動物(rodents)和靈長類動物(primates)的藥物試驗發現到,夠高的安非他明劑量會導致多巴胺神經中毒甚或致使多巴胺末梢神經受損退化並降低轉運體和接收器的功能。[148][149]目前並無證據顯示安非他明會直接荼毒人類的神經。[150][151]然而,超高劑量(large doses)的安非他明攝取量可能會產生高熱(體溫過高)的現象並間接導致:多巴胺的神經性中毒(dopaminergic neurotoxicity)、過多的活性氧類(reactive oxygen species)生成、自然氧化(autoxidation)增加。[來源組 8]從高劑量的安非他明攝取量引起的神經中毒的生物模式中發現,人體核心體溫高於40°C是「高劑量的安非他明攝取量」是否引起神經中毒的「必要條件」。[149]在動物試驗中,若動物的腦溫長期超過40°C,容易因過多的活性氧類生成、受干擾的細胞蛋白功能和短暫的血腦屏障標準放寬而促使安非他明性的神經中毒發生。[149]
致病
[編輯]嚴重的安非他明過量可能造成「中樞神經刺激劑過量所引發的精神異常(stimulant psychosis)」,徵狀包含但不限於幻覺(delusion)和被害妄想、疑神疑鬼、妄想、偏執等。[98]此機制用於精神分裂症研究時誘發精神病發作的藥物。[154]一篇由考科藍協作組織所做的論文回顧及統整發現在所有因攝取嚴重過量的「安非他明、dextro-安非他明、及甲基安非他明」而導致精神異常的患者中,有5%-15%的患者即便經過治療,仍無法完全康復。[98][155]
根據同一篇由考科藍協作組織所做的論文回顧及統整,至少一個實驗(trial)顯示抗精神病藥物(antipsychotic)能有效解決因嚴重過量的安非他明所致的急性精神異常徵狀。 [98]另外,穩定在服用抗精神病藥物的人使用安非他明後心跳血壓等生理變化也較一般人來的少。[154]
「攝取醫療劑量的安非他明所致的急性精神異常」是非常罕見的(very rare)。
(非常罕見 very rare: < 1/10000 ;罕見 rare:>= 1/10000 & <1/1000)[99][83]
交互作用
[編輯]目前已知許多種物質都會和安非他明發生藥物相互作用,導致安非他明或參與作用的另一物質的藥效或代謝過程發生改變。[4][156]用於分解安非他明的酶的抑制劑(如CYP2D6、FMO3)都會延長其半衰期,這意味着藥效會更持久。[17][156]安非他明也會和MAOIs產生交互作用,特別是MAOI類中的monoamine oxidase A抑制劑(monoamine oxidase A inhibitors)。
因為MAOIs和安非他明兩者都會增加兒茶酚胺(i.e.,正腎上腺素和多巴胺)在血漿中的濃度[156];因此MAOIs與安非他明合併使用是危險的[156]。
安非他明會調節幾乎所有作用於中樞神經的藥物的活動。特別需要注意的是,安非他明可能會降低鎮靜劑和中樞神經抑制劑)的效果,並增加其他中樞神經刺激劑和抗憂鬱藥的效果。[156]
安非他明也可能降低抗高血壓藥和抗精神病藥(anti-psychotics)的藥效,這是因為安非他明本身對於血壓及多巴胺系統的作用。[156]
鋅的補充劑可能會降低安非他明用於治療注意力不足過動症時的最小有效劑量(minimum effective dose)。[XIV][160]
整體來說,安非他明並不會與日常生活中常見的食物起任何重大的交互作用,但安非他明的吸收和排泄會分別受到腸胃內容物(gastrointestinal content)的pH值和尿液的酸鹼值影響。[156] 酸性物質會減少安非他明的吸收並增加尿液的排泄;鹼性物質則相反。
由於pH值在安非他明的吸收這件事上具有影響力,所以安非他明也會和 氫離子泵阻斷劑(PPI, proton pump inhibitors)和H2受體阻抗劑(H2 antihistamines)等中和胃酸的制酸劑產生交互作用。[156]
藥學
[編輯]藥物代謝動力學
[編輯]安非他明的口服生物體可利用率[參 17]與腸胃的pH值連動;[156]安非他明非常容易在腸道被吸收,右旋苯丙胺的生體可利用率在多數的情況下高於75%。[2]安非他明呈弱鹼性,其pKa值介於9–10之間;[4]因此,當pH值呈鹼性時,多數的安非他明會以其易溶於脂類的純胺類型態形式存在。在此情況下,身體會通過腸道上皮組織富含脂類的細胞膜[參 18]來吸收安非他明。[4][156]相反地,酸性的pH值表示安非他明主要以易溶於水的離子(鹽)形式存在,因此較少能被吸收。[4]大約15–40%循環於血管中的安非他明與血漿蛋白[參 19]相連接。[3]安非他明的對映異構體的半衰期會隨着尿液的pH值而有所不同。[4]當尿液的酸鹼值落在正常範圍中,右旋苯丙胺和左旋苯丙胺的半衰期分別為9–11小時及11–14小時。[4]酸性飲食會導致安非他明的對映異構體的半衰期降低至8–11小時;鹼性飲食則會使安非他明的對映異構體的半衰期增加到16–31小時。[5][12]
成分為安非他明或其衍生物的短效藥品大約在口服後三小時在體內達到最高血漿濃度;而成分為安非他明或其衍生物的長效藥品則在口服後大約七小時在體內達到最高血漿濃度。[4]
安非他明主要透過腎臟來代謝,大約30–40%的藥物以藥物本身原始的型態從酸鹼度正常的尿液中排出。[4] 尿液是鹼性時,安非他明傾向以其純胺類型態存在,因此較少被排泄。[4]
當尿液的pH值失常時,各種安非他明的分解物在尿液中重新結合的程度將從最低1%到最高75%。該程度的高低大多取決於於尿液的酸鹼值,尿液越酸,結合率越高;尿液愈鹼,結合率越低。[4]安非他明通常於口服後兩天內自體內完全代謝完畢。[5]安非他明確切的半衰期及藥效作用期隨着(小於兩天的)重複服用導致的血漿內安非他明濃度(plasma concentration of amphetamine)的增加而延長。[161]
對人體無藥效的前藥(prodrug):賴氨酸安非他明並不若安非他明一樣容易受腸胃道環境的pH值影響;[162]賴氨酸安非他明在腸道被吸收進入血管的血液後很快就會透過水解的方式轉化為右旋安非他明。而參與這水解反應的酶與紅血球有關。[162]
Lisdexamfetamine的半衰期通常小於一個小時。[162]
細胞色素 P450 2D6(Cytochrome P450 2D6、或CYP2D6)、多巴胺β羥化酶(Dopamine β-hydroxylase、或DBH)、flavin-containing monooxygenase 3、butyrate-CoA ligase、和glycine N-acyltransferase為已知在人體中參與[註 3]「安非他明」及「安非他明代謝後之產物」的代謝反應的酶。[來源組 9]
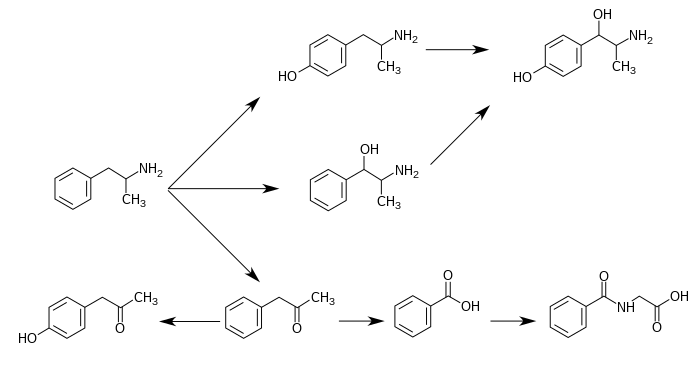
「安非他明代謝後之產物」包含:4-hydroxyamphetamine、4-hydroxynorephedrine、4-羥基苯基丙酮、苯甲酸、馬尿酸、苯丙醇胺、苯基丙酮[註 4][4][5][6]。
在這些「安非他明代謝後之產物」之中,有實際藥效的產物(sympathomimetics)為:4‑hydroxyamphetamine[166]、4‑hydroxynorephedrine[6]、和norephedrine[167]。 [166]4‑hydroxynorephedrine,[168]and norephedrine.[167]
安非他明的主要代謝途徑包含:芳香對羥基化、脂肪族α-、β-羥基化、N-氧化、N-脫烷基、和脫氨基。[4][5]
下圖為已知的「安非他明」代謝途徑和「安非他明代謝後之產物」:[4][17][6]
苯丙胺的代謝途徑
從酸鹼度正常的尿液中可發現,大約30–40%的「安非他明」以本身原始的型態排出;大約50%的安非他明以不具藥效的「安非他明代謝後之產物」(即為圖片中最下列的產物)的型態排出。 [4] 剩下的10–20%則為「安非他明代謝後之產物」之中,有實際藥效的產物。 [4] 苯甲酸(Benzoic acid)被butyrate-CoA連接酶(butyrate-CoA ligase)代謝後成為一個中介物質/中間產物(intermediate product):benzoyl-CoA [164] 隨後透過glycine N-acyltransferase代謝並轉化為馬尿酸(hippuric acid)。[165] |
藥物效應動力學
[編輯]苯丙胺在多巴胺能神經元的藥物效應動力學
|
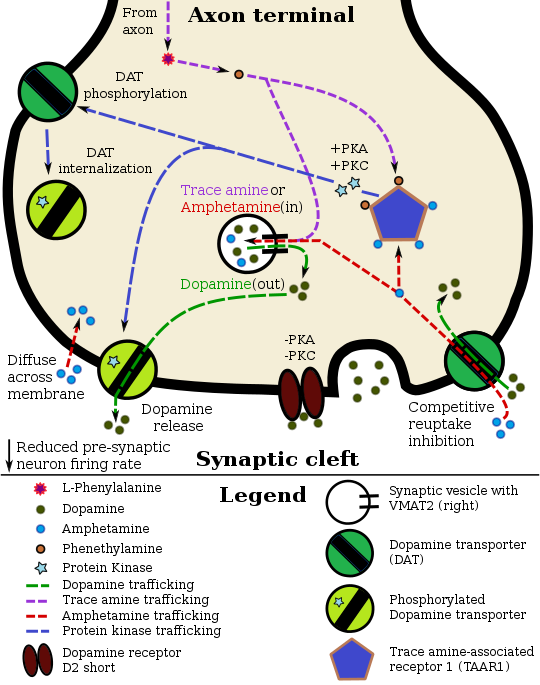
安非他明藉由將單胺類神經遞質在腦中(主要於腦中那酬賞與執行功能路徑上的兒茶酚胺的神經元)的用途改變為神經訊號來產生療效。[30][49]
安非他明作用於monoamine transporter上的效應使得犒賞系統和管控功能中的主要神經傳導物質(多巴胺、正腎上腺素)依安非他明的劑量,成比例的迅速上升。[30][49][169]
服用安非他明所帶來的增強和促進motivational salience的效果起因於中腦邊緣通路中的多巴胺活動變得更加活躍之故。[31]
安非他明帶給使用者的欣快感和運動協調激勵之效果和安非他明刺激紋狀體的神經突觸之多巴胺和正腎上腺素濃度增加的速度和規模成正比[1]。
安非他明已經被確認為trace amine-associated receptor 1(TAAR1)[註 5]的強刺激劑(potent full agonist)。[30][170]
TAAR1受額外刺激後會藉由活化腺苷酸環化酶和抑制單胺轉運蛋白的功能而增加cAMP的生成。[30][171]
單胺的自身受體(例如:D2 short、presynaptic α2、和presynaptic 5-HT1A))具有與TAAR1相反的效果,而這些受體形成了一個對於單胺的管理系統。[30][137]
值得注意的是,安非他明和痕量胺擁有對TAAR1相當高的連結親緣性(連結吸引力),但對於單胺之autoreceptors則不然。[30][137]
神經造影研究表示,安非他明和痕量胺對單胺類神經遞質的再回收抑制作用具有位置上的針對性,進一步來說,就是針對那些相關且有TAAR1同時存在的單胺類神經遞質神經元。[30]
截至2010年[update],TAAR1和多巴胺轉運體的「共同位置化」co-localization現象已可在恆河猴身上透過視覺化的方式觀察到,但【帶有去甲腎上腺素轉運體(NET)的TAAR1】和【血清素轉運體(SERT)】的共同位置化現象迄今只能透過信使核糖核酸(mRNA)的表現(expression)來證明。[30]
In addition to the neuronal monoamine transporters, amphetamine also inhibits both vesicular monoamine transporters, VMAT1 and VMAT2, as well as SLC1A1, SLC22A3, and SLC22A5.[來源組 10] SLC1A1 is excitatory amino acid transporter 3 (EAAT3), a glutamate transporter located in neurons, SLC22A3 is an extraneuronal monoamine transporter that is present in astrocytes, and SLC22A5 is a high-affinity carnitine transporter.[來源組 10]Amphetamine is known to strongly induce cocaine- and amphetamine-regulated transcript (CART) gene expression,[3][178]a neuropeptide involved in feeding behavior, stress, and reward, which induces observable increases in neuronal development and survival in vitro.[3][179][180]The CART receptor has yet to be identified, but there is significant evidence that CART binds to a unique Gi/Go-coupled GPCR.[180][181]Amphetamine also inhibits monoamine oxidases at very high doses, resulting in less monoamine and trace amine metabolism and consequently higher concentrations of synaptic monoamines.[19][182]In humans, the only post-synaptic receptor at which amphetamine is known to bind is the 5-HT1A receptor, where it acts as an agonist with micromolar affinity.[183][184]
The full profile of amphetamine's short-term drug effects in humans is mostly derived through increased cellular communication or neurotransmission of dopamine,[30]serotonin,[30]norepinephrine,[30]epinephrine,[169]histamine,[169]CART peptides,[3][178]endogenous opioids,[185][186][187]adrenocorticotropic hormone,[188][189]corticosteroids,[188][189]and glutamate,[172][174]which it effects through interactions with CART, 5-HT1A, EAAT3, TAAR1, VMAT1, VMAT2, and possibly other biological targets.[來源組 11]
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[190]Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[73][190]
歷史、社會與文化
[編輯]成藥
[編輯]目前幾種安非他明的處方藥配方含有兩種對映異構體,包括阿得拉爾,Dyanavel XR和Evekeo,其中最後一種是外消旋的安非他明硫酸鹽。[1][37][90]目前的一些品牌及其通用等同物如下。
| 藥物商品名稱 | United States Adopted Name |
(D:L) ratio | 藥劑型 Dosage form |
上市日期Marketing start date |
來源 |
|---|---|---|---|---|---|
| Adderall | – | 3:1 (salts) | 錠劑 tablet |
1996 | [1][29] |
| Adderall XR | – | 3:1 (salts) | 膠囊 capsule |
2001 | [1][29] |
| Adzenys XR | amphetamine | 3:1 (base) | ODT | 2016 | [191][192] |
| Dyanavel XR | amphetamine | 3.2:1 (base) | suspension | 2015 | [90][193] |
| Evekeo | amphetamine sulfate | 1:1 (salts) | tablet | 2012 | [37][194] |
| Dexedrine | dextroamphetamine sulfate | 1:0 (salts) | capsule | 1976 | [1][29] |
| ProCentra | dextroamphetamine sulfate | 1:0 (salts) | liquid | 2010 | [29] |
| Zenzedi | dextroamphetamine sulfate | 1:0 (salts) | tablet | 2013 | [29] |
| Vyvanse | lisdexamfetamine dimesylate | 1:0 (prodrug) | capsule | 2007 | [1][162] |
| tablet |

| 藥物 | 化學式 | 分子量[XV] | amphetamine base [XVI] |
amphetamine base in equal doses |
doses with equal base content[XVII] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[196][197] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[198] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[196][197] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[198] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[199] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[200] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[201] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[XVIII][90] | C9H13N | |||||||||
備註A
[編輯]- ^ 對映異構體指的是兩個形狀相同但方向相反的兩個分子,二個分子互為鏡像。[23]Levoamphetamine 和 dextroamphetamine 分別被簡稱為 L-amph 或 levamfetamine (INN) 和 D-amph 或 dexamfetamine (INN) [19]
- ^ "阿得拉爾"是一個品牌名稱而非公有領域的稱呼。但因為以下幾個安非他明的異構體的名稱及其英文名稱 ("dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate, and amphetamine aspartate") 太長了,因此本文將以此品牌名稱來表示此種安非他明的混合物。[29])
- ^ 「安非他明」一詞也意指一個化學分類,但與「安非他明衍生物」這個化學分類不同的是,「安非他明」類在學術上並無標準的定義。[14][25]
有一個「安非他明」類的定義嚴格限定分類中僅有:安非他明的racemate and enantiomers和甲基安非他明methamphetamine的racemate and enantiomers。[25]
大多數「安非他明」類的定義為那些在藥理學上以及結構上與安非他明相關的化合物。[25]
為避免讀者混淆,本條目中僅會使用amphetamine、amphetamines來表示racemic amphetamine, levoamphetamine, and dextroamphetamine;安非他明衍生物(substituted amphetamines)來表示安非他明的結構分類。 - ^ 研究證實,長期以中樞神經興奮劑治療ADHD能在下列這些方面產生大幅的進步:學業、駕駛、降低藥物濫用、降低肥胖、自尊、和社交功能等。[45]
在上述領域中,最為突出的領域為: 學業(例如:GPA分數、成果測驗分數、受教育的時間長度、和教育程度)、自尊(例如:自尊心測驗分數、嘗試自殺的次數、自殺率等)和社交功能(例如:peer nomination scores、社交技巧、家庭關係、同儕關係、和浪漫關係/情侶關係)[45]
長期以「藥物治療合併行為治療」的模式來治療ADHD,能夠比單獨以藥物治療,產生更全面且更長足的進步。 [45] - ^ 考科藍協作組織對於歷年眾多的「隨機對照試驗」的系統性回顧、數據統整分析後所得出的總結,基本上都是非常有水準且深具參考價值的。 [53]
- ^ 美國食品藥物管理局核准的藥品使用指引及醫療上的禁忌(放在藥盒中的仿單/說明書)並非為了限制醫師的決策而是為了避免藥商恣意宣稱藥物的作用。醫師可以此為參考,並依照每位病人的實際情況做出獨立的判斷。 [82]
- ^ 然而根據一篇回顧性論文,安非他明可以處方給曾有藥物濫用歷史的人,不過需要有對患者適度的藥品控管,例如:每天由醫護人員配給處方劑量。[1]
- ^ 曾受此副作用的用藥者,身高及體重在在短暫停藥後恢復至應有水準是可以被預期的。[44][47][88] 根據追蹤,持續三年過程不停歇的安非他明治療(沒有合併任何積極減少安非他明副作用的療法的情況下)平均會減少 2公分的最終身高。 [88]
- ^ 「95% 信賴區間」指的是:有95%的概率,真實的死亡人數介於3,425 和 4,145 之間。
- ^ 轉錄因子是一種可以增加或降低一個特定基因的基因表現的蛋白。[127]
- ^ 簡單來說,這裏的「充分且必要(necessary and sufficient)」關係指的是「ΔFosB在伏隔核中的過度表達(over-expression)」與「成癮衍生的行為」及「神經元為了適應新常態所做的調適」永遠都是一起發生。
- ^ NMDA接受器們為與電壓相關的ligand-gated ion channels。ligand-gated ion channels這個通道需要glutamate 以及一個共同促進劑(co-agonist ):(D-serine 或 大豆屬glycine)的同時連接才能被開啟。
[139] - ^ 該篇回顧表示magnesium L-aspartate 及 氯化鎂能大幅改善成癮行為。
- ^ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[157][158][159]The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[159]
- ^ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[195] and were within 0.01g/mol of published pharmaceutical values.
- ^ Amphetamine base percentage=molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ^ (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- ^ This product (Dyanavel XR) is an oral suspension (i.e., a drug that is suspended in a liquid and taken by mouth) that contains 2.5 mg/mL of amphetamine base.[90] The product uses an ion exchange resin to achieve extended release of the amphetamine base.[90]
備註B
[編輯]- ^ 智力測驗結果與專注力有關,詳見注意力不足過動症#智力
- ^ 因成癮所致的行為
- ^ 酶做為反應的催化劑catalyst,並不實際參與反應。
- ^ 不是苯丙酮
- ^ a Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) discovered in 2001, which is important for regulation of brain monoamines.
備註C
[編輯]註釋
[編輯]英文名稱對照
[編輯]- ^ 英文名稱為:Pharmaceutical amphetamine
- ^ 英文名稱為:racemic amphetamine
- ^ 英文名稱為:substituted amphetamine
- ^ 英文名稱為:Bupropion
- ^ 英文名稱為:meth-amphetamine
- ^ 英文名稱為:Randomized controlled trials
- ^ 英文名稱為:follow-up studies
- ^ 英文名稱為:neurotransmitter systems
- ^ 英文名稱為:dopamine
- ^ 英文名稱為:locus coeruleus
- ^ 英文名稱為:prefrontal cortex
- ^ 英文名稱為:nor-epinephrine或nor-adrenaline
- ^ 英文名稱為:Cochrane Collaboration
- ^ 英文名稱為:systematic review
- ^ 英文名稱為:meta-analysis
- ^ 英文名稱為:clinical trial
- ^ 英文名稱為:bioavailability
- ^ 英文名稱為:cell membrane
- ^ 英文名稱為:plasma protein
引用
[編輯]- ^ [89][73][88][90][91]
- ^ [92][93][94][95]
- ^ [83][84][92][94]
- ^ [96][89][73][97]
- ^ 5.0 5.1 [110][111][112][113][140]
- ^ [115][33][73][103][116]
- ^ [107][110][125][128][129]
- ^ [149][152][153]
- ^ [4][14][163][16][17][18][164][165]
- ^ 10.0 10.1 [169][172][173][174][175][176][177]
- ^ [30][169][173][174][178][183]
來源
[編輯]- ^ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 Heal DJ, Smith SL, Gosden J, Nutt DJ. Amphetamine, past and present – a pharmacological and clinical perspective. J. Psychopharmacol. June 2013, 27 (6): 479–496. PMC 3666194
 . PMID 23539642. doi:10.1177/0269881113482532.
. PMID 23539642. doi:10.1177/0269881113482532. The intravenous use of d-amphetamine and other stimulants still pose major safety risks to the individuals indulging in this practice. Some of this intravenous abuse is derived from the diversion of ampoules of d-amphetamine, which are still occasionally prescribed in the UK for the control of severe narcolepsy and other disorders of excessive sedation. ... For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
- ^ 2.0 2.1 Dextroamphetamine. DrugBank. University of Alberta. 2013-02-08. (原始內容存檔於2019-08-06) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 3.0 3.1 3.2 3.3 3.4 Amphetamine. DrugBank. University of Alberta. 2013-02-08. (原始內容存檔於2013-10-12) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 4.20 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 12–13. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ 5.0 5.1 5.2 5.3 5.4 Amphetamine. Pubchem Compound. National Center for Biotechnology Information. (原始內容存檔於2014-10-09) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 6.0 6.1 6.2 6.3 6.4 Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G. Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection. J. Pharm. Biomed. Anal. September 2002, 30 (2): 247–255. PMID 12191709. doi:10.1016/S0731-7085(02)00330-8.
- ^ amphetamine/dextroamphetamine. Medscape. WebMD. (原始內容存檔於2021-08-27) 使用
|archiveurl=需要含有|url=(幫助).Onset of action: 30–60 min
|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 8.0 8.1 8.2 Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (編). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 112. ISBN 9781441913968.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ Brams M, Mao AR, Doyle RL. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad. Med. September 2008, 120 (3): 69–88. PMID 18824827. doi:10.3810/pgm.2008.09.1909.
- ^ 10.0 10.1 Brams M, Mao AR, Doyle RL. Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder. Postgrad. Med. September 2008, 120 (3): 69–88. PMID 18824827. doi:10.3810/pgm.2008.09.1909.
- ^ 11.0 11.1 11.2 11.3 11.4 Adderall IR Prescribing Information (PDF). United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc.: 1–6. October 2015 [2016-05-18]. (原始內容存檔 (PDF)於2018-09-15).
- ^ 12.0 12.1 AMPHETAMINE. United States National Library of Medicine – Toxnet. Hazardous Substances Data Bank. (原始內容存檔於2017-10-02) 使用
|archiveurl=需要含有|url=(幫助).Concentrations of (14)C-amphetamine declined less rapidly in the plasma of human subjects maintained on an alkaline diet (urinary pH > 7.5) than those on an acid diet (urinary pH < 6). Plasma half-lives of amphetamine ranged between 16-31 hr & 8-11 hr, respectively, & the excretion of (14)C in 24 hr urine was 45 & 70%.
|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 13.0 13.1 Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. October 2012, 9 (4): 739–752. PMC 3480574
 . PMID 23065655. doi:10.1007/s13311-012-0150-9.
. PMID 23065655. doi:10.1007/s13311-012-0150-9.
- ^ 14.0 14.1 14.2 14.3 Glennon RA. Phenylisopropylamine stimulants: amphetamine-related agents. Lemke TL, Williams DA, Roche VF, Zito W (編). Foye's principles of medicinal chemistry 7th. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013: 646–648 [2015-09-11]. ISBN 9781609133450. (原始內容存檔於2019-06-09).
The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB. Dopamine-beta-hydroxylase. Stereochemical course of the reaction (PDF). J. Biol. Chem. 1974-01, 249 (2): 454–458 [2014-11-06]. PMID 4809526. (原始內容存檔 (PDF)於2019-04-04).
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ 16.0 16.1 Horwitz D, Alexander RW, Lovenberg W, Keiser HR. Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity. Circ. Res. May 1973, 32 (5): 594–599. PMID 4713201. doi:10.1161/01.RES.32.5.594.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ^ 17.0 17.1 17.2 17.3 Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol. Ther. 2005-06, 106 (3): 357–387. PMC 1828602
 . PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
. PMID 15922018. doi:10.1016/j.pharmthera.2005.01.001.
"Table 5: N-containing drugs and xenobiotics oxygenated by FMO (頁面存檔備份,存於互聯網檔案館)" - ^ 18.0 18.1 Cashman JR, Xiong YN, Xu L, Janowsky A. N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication. J. Pharmacol. Exp. Ther. March 1999, 288 (3): 1251–1260. PMID 10027866.
- ^ 19.0 19.1 19.2 Amphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 11 April 2015. (原始內容存檔於2013-10-13) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); 引用錯誤:帶有name屬性「PubChem Header」的<ref>標籤用不同內容定義了多次 - ^ Amphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 5 November 2016 [9 November 2016]. (原始內容存檔於2021-01-29).
|section-url=被忽略 (幫助);|section=被忽略 (幫助) - ^ Amphetamine. PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. (原始內容存檔於2013-10-13) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ Amphetamine. Chemspider. (原始內容存檔於2020-12-22) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ Enantiomer. IUPAC Goldbook. International Union of Pure and Applied Chemistry. [2014-03-14]. doi:10.1351/goldbook.E02069. (原始內容存檔於2013-03-17).
One of a pair of molecular entities which are mirror images of each other and non-superposable.
- ^ 24.0 24.1 Guidelines on the Use of International Nonproprietary Names (INNS) for Pharmaceutical Substances. World Health Organization. 1997 [1 December 2014]. (原始內容存檔於2015-01-09).
In principle, INNs are selected only for the active part of the molecule which is usually the base, acid or alcohol. In some cases, however, the active molecules need to be expanded for various reasons, such as formulation purposes, bioavailability or absorption rate. In 1975 the experts designated for the selection of INN decided to adopt a new policy for naming such molecules. In future, names for different salts or esters of the same active substance should differ only with regard to the inactive moiety of the molecule. ... The latter are called modified INNs (INNMs).
- ^ 25.0 25.1 25.2 25.3 25.4 25.5 Yoshida T. Chapter 1: Use and Misuse of Amphetamines: An International Overview. Klee H (編). Amphetamine Misuse: International Perspectives on Current Trends. Amsterdam, Netherlands: Harwood Academic Publishers. 1997: 2 [1 December 2014]. ISBN 9789057020810.
Amphetamine, in the singular form, properly applies to the racemate of 2-amino-1-phenylpropane. ... In its broadest context, however, the term [amphetamines] can even embrace a large number of structurally and pharmacologically related substances.
- ^ 引用錯誤:沒有為名為
DrugBank1的參考文獻提供內容 - ^ Amphetamine. Medical Subject Headings. United States National Library of Medicine. [16 December 2013]. (原始內容存檔於2015-09-27).
- ^ 引用錯誤:沒有為名為
MeSHAmphetaminer的參考文獻提供內容 - ^ 29.0 29.1 29.2 29.3 29.4 29.5 29.6 National Drug Code Amphetamine Search Results. National Drug Code Directory. United States Food and Drug Administration. [2013-12-16]. (原始內容存檔於2013-12-16).
- ^ 30.00 30.01 30.02 30.03 30.04 30.05 30.06 30.07 30.08 30.09 30.10 30.11 30.12 Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. January 2011, 116 (2): 164–176. PMC 3005101
 . PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
. PMID 21073468. doi:10.1111/j.1471-4159.2010.07109.x.
- ^ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 31.7
Malenka RC, Nestler EJ, Hyman SE. Chapter 13: Higher Cognitive Function and Behavioral Control. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 318, 321. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in normal subjects and those with ADHD. ... stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks ... through indirect stimulation of dopamine and norepinephrine receptors. ...
Beyond these general permissive effects, dopamine (acting via D1 receptors) and norepinephrine (acting at several receptors) can, at optimal levels, enhance working memory and aspects of attention. - ^ 32.0 32.1 32.2
Liddle DG, Connor DJ. Nutritional supplements and ergogenic AIDS. Prim. Care. June 2013, 40 (2): 487–505. PMID 23668655. doi:10.1016/j.pop.2013.02.009.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
· Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
· Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
· Improved reaction time
· Increased muscle strength and delayed muscle fatigue
· Increased acceleration
· Increased alertness and attention to task - ^ 33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 11. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. March 2010, 125 (3): 363–375. PMID 19948186. doi:10.1016/j.pharmthera.2009.11.005.
- ^ Amphetamine. European Monitoring Centre for Drugs and Drug Addiction. [2013-10-19]. (原始內容存檔於2020-02-27).
- ^ Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ. Biosynthesis of amphetamine analogs in plants. Trends Plant Sci. 2012, 17 (7): 404–412. PMID 22502775. doi:10.1016/j.tplants.2012.03.004.
Substituted amphetamines, which are also called phenylpropylamino alkaloids, are a diverse group of nitrogen-containing compounds that feature a phenethylamine backbone with a methyl group at the α-position relative to the nitrogen (Figure 1). ... Beyond (1R,2S)-ephedrine and (1S,2S)-pseudoephedrine, myriad other substituted amphetamines have important pharmaceutical applications. ... For example, (S)-amphetamine (Figure 4b), a key ingredient in Adderall® and Dexedrine®, is used to treat attention deficit hyperactivity disorder (ADHD) [79]. ...
[Figure 4](b) Examples of synthetic, pharmaceutically important substituted amphetamines. - ^ 37.0 37.1 37.2 Evekeo Prescribing Information (PDF). Arbor Pharmaceuticals LLC: 1–2. April 2014 [2015-08-11]. (原始內容存檔 (PDF)於2016-03-04).
- ^ Obsessive compulsive disorder (OCD). NHS Choice. 2016-09-28 [2017-04-04]. (原始內容存檔於2017-10-09).
- ^ Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L. Toxicity of amphetamines: an update. Arch. Toxicol. August 2012, 86 (8): 1167–1231. PMID 22392347. doi:10.1007/s00204-012-0815-5.
- ^ Berman S, O'Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann. N. Y. Acad. Sci. October 2008, 1141: 195–220. PMC 2769923
 . PMID 18991959. doi:10.1196/annals.1441.031.
. PMID 18991959. doi:10.1196/annals.1441.031.
- ^ 41.0 41.1 Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. February 2013, 70 (2): 185–198. PMID 23247506. doi:10.1001/jamapsychiatry.2013.277.
- ^ 42.0 42.1
Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J. Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies. J. Clin. Psychiatry. September 2013, 74 (9): 902–917. PMC 3801446
 . PMID 24107764. doi:10.4088/JCP.12r08287.
. PMID 24107764. doi:10.4088/JCP.12r08287.
- ^ 43.0 43.1
Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects.. Acta psychiatrica Scand. February 2012, 125 (2): 114–126. PMID 22118249. doi:10.1111/j.1600-0447.2011.01786.x.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- ^ 44.0 44.1 44.2 44.3
Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (編). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 121–123, 125–127. ISBN 9781441913968.
Ongoing research has provided answers to many of the parents』 concerns, and has confirmed the effectiveness and safety of the long-term use of medication.
- ^ 45.0 45.1 45.2 45.3 45.4 Arnold LE, Hodgkins P, Caci H, Kahle J, Young S. Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review. PLoS ONE. February 2015, 10 (2): e0116407. PMC 4340791
 . PMID 25714373. doi:10.1371/journal.pone.0116407.
. PMID 25714373. doi:10.1371/journal.pone.0116407. The highest proportion of improved outcomes was reported with combination treatment (83% of outcomes). Among significantly improved outcomes, the largest effect sizes were found for combination treatment. The greatest improvements were associated with academic, self-esteem, or social function outcomes.
Figure 3: Treatment benefit by treatment type and outcome group (頁面存檔備份,存於互聯網檔案館) - ^ Arnold, L. Eugene; Hodgkins, Paul; Caci, Hervé; Kahle, Jennifer; Young, Susan. Effect of Treatment Modality on Long-Term Outcomes in Attention-Deficit/Hyperactivity Disorder: A Systematic Review. PLoS ONE. [2017-05-15]. PMID 25714373. doi:10.1371/journal.pone.0116407. (原始內容存檔於2020-11-09).
- ^ 47.0 47.1 47.2 47.3 47.4 Huang YS, Tsai MH. Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge. CNS Drugs. July 2011, 25 (7): 539–554. PMID 21699268. doi:10.2165/11589380-000000000-00000.
Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. ... In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ^ 48.0 48.1 48.2 Malenka RC, Nestler EJ, Hyman SE. Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 154–157. ISBN 9780071481274.
- ^ 49.0 49.1 49.2 Bidwell LC, McClernon FJ, Kollins SH. Cognitive enhancers for the treatment of ADHD. Pharmacol. Biochem. Behav. August 2011, 99 (2): 262–274. PMC 3353150
 . PMID 21596055. doi:10.1016/j.pbb.2011.05.002.
. PMID 21596055. doi:10.1016/j.pbb.2011.05.002.
- ^
Parker J, Wales G, Chalhoub N, Harpin V. The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials. Psychol. Res. Behav. Manag. (systematic review (secondary source)). September 2013, 6: 87–99. PMC 3785407
 . PMID 24082796. doi:10.2147/PRBM.S49114.
. PMID 24082796. doi:10.2147/PRBM.S49114. Only one paper53 examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- ^ Millichap JG. Chapter 9: Medications for ADHD. Millichap JG (編). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD 2nd. New York, USA: Springer. 2010: 111–113. ISBN 9781441913968.
- ^ Stimulants for Attention Deficit Hyperactivity Disorder. WebMD. Healthwise. 2010-04-12 [2013-11-12]. (原始內容存檔於2013-11-13).
- ^ Scholten RJ, Clarke M, Hetherington J. The Cochrane Collaboration. Eur. J. Clin. Nutr. August 2005,. 59 Suppl 1: S147–S149; discussion S195–S196. PMID 16052183. doi:10.1038/sj.ejcn.1602188.
- ^ 54.0 54.1 Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M. Castells X , 編. Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults. Cochrane Database Syst. Rev. June 2011, (6): CD007813. PMID 21678370. doi:10.1002/14651858.CD007813.pub2.
- ^ Punja S, Shamseer L, Hartling L, Urichuk L, Vandermeer B, Nikles J, Vohra S. Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst. Rev. February 2016, 2: CD009996. PMID 26844979. doi:10.1002/14651858.CD009996.pub2.
- ^ Pringsheim T, Steeves T. Pringsheim T , 編. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst. Rev. April 2011, (4): CD007990. PMID 21491404. doi:10.1002/14651858.CD007990.pub2.
- ^ Home of MedlinePlus→ Health Topics → Attention Deficit Hyperactivity Disorder Attention Deficit Hyperactivity Disorder Also called: ADHD. Medlineplus.gov. [2016-12-27]. (原始內容存檔於2016-12-25).
- ^ 衛生福利部-食品藥物管理署-管制藥品. Fda.gov.tw. 2013-12-30 [2016-12-27]. (原始內容存檔於2017-01-20).
- ^ 59.0 59.1 59.2 Abuse, National Institute on Drug. Stimulant ADHD Medications: Methylphenidate and Amphetamines. [2017-04-05]. (原始內容存檔於2020-04-03).
- ^ Choices, N. H. S. What is a controlled medicine (drug)? - Health questions - NHS Choices. 2016-12-12 [2017-04-05]. (原始內容存檔於2017-05-03).
- ^ Methylphenidate. Home of MedlinePlus → Drugs, Herbs and Supplements → Methylphenidate Methylphenidate pronounced as (meth il fen i date). 2016-02-15 [2017-02-27]. (原始內容存檔於2017-07-04).
- ^ Combining medications could offer better results for ADHD patients. Science News. Elsevier. 2016-08-01 [January 2017]. (原始內容存檔於2017-01-02).
"Three studies to be published in the August 2016 issue of the Journal of the American Academy of Child and Adolescent Psychiatry (JAACAP) report that combining two standard medications could lead to greater clinical improvements for children with attention-deficit/hyperactivity disorder (ADHD) than either ADHD therapy alone.", August, 2016
- ^ Adults with ADHD. MedlinePlus the Magazine 9. 8600 Rockville Pike · Bethesda, MD 20894, United States of America: NATIONAL LIBRARY OF MEDICINE at the NATIONAL INSTITUTES OF HEALTH. Spring 2014: 19 [2017-04-05]. ISSN 1937-4712. (原始內容存檔於2017-07-15) (美國英語).
- ^ Attention deficit hyperactivity disorder. Home → Medical Encyclopedia → Attention deficit hyperactivity disorder. NATIONAL LIBRARY OF MEDICINE at the NATIONAL INSTITUTES OF HEALTH. 2016-05-25 [2017-02-27]. (原始內容存檔於2017-01-26).
- ^ All Disorders. National Institute of Neurological Disorders and Stroke. [2017-02-27]. (原始內容存檔於2016-12-02).
- ^ 66.0 66.1 Spencer RC, Devilbiss DM, Berridge CW. The Cognition-Enhancing Effects of Psychostimulants Involve Direct Action in the Prefrontal Cortex. Biol. Psychiatry. June 2015, 77 (11): 940–950. PMID 25499957. doi:10.1016/j.biopsych.2014.09.013.
The procognitive actions of psychostimulants are only associated with low doses. Surprisingly, despite nearly 80 years of clinical use, the neurobiology of the procognitive actions of psychostimulants has only recently been systematically investigated. Findings from this research unambiguously demonstrate that the cognition-enhancing effects of psychostimulants involve the preferential elevation of catecholamines in the PFC and the subsequent activation of norepinephrine α2 and dopamine D1 receptors. ... This differential modulation of PFC-dependent processes across dose appears to be associated with the differential involvement of noradrenergic α2 versus α1 receptors. Collectively, this evidence indicates that at low, clinically relevant doses, psychostimulants are devoid of the behavioral and neurochemical actions that define this class of drugs and instead act largely as cognitive enhancers (improving PFC-dependent function). ... In particular, in both animals and humans, lower doses maximally improve performance in tests of working memory and response inhibition, whereas maximal suppression of overt behavior and facilitation of attentional processes occurs at higher doses.
- ^ Ilieva IP, Hook CJ, Farah MJ. Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis. J. Cogn. Neurosci. January 2015: 1–21. PMID 25591060. doi:10.1162/jocn_a_00776.
Specifically, in a set of experiments limited to high-quality designs, we found significant enhancement of several cognitive abilities. ... The results of this meta-analysis ... do confirm the reality of cognitive enhancing effects for normal healthy adults in general, while also indicating that these effects are modest in size.
- ^
Bagot KS, Kaminer Y. Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: a systematic review. Addiction. April 2014, 109 (4): 547–557. PMC 4471173
 . PMID 24749160. doi:10.1111/add.12460.
. PMID 24749160. doi:10.1111/add.12460. Amphetamine has been shown to improve consolidation of information (0.02 ≥ P ≤ 0.05), leading to improved recall.
- ^ Devous MD, Trivedi MH, Rush AJ. Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers. J. Nucl. Med. April 2001, 42 (4): 535–542. PMID 11337538.
- ^
Malenka RC, Nestler EJ, Hyman SE. Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ^ 71.0 71.1 71.2 Wood S, Sage JR, Shuman T, Anagnostaras SG. Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol. Rev. January 2014, 66 (1): 193–221. PMID 24344115. doi:10.1124/pr.112.007054.
- ^ Twohey M. Pills become an addictive study aid. JS Online. 2006-03-26 [2007-12-02]. (原始內容存檔於2007-08-15).
- ^ 73.00 73.01 73.02 73.03 73.04 73.05 73.06 73.07 73.08 73.09 73.10 73.11 73.12 73.13 73.14 73.15 73.16 73.17 73.18 73.19 73.20 Westfall DP, Westfall TC. Miscellaneous Sympathomimetic Agonists. Brunton LL, Chabner BA, Knollmann BC (編). Goodman & Gilman's Pharmacological Basis of Therapeutics 12th. New York, USA: McGraw-Hill. 2010. ISBN 9780071624428.
- ^ 74.0 74.1 74.2 74.3
Parr JW. Attention-deficit hyperactivity disorder and the athlete: new advances and understanding. Clin. Sports Med. July 2011, 30 (3): 591–610. PMID 21658550. doi:10.1016/j.csm.2011.03.007.
In 1980, Chandler and Blair47 showed significant increases in knee extension strength, acceleration, anaerobic capacity, time to exhaustion during exercise, pre-exercise and maximum heart rates, and time to exhaustion during maximal oxygen consumption (VO2 max) testing after administration of 15 mg of dextroamphetamine versus placebo. Most of the information to answer this question has been obtained in the past decade through studies of fatigue rather than an attempt to systematically investigate the effect of ADHD drugs on exercise.
- ^ 75.0 75.1 75.2
Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R. Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing. Sports Med. May 2013, 43 (5): 301–311. PMID 23456493. doi:10.1007/s40279-013-0030-4.
In high-ambient temperatures, dopaminergic manipulations clearly improve performance. The distribution of the power output reveals that after dopamine reuptake inhibition, subjects are able to maintain a higher power output compared with placebo. ... Dopaminergic drugs appear to override a safety switch and allow athletes to use a reserve capacity that is 『off-limits』 in a normal (placebo) situation.
- ^ Bracken NM. National Study of Substance Use Trends Among NCAA College Student-Athletes (PDF). NCAA Publications. National Collegiate Athletic Association. January 2012 [2013-10-08]. (原始內容存檔 (PDF)於2020-08-20).
- ^
Docherty JR. Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA). Br. J. Pharmacol. June 2008, 154 (3): 606–622. PMC 2439527
 . PMID 18500382. doi:10.1038/bjp.2008.124.
. PMID 18500382. doi:10.1038/bjp.2008.124.
- ^
Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson's disease and timing deficits. Front. Integr. Neurosci. October 2013, 7: 75. PMC 3813949
 . PMID 24198770. doi:10.3389/fnint.2013.00075.
. PMID 24198770. doi:10.3389/fnint.2013.00075. Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or 「clock,」 activity. For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft advances the start of responding during interval timing, whereas antagonists of D2 type dopamine receptors typically slow timing;... Depletion of dopamine in healthy volunteers impairs timing, while amphetamine releases synaptic dopamine and speeds up timing.
- ^
Rattray B, Argus C, Martin K, Northey J, Driller M. Is it time to turn our attention toward central mechanisms for post-exertional recovery strategies and performance?. Front. Physiol. March 2015, 6: 79. PMC 4362407
 . PMID 25852568. doi:10.3389/fphys.2015.00079.
. PMID 25852568. doi:10.3389/fphys.2015.00079. Aside from accounting for the reduced performance of mentally fatigued participants, this model rationalizes the reduced RPE and hence improved cycling time trial performance of athletes using a glucose mouthwash (Chambers et al., 2009) and the greater power output during a RPE matched cycling time trial following amphetamine ingestion (Swart, 2009). ... Dopamine stimulating drugs are known to enhance aspects of exercise performance (Roelands et al., 2008)
- ^
Roelands B, De Pauw K, Meeusen R. Neurophysiological effects of exercise in the heat. Scand. J. Med. Sci. Sports. June 2015,. 25 Suppl 1: 65–78. PMID 25943657. doi:10.1111/sms.12350.
This indicates that subjects did not feel they were producing more power and consequently more heat. The authors concluded that the 「safety switch」 or the mechanisms existing in the body to prevent harmful effects are overridden by the drug administration (Roelands et al., 2008b). Taken together, these data indicate strong ergogenic effects of an increased DA concentration in the brain, without any change in the perception of effort.
- ^ 引用錯誤:沒有為名為
FDA Effect的參考文獻提供內容 - ^
Kessler S. Drug therapy in attention-deficit hyperactivity disorder. South. Med. J. January 1996, 89 (1): 33–38. PMID 8545689. doi:10.1097/00007611-199601000-00005.
statements on package inserts are not intended to limit medical practice. Rather they are intended to limit claims by pharmaceutical companies. ... the FDA asserts explicitly, and the courts have upheld that clinical decisions are to be made by physicians and patients in individual situations.
- ^ 83.0 83.1 83.2 83.3 83.4 83.5 83.6 83.7 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 4–6. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ 84.00 84.01 84.02 84.03 84.04 84.05 84.06 84.07 84.08 84.09 84.10 Heedes G, Ailakis J. Amphetamine (PIM 934). INCHEM. International Programme on Chemical Safety. [2014-06-24]. (原始內容存檔於2019-04-18).
- ^ 85.0 85.1 Dexedrine Prescribing Information (PDF). United States Food and Drug Administration. Amedra Pharmaceuticals LLC. October 2013 [2013-11-04]. (原始內容存檔 (PDF)於2016-03-04).
- ^ Feinberg SS. Combining stimulants with monoamine oxidase inhibitors: a review of uses and one possible additional indication. J. Clin. Psychiatry. November 2004, 65 (11): 1520–1524. PMID 15554766. doi:10.4088/jcp.v65n1113.
- ^ Stewart JW, Deliyannides DA, McGrath PJ. How treatable is refractory depression?. J. Affect. Disord. June 2014, 167: 148–152. PMID 24972362. doi:10.1016/j.jad.2014.05.047.
- ^ 88.0 88.1 88.2 88.3 Vitiello B. Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function. Child Adolesc. Psychiatr. Clin. N. Am. April 2008, 17 (2): 459–474. PMC 2408826
 . PMID 18295156. doi:10.1016/j.chc.2007.11.010.
. PMID 18295156. doi:10.1016/j.chc.2007.11.010.
- ^ 89.00 89.01 89.02 89.03 89.04 89.05 89.06 89.07 89.08 89.09 89.10 89.11 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 4–8. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa (PDF). J. Investig. Allergol. Clin. Immunol. 2006, 16 (3): 148–155 [2015-04-29]. PMID 16784007. (原始內容存檔 (PDF)於2020-05-10).
Table 2. Decongestants Causing Rhinitis Medicamentosa
– Nasal decongestants:
– Sympathomimetic:
· Amphetamine - ^ 92.0 92.1 FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults. United States Food and Drug Administration. 2011-12-20 [2013-11-04]. (原始內容存檔於2013-10-30).
- ^ Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O'Duffy A, Connell FA, Ray WA. ADHD drugs and serious cardiovascular events in children and young adults. N. Engl. J. Med. 2011-11, 365 (20): 1896–1904. PMC 4943074
 . PMID 22043968. doi:10.1056/NEJMoa1110212.
. PMID 22043968. doi:10.1056/NEJMoa1110212.
- ^ 94.0 94.1 FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults. United States Food and Drug Administration. 2011-12-15 [2013-11-04]. (原始內容存檔於2013-10-30).
- ^ Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011-12, 306 (24): 2673–2683. PMC 3350308
 . PMID 22161946. doi:10.1001/jama.2011.1830.
. PMID 22161946. doi:10.1001/jama.2011.1830.
- ^ Montgomery KA. Sexual desire disorders. Psychiatry (Edgmont). 2008-06, 5 (6): 50–55. PMC 2695750
 . PMID 19727285.
. PMID 19727285.
- ^ O'Connor PG. Amphetamines. Merck Manual for Health Care Professionals. Merck. 2012-02 [2012-05-08]. (原始內容存檔於2012-05-06).
- ^ 98.0 98.1 98.2 98.3 Shoptaw SJ, Kao U, Ling W. Shoptaw SJ, Ali R , 編. Treatment for amphetamine psychosis. Cochrane Database Syst. Rev. January 2009, (1): CD003026. PMID 19160215. doi:10.1002/14651858.CD003026.pub3.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - ^ 99.0 99.1 Greydanus D. Stimulant Misuse: Strategies to Manage a Growing Problem (PDF). American College Health Association (Review Article). ACHA Professional Development Program: 20. [2013-11-02]. (原始內容 (PDF)存檔於2013-11-03).
- ^ 100.0 100.1 Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol. Psychiatry. May 2009, 65 (10): 900–904. PMC 2693956
 . PMID 19111278. doi:10.1016/j.biopsych.2008.11.016.
. PMID 19111278. doi:10.1016/j.biopsych.2008.11.016. This study demonstrates that humans, like nonhumans, prefer a place associated with amphetamine administration. These findings support the idea that subjective responses to a drug contribute to its ability to establish place conditioning.
- ^ 101.0 101.1 Nestler, Eric J.; Malenka, Robert C. Chapter 15: Reinforcement and Addictive Disorders. Molecular neuropharmacology : a foundation for clinical neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 364–375. ISBN 978-0-07-164119-7. OCLC 273018757.
- ^ 毒品危害防制條例. 全國法規資料庫. 中華民國法務部. [2022-09-05]. (原始內容存檔於2019-06-11).
- ^ 103.0 103.1 Spiller HA, Hays HL, Aleguas A. Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management. CNS Drugs. June 2013, 27 (7): 531–543. PMID 23757186. doi:10.1007/s40263-013-0084-8.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- ^ Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013 (PDF). Lancet. 2015, 385 (9963): 117–171 [2015-03-03]. PMC 4340604
 . PMID 25530442. doi:10.1016/S0140-6736(14)61682-2.
. PMID 25530442. doi:10.1016/S0140-6736(14)61682-2. Amphetamine use disorders ... 3,788 (3,425–4,145)
- ^ Kanehisa Laboratories. Amphetamine – Homo sapiens (human). KEGG Pathway. 2014-10-10 [2014-10-31]. (原始內容存檔於2020-08-08).
- ^ 106.0 106.1 106.2 106.3 106.4 Nechifor M. Magnesium in drug dependences. Magnes. Res. March 2008, 21 (1): 5–15. PMID 18557129.
- ^ 107.0 107.1 107.2 107.3 107.4 Ruffle JK. Molecular neurobiology of addiction: what's all the (Δ)FosB about?. Am. J. Drug Alcohol Abuse. November 2014, 40 (6): 428–437. PMID 25083822. doi:10.3109/00952990.2014.933840.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure.
- ^ 108.0 108.1 108.2 108.3 108.4 Nestler, Eric J. Cellular basis of memory for addiction. Dialogues in Clinical Neuroscience. 2013-12, 15 (4): 431–443. ISSN 1294-8322. PMC 3898681
 . PMID 24459410. doi:10.31887/DCNS.2013.15.4/enestler.
. PMID 24459410. doi:10.31887/DCNS.2013.15.4/enestler.
- ^ Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. November 2011, 12 (11): 623–637. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111. ΔFosB serves as one of the master control proteins governing this structural plasticity.
- ^ 110.00 110.01 110.02 110.03 110.04 110.05 110.06 110.07 110.08 110.09 110.10 110.11 110.12 110.13 110.14 110.15 110.16 110.17 110.18 110.19 110.20 110.21 Olsen CM. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology. December 2011, 61 (7): 1109–1122. PMC 3139704
 . PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010.
. PMID 21459101. doi:10.1016/j.neuropharm.2011.03.010. Similar to environmental enrichment, studies have found that exercise reduces self-administration and relapse to drugs of abuse (Cosgrove et al., 2002; Zlebnik et al., 2010). There is also some evidence that these preclinical findings translate to human populations, as exercise reduces withdrawal symptoms and relapse in abstinent smokers (Daniel et al., 2006; Prochaska et al., 2008), and one drug recovery program has seen success in participants that train for and compete in a marathon as part of the program (Butler, 2005). ... In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008).
- ^ 111.0 111.1 111.2 111.3 Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA. Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci. Biobehav. Rev. September 2013, 37 (8): 1622–1644. PMC 3788047
 . PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011.
. PMID 23806439. doi:10.1016/j.neubiorev.2013.06.011. These findings suggest that exercise may 「magnitude」-dependently prevent the development of an addicted phenotype possibly by blocking/reversing behavioral and neuroadaptive changes that develop during and following extended access to the drug. ... Exercise has been proposed as a treatment for drug addiction that may reduce drug craving and risk of relapse. Although few clinical studies have investigated the efficacy of exercise for preventing relapse, the few studies that have been conducted generally report a reduction in drug craving and better treatment outcomes ... Taken together, these data suggest that the potential benefits of exercise during relapse, particularly for relapse to psychostimulants, may be mediated via chromatin remodeling and possibly lead to greater treatment outcomes.
- ^ 112.0 112.1 112.2 Zhou Y, Zhao M, Zhou C, Li R. Sex differences in drug addiction and response to exercise intervention: From human to animal studies. Front. Neuroendocrinol. July 2015, 40: 24–41. PMID 26182835. doi:10.1016/j.yfrne.2015.07.001.
Collectively, these findings demonstrate that exercise may serve as a substitute or competition for drug abuse by changing ΔFosB or cFos immunoreactivity in the reward system to protect against later or previous drug use. ... The postulate that exercise serves as an ideal intervention for drug addiction has been widely recognized and used in human and animal rehabilitation.
- ^ 113.0 113.1 113.2 Linke SE, Ussher M. Exercise-based treatments for substance use disorders: evidence, theory, and practicality. Am. J. Drug Alcohol Abuse. January 2015, 41 (1): 7–15. PMC 4831948
 . PMID 25397661. doi:10.3109/00952990.2014.976708.
. PMID 25397661. doi:10.3109/00952990.2014.976708. The limited research conducted suggests that exercise may be an effective adjunctive treatment for SUDs. In contrast to the scarce intervention trials to date, a relative abundance of literature on the theoretical and practical reasons supporting the investigation of this topic has been published. ... numerous theoretical and practical reasons support exercise-based treatments for SUDs, including psychological, behavioral, neurobiological, nearly universal safety profile, and overall positive health effects.
- ^ 114.0 114.1 Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and Addictive Disorders. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 386. ISBN 9780071481274.
Currently, cognitive–behavioral therapies are the most successful treatment available for preventing the relapse of psychostimulant use.
- ^ Greene SL, Kerr F, Braitberg G. Review article: amphetamines and related drugs of abuse. Emerg. Med. Australas. 2008-10, 20 (5): 391–402. PMID 18973636. doi:10.1111/j.1742-6723.2008.01114.x.
- ^ Albertson TE. Amphetamines. Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AH (編). Poisoning & Drug Overdose 6th. New York: McGraw-Hill Medical. 2011: 77–79. ISBN 9780071668330.
- ^ Glossary. Icahn School of Medicine. [2021-04-29].
- ^ Volkow, Nora D.; Koob, George F.; McLellan, A. Thomas. Longo, Dan L. , 編. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine. 2016-01-28, 374 (4): 363–371. ISSN 0028-4793. PMC 6135257
 . PMID 26816013. doi:10.1056/NEJMra1511480 (英語).
. PMID 26816013. doi:10.1056/NEJMra1511480 (英語).
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and Addictive Disorders. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York: McGraw-Hill Medical. 2009: 368. ISBN 9780071481274.
Such agents also have important therapeutic uses; cocaine, for example, is used as a local anesthetic (Chapter 2), and amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction, especially when they are rapidly administered or when high-potency forms are given.
- ^ Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr. Med. Res. Opin. May 2008, 24 (5): 1345–1357. PMID 18384709. doi:10.1185/030079908X280707.
When oral formulations of psychostimulants are used at recommended doses and frequencies, they are unlikely to yield effects consistent with abuse potential in patients with ADHD.
- ^ Stolerman IP. Stolerman IP , 編. Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. 2010: 78. ISBN 9783540686989.
- ^ Amphetamines: Drug Use and Abuse. Merck Manual Home Edition. Merck. February 2003 [2007-02-28]. (原始內容存檔於2007-02-17).
- ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M. Pérez-Mañá C , 編. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev. September 2013, 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
- ^ Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. July 2006, 29: 565–598. PMID 16776597. doi:10.1146/annurev.neuro.29.051605.113009.
- ^ 125.0 125.1 125.2 125.3 125.4 125.5 125.6 125.7 Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. November 2011, 12 (11): 623–637. PMC 3272277
 . PMID 21989194. doi:10.1038/nrn3111.
. PMID 21989194. doi:10.1038/nrn3111.
- ^ 126.0 126.1 126.2 126.3 Steiner H, Van Waes V. Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. January 2013, 100: 60–80. PMC 3525776
 . PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
. PMID 23085425. doi:10.1016/j.pneurobio.2012.10.001.
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 4: Signal Transduction in the Brain. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 94. ISBN 9780071481274.
- ^ Kanehisa Laboratories. Alcoholism – Homo sapiens (human). KEGG Pathway. 2014-10-29 [2014-10-31]. (原始內容存檔於2019-11-12).
- ^ Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 2009-02, 106 (8): 2915–2920. PMC 2650365
 . PMID 19202072. doi:10.1073/pnas.0813179106.
. PMID 19202072. doi:10.1073/pnas.0813179106.
- ^ Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. January 2014,. 76 Pt B: 259–268. PMC 3766384
 . PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004.
. PMID 23643695. doi:10.1016/j.neuropharm.2013.04.004.
- ^ 131.0 131.1 Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M. Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. J. Psychoactive Drugs. March 2012, 44 (1): 38–55. PMC 4040958
 . PMID 22641964. doi:10.1080/02791072.2012.662112.
. PMID 22641964. doi:10.1080/02791072.2012.662112.
- ^ Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J. Neurosci. February 2013, 33 (8): 3434–3442. PMC 3865508
 . PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
. PMID 23426671. doi:10.1523/JNEUROSCI.4881-12.2013.
- ^ Beloate LN, Weems PW, Casey GR, Webb IC, Coolen LM. Nucleus accumbens NMDA receptor activation regulates amphetamine cross-sensitization and deltaFosB expression following sexual experience in male rats. Neuropharmacology. February 2016, 101: 154–164. PMID 26391065. doi:10.1016/j.neuropharm.2015.09.023.
- ^ Stoops WW, Rush CR. Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research. Expert Rev Clin Pharmacol. May 2014, 7 (3): 363–374. PMC 4017926
 . PMID 24716825. doi:10.1586/17512433.2014.909283.
. PMID 24716825. doi:10.1586/17512433.2014.909283. Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- ^ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M. Efficacy of psychostimulant drugs for amphetamine abuse or dependence. Cochrane Database Syst. Rev. September 2013, 9: CD009695. PMID 23996457. doi:10.1002/14651858.CD009695.pub2.
To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. ... Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy
- ^ Forray A, Sofuoglu M. Future pharmacological treatments for substance use disorders. Br. J. Clin. Pharmacol. February 2014, 77 (2): 382–400. PMC 4014020
 . PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x.
. PMID 23039267. doi:10.1111/j.1365-2125.2012.04474.x.
- ^ 137.0 137.1 137.2 137.3 Grandy DK, Miller GM, Li JX. "TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug Alcohol Depend. February 2016, 159: 9–16. PMID 26644139. doi:10.1016/j.drugalcdep.2015.11.014.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ 138.0 138.1 Jing L, Li JX. Trace amine-associated receptor 1: A promising target for the treatment of psychostimulant addiction. Eur. J. Pharmacol. August 2015, 761: 345–352. PMC 4532615
 . PMID 26092759. doi:10.1016/j.ejphar.2015.06.019.
. PMID 26092759. doi:10.1016/j.ejphar.2015.06.019. Existing data provided robust preclinical evidence supporting the development of TAAR1 agonists as potential treatment for psychostimulant abuse and addiction.
- ^ 139.0 139.1 Malenka RC, Nestler EJ, Hyman SE. Chapter 5: Excitatory and Inhibitory Amino Acids. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 124–125. ISBN 9780071481274.
- ^ 140.0 140.1 140.2 Carroll ME, Smethells JR. Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments. Front. Psychiatry. February 2016, 6: 175. PMC 4745113
 . PMID 26903885. doi:10.3389/fpsyt.2015.00175.
. PMID 26903885. doi:10.3389/fpsyt.2015.00175. Physical Exercise
There is accelerating evidence that physical exercise is a useful treatment for preventing and reducing drug addiction ... In some individuals, exercise has its own rewarding effects, and a behavioral economic interaction may occur, such that physical and social rewards of exercise can substitute for the rewarding effects of drug abuse. ... The value of this form of treatment for drug addiction in laboratory animals and humans is that exercise, if it can substitute for the rewarding effects of drugs, could be self-maintained over an extended period of time. Work to date in [laboratory animals and humans] regarding exercise as a treatment for drug addiction supports this hypothesis. ... Animal and human research on physical exercise as a treatment for stimulant addiction indicates that this is one of the most promising treatments on the horizon. - ^ 141.0 141.1 141.2 Shoptaw SJ, Kao U, Heinzerling K, Ling W. Shoptaw SJ , 編. Treatment for amphetamine withdrawal. Cochrane Database Syst. Rev. April 2009, (2): CD003021. PMID 19370579. doi:10.1002/14651858.CD003021.pub2.
The prevalence of this withdrawal syndrome is extremely common (Cantwell 1998; Gossop 1982) with 87.6% of 647 individuals with amphetamine dependence reporting six or more signs of amphetamine withdrawal listed in the DSM when the drug is not available (Schuckit 1999) ... The severity of withdrawal symptoms is greater in amphetamine dependent individuals who are older and who have more extensive amphetamine use disorders (McGregor 2005). Withdrawal symptoms typically present within 24 hours of the last use of amphetamine, with a withdrawal syndrome involving two general phases that can last 3 weeks or more. The first phase of this syndrome is the initial "crash" that resolves within about a week (Gossop 1982;McGregor 2005) ...
- ^ Cantwell, Bernadette; McBride, Andrew J. Self detoxication by amphetamine dependent patients: a pilot study. Drug and Alcohol Dependence (Elsevier BV). 1998, 49 (2): 157–163 [2017-05-09]. doi:10.1016/s0376-8716(97)00160-9.
- ^ Amphetamine-Related Psychiatric Disorders Clinical Presentation: History, Physical, Causes. Medscape Reference. 2015-12-03 [2017-05-09]. (原始內容存檔於2020-08-10).
- ^ Adderall IR Prescribing Information (PDF). United States Food and Drug Administration. Teva Pharmaceuticals USA, Inc. October 2015 [2016-05-18]. (原始內容存檔 (PDF)於2018-09-15).
- ^ Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ The amphetamine withdrawal syndrome. Department of Health. [2017-05-09]. (原始內容存檔於2017-02-08).
- ^ 147.0 147.1 147.2 Self detoxication by amphetamine dependent patients: a pilot study. ScienceDirect.com. 2016-01-01 [2017-05-09].
- ^ Advokat C. Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD. J. Atten. Disord. July 2007, 11 (1): 8–16. PMID 17606768. doi:10.1177/1087054706295605.
- ^ 149.0 149.1 149.2 149.3 Bowyer JF, Hanig JP. Amphetamine- and methamphetamine-induced hyperthermia: Implications of the effects produced in brain vasculature and peripheral organs to forebrain neurotoxicity. Temperature (Austin). November 2014, 1 (3): 172–182. PMC 5008711
 . PMID 27626044. doi:10.4161/23328940.2014.982049.
. PMID 27626044. doi:10.4161/23328940.2014.982049. Hyperthermia alone does not produce amphetamine-like neurotoxicity but AMPH and METH exposures that do not produce hyperthermia (≥40°C) are minimally neurotoxic. Hyperthermia likely enhances AMPH and METH neurotoxicity directly through disruption of protein function, ion channels and enhanced ROS production. ... The hyperthermia and the hypertension produced by high doses amphetamines are a primary cause of transient breakdowns in the blood-brain barrier (BBB) resulting in concomitant regional neurodegeneration and neuroinflammation in laboratory animals. ... In animal models that evaluate the neurotoxicity of AMPH and METH, it is quite clear that hyperthermia is one of the essential components necessary for the production of histological signs of dopamine terminal damage and neurodegeneration in cortex, striatum, thalamus and hippocampus.
- ^ Amphetamine. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. [2014-02-26]. (原始內容存檔於2017-10-02).
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- ^ Malenka RC, Nestler EJ, Hyman SE. Chapter 15: Reinforcement and addictive disorders. Sydor A, Brown RY (編). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience 2nd. New York, USA: McGraw-Hill Medical. 2009: 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- ^ Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox. Res. February 2000, 1 (3): 181–195. PMID 12835101. doi:10.1007/BF03033289.
- ^ Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself (PDF). Acta Med. Okayama. 2008-06, 62 (3): 141–150 [2017-04-30]. PMID 18596830. (原始內容存檔 (PDF)於2020-12-22).
- ^ 154.0 154.1 Neal R. Swerdlow. Savita G. Bhakta , Jo Talledo, Lindsay Benster, Juliana Kotz, Maria Lavadia and Gregory A. Light. Lessons learned by giving amphetamine to antipsychotic-medicated schizophrenia patients. Neurophsychopharmacology. 2019.
- ^ Hofmann FG. A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects 2nd. New York, USA: Oxford University Press. 1983: 329. ISBN 9780195030570.
- ^ 156.00 156.01 156.02 156.03 156.04 156.05 156.06 156.07 156.08 156.09 Adderall XR Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 8–10. December 2013 [2013-12-30]. (原始內容存檔 (PDF)於2018-07-18).
- ^ Krause J. SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder. Expert Rev. Neurother. April 2008, 8 (4): 611–625. PMID 18416663. doi:10.1586/14737175.8.4.611.
Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.
- ^ Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. February 2011, 69 (4): 628–649. PMC 3065181
 . PMID 21338876. doi:10.1016/j.neuron.2011.02.010.
. PMID 21338876. doi:10.1016/j.neuron.2011.02.010. They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).
- ^ 159.0 159.1 Scholze P, Nørregaard L, Singer EA, Freissmuth M, Gether U, Sitte HH. The role of zinc ions in reverse transport mediated by monoamine transporters. J. Biol. Chem. June 2002, 277 (24): 21505–21513. PMID 11940571. doi:10.1074/jbc.M112265200.
The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET).
- ^ Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry. October 2012, 51 (10): 1003–1019.e20. PMID 23021477. doi:10.1016/j.jaac.2012.08.015.
With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110
- ^ Richard RA. Chapter 5—Medical Aspects of Stimulant Use Disorders. National Center for Biotechnology Information Bookshelf. Treatment Improvement Protocol 33. Substance Abuse and Mental Health Services Administration. 1999. (原始內容存檔於2020-11-11) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 162.0 162.1 162.2 162.3 Vyvanse Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc: 12–16. January 2015 [2015-02-24]. (原始內容存檔 (PDF)於2015-02-25).
- ^ 引用錯誤:沒有為名為
DBH amph primary"的參考文獻提供內容 - ^ 164.0 164.1 butyrate-CoA ligase. BRENDA. Technische Universität Braunschweig. (原始內容存檔於2017-06-22) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 165.0 165.1 glycine N-acyltransferase. BRENDA. Technische Universität Braunschweig. (原始內容存檔於2017-06-23) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 166.0 166.1 p-Hydroxyamphetamine. PubChem Compound. National Center for Biotechnology Information. (原始內容存檔於2013-06-07) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 167.0 167.1 Phenylpropanolamine. PubChem Compound. National Center for Biotechnology Information. (原始內容存檔於2014-10-18) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ p-Hydroxynorephedrine. PubChem Compound. National Center for Biotechnology Information. (原始內容存檔於2013-10-15) 使用
|archiveurl=需要含有|url=(幫助).|section-url=被忽略 (幫助);|section=被忽略 (幫助); - ^ 169.0 169.1 169.2 169.3 169.4 Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci. January 2011, 1216: 86–98. PMC 4183197
 . PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x.
. PMID 21272013. doi:10.1111/j.1749-6632.2010.05906.x. VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC). ... AMPH release of DA from synapses requires both an action at VMAT2 to release DA to the cytoplasm and a concerted release of DA from the cytoplasm via "reverse transport" through DAT.
- ^ Maguire JJ, Davenport AP. TA1 receptor. IUPHAR database. International Union of Basic and Clinical Pharmacology. 2 December 2014 [8 December 2014]. (原始內容存檔於2015-06-29).
- ^ Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Lakhlani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. July 2001, 98 (16): 8966–8971. PMC 55357
 . PMID 11459929. doi:10.1073/pnas.151105198.
. PMID 11459929. doi:10.1073/pnas.151105198.
- ^ 172.0 172.1 Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG. Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron. 2014-07, 83 (2): 404–416. PMC 4159050
 . PMID 25033183. doi:10.1016/j.neuron.2014.05.043.
. PMID 25033183. doi:10.1016/j.neuron.2014.05.043. AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH
- ^ 173.0 173.1 SLC18 family of vesicular amine transporters. IUPHAR database. International Union of Basic and Clinical Pharmacology. [2015-11-13]. (原始內容存檔於2020-10-28).
- ^ 174.0 174.1 174.2 SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 [ Homo sapiens (human) ]. NCBI Gene. United States National Library of Medicine – National Center for Biotechnology Information. [2014-11-11]. (原始內容存檔於2020-10-21).
Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. ... internalization of EAAT3 triggered by amphetamine increases glutamatergic signaling and thus contributes to the effects of amphetamine on neurotransmission.
- ^ Zhu HJ, Appel DI, Gründemann D, Markowitz JS. Interaction of organic cation transporter 3 (SLC22A3) and amphetamine. J. Neurochem. 2010-07, 114 (1): 142–149. PMC 3775896
 . PMID 20402963. doi:10.1111/j.1471-4159.2010.06738.x.
. PMID 20402963. doi:10.1111/j.1471-4159.2010.06738.x.
- ^ Rytting E, Audus KL. Novel organic cation transporter 2-mediated carnitine uptake in placental choriocarcinoma (BeWo) cells. J. Pharmacol. Exp. Ther. January 2005, 312 (1): 192–198. PMID 15316089. doi:10.1124/jpet.104.072363.
- ^ Inazu M, Takeda H, Matsumiya T. [The role of glial monoamine transporters in the central nervous system]. Nihon Shinkei Seishin Yakurigaku Zasshi. August 2003, 23 (4): 171–178. PMID 13677912 (日語).
- ^ 178.0 178.1 178.2 Vicentic A, Jones DC. The CART (cocaine- and amphetamine-regulated transcript) system in appetite and drug addiction. J. Pharmacol. Exp. Ther. February 2007, 320 (2): 499–506. PMID 16840648. doi:10.1124/jpet.105.091512.
The physiological importance of CART was further substantiated in numerous human studies demonstrating a role of CART in both feeding and psychostimulant addiction. ... Colocalization studies also support a role for CART in the actions of psychostimulants. ... CART and DA receptor transcripts colocalize (Beaudry et al., 2004). Second, dopaminergic nerve terminals in the NAc synapse on CART-containing neurons (Koylu et al., 1999), hence providing the proximity required for neurotransmitter signaling. These studies suggest that DA plays a role in regulating CART gene expression possibly via the activation of CREB.
- ^ Zhang M, Han L, Xu Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin. Exp. Pharmacol. Physiol. June 2012, 39 (6): 586–592. PMID 22077697. doi:10.1111/j.1440-1681.2011.05642.x.
Recently, it was demonstrated that CART, as a neurotrophic peptide, had a cerebroprotective against focal ischaemic stroke and inhibited the neurotoxicity of β-amyloid protein, which focused attention on the role of CART in the central nervous system (CNS) and neurological diseases. ... The literature indicates that there are many factors, such as regulation of the immunological system and protection against energy failure, that may be involved in the cerebroprotection afforded by CART
- ^ 180.0 180.1 Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat. Rev. Neurosci. October 2008, 9 (10): 747–758. PMC 4418456
 . PMID 18802445. doi:10.1038/nrn2493.
. PMID 18802445. doi:10.1038/nrn2493. Several studies on CART (cocaine- and amphetamine-regulated transcript)-peptide-induced cell signalling have demonstrated that CART peptides activate at least three signalling mechanisms. First, CART 55–102 inhibited voltage-gated L-type Ca2+ channels ...
- ^ Lin Y, Hall RA, Kuhar MJ. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6–38 as a CART receptor antagonist. Neuropeptides. October 2011, 45 (5): 351–358. PMC 3170513
 . PMID 21855138. doi:10.1016/j.npep.2011.07.006.
. PMID 21855138. doi:10.1016/j.npep.2011.07.006.
- ^ Monoamine oxidase (Homo sapiens). BRENDA. Technische Universität Braunschweig. 1 January 2014 [4 May 2014]. (原始內容存檔於2020-07-27).
- ^ 183.0 183.1 引用錯誤:沒有為名為
5HT1A secondary的參考文獻提供內容 - ^ 引用錯誤:沒有為名為
5HT1A Primary的參考文獻提供內容 - ^ Finnema SJ, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, Sallinen J, Wong E, Farde L, Halldin C, Grimwood S. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology. November 2015, 232 (21–22): 4129–4157. PMC 4600473
 . PMID 25921033. doi:10.1007/s00213-015-3938-6.
. PMID 25921033. doi:10.1007/s00213-015-3938-6. More recently, Colasanti and colleagues reported that a pharmacologically induced elevation in endogenous opioid release reduced [11C]carfentanil binding in several regions of the human brain, including the basal ganglia, frontal cortex, and thalamus (Colasanti et al. 2012). Oral administration of d-amphetamine, 0.5 mg/kg, 3 h before [11C]carfentanil injection, reduced BPND values by 2–10 %. The results were confirmed in another group of subjects (Mick et al. 2014). However, Guterstam and colleagues observed no change in [11C]carfentanil binding when d-amphetamine, 0.3 mg/kg, was administered intravenously directly before injection of [11C]carfentanil (Guterstam et al. 2013). It has been hypothesized that this discrepancy may be related to delayed increases in extracellular opioid peptide concentrations following amphetamine-evoked monoamine release (Colasanti et al. 2012; Mick et al. 2014).
- ^ Loseth GE, Ellingsen DM, Leknes S. State-dependent μ-opioid modulation of social motivation. Front. Behav. Neurosci. December 2014, 8: 1–15. PMC 4264475
 . PMID 25565999. doi:10.3389/fnbeh.2014.00430.
. PMID 25565999. doi:10.3389/fnbeh.2014.00430. Similar MOR activation patterns were reported during positive mood induced by an amusing video clip (Koepp et al., 2009) and following amphetamine administration in humans (Colasanti et al., 2012).
- ^ Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, Erritzoe D, Tziortzi AC, Reed LJ, Lingford-Hughes AR, Waldman AD, Schruers KR, Matthews PM, Gunn RN, Nutt DJ, Rabiner EA. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol. Psychiatry. September 2012, 72 (5): 371–377. PMID 22386378. doi:10.1016/j.biopsych.2012.01.027.
- ^ 188.0 188.1 引用錯誤:沒有為名為
Human amph effects的參考文獻提供內容 - ^ 189.0 189.1 引用錯誤:沒有為名為
Primary: Human HPA axis的參考文獻提供內容 - ^ 190.0 190.1 Lewin AH, Miller GM, Gilmour B. Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class. Bioorg. Med. Chem. December 2011, 19 (23): 7044–7048. PMC 3236098
 . PMID 22037049. doi:10.1016/j.bmc.2011.10.007.
. PMID 22037049. doi:10.1016/j.bmc.2011.10.007.
- ^ Adzenys XR Prescribing Information (PDF). United States Food and Drug Administration. Neos Therapeutics, Inc.: 15. January 2016 [2016-03-07]. (原始內容存檔 (PDF)於2020-10-30).
ADZENYS XR-ODT (amphetamine extended-release orally disintegrating tablet) contains a 3 to 1 ratio of d- to l-amphetamine, a central nervous system stimulant.
- ^ Adzenys XR. United States Food and Drug Administration. [2016-03-07].
- ^ Evekeo. United States Food and Drug Administration. [2015-08-11].
- ^ Molecular Weight Calculator. Lenntech. [19 August 2015].
- ^ 196.0 196.1 Dextroamphetamine Sulfate USP. Mallinckrodt Pharmaceuticals. March 2014 [19 August 2015].
- ^ 197.0 197.1 D-amphetamine sulfate. Tocris. 2015 [19 August 2015].
- ^ 198.0 198.1 Amphetamine Sulfate USP. Mallinckrodt Pharmaceuticals. March 2014 [19 August 2015].
- ^ Dextroamphetamine Saccharate. Mallinckrodt Pharmaceuticals. March 2014 [19 August 2015].
- ^ Amphetamine Aspartate. Mallinckrodt Pharmaceuticals. March 2014 [19 August 2015].
- ^ Vyvanse Prescribing Information (PDF). United States Food and Drug Administration. Shire US Inc.: 17–21. May 2017 [10 July 2017].
參見
[編輯]外部連結
[編輯]- CID 3007 from PubChem – Amphetamine
- CID 5826 from PubChem – Dextroamphetamine
- CID 32893 from PubChem – Levoamphetamine
- Comparative Toxicogenomics Database entry: Amphetamine (頁面存檔備份,存於互聯網檔案館)
- Comparative Toxicogenomics Database entry: CARTPT(頁面存檔備份,存於互聯網檔案館)
- YouTube上的Drug abuse and drug addictions
- YouTube上的Mechanism of Drug Addiction in the Brain, Animation.
- YouTube上的Drug dependence
毒品危害防制條例-全國法規資料庫 (頁面存檔備份,存於互聯網檔案館)